Auditory Cueing of Pre-Learned Skills and Role of Subcortical Information Processing to Maximize Rehabilitative Outcomes Bridging Science and Music-Based Interventions
Abstract
:1. Introduction Bridging Science and Music-Based Interventions
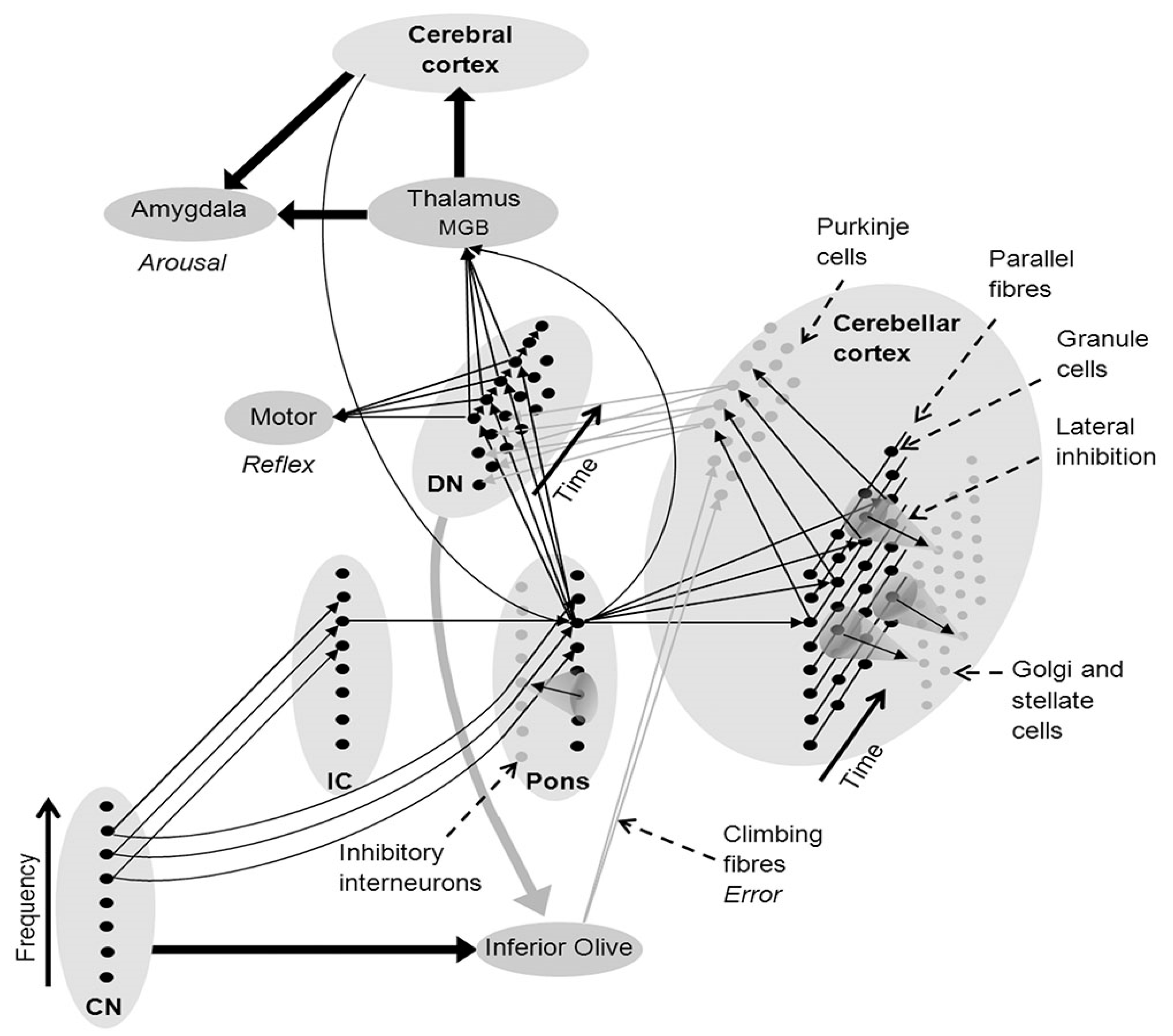
2. Auditory Entrainment Is a Fundamental Agent of Action in NMT
Engagement of Subcortical Systems by Auditory Entrainment Is Key Factor
3. Discussion: Implications for Neurologic Music Therapy in Neurorehabilitation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altenmuller, E.; Schlaug, G. Neurobiological aspects of neurologic music therapy. Music. Med. 2013, 5, 210–216. [Google Scholar] [CrossRef] [Green Version]
- O’Kelly, J.; Fachner, J.C.; Tervaniemi, M. Editorial: Dialogues in music therapy and music neuroscience: Collaborative understanding driving clinical advances. Front. Hum. Neurosci. 2016, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaut, M.H.; Hoemberg, V. (Eds.) Handbook of Neurologic Music Therapy; Oxford University Press: New York, NY, USA, 2014; p. 2. [Google Scholar]
- Thaut, M.; McIntosh, G.; Rice, R.; Miller, R.; Rathbun, J.; Brault, J. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. Off. J. Mov. Disord. Soc. 1996, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, C.M. Using rhythmic auditory stimulation for rehabilitation. In Music, Science and the Rhythmic Brain; Berger, J., Turow, G., Eds.; Rutledge: New York, NY, USA, 2011; pp. 111–121. [Google Scholar]
- Cochen De Cock, V.; Dotov, D.G.; Ihalainen, P.; Begel, V.; Galtier, F.; Lebrun, C.; Picot, M.C.; Driss, V.; Landragin, N.; Geny, C.; et al. Rhythmic abilities and musical training in Parkinson’s Disease: Do they help? NPJ Park. Dis. 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, R.S.; Naro, A.; Filoni, S.; Pullia, M.; Billeri, L.; Tomasello, P.; Portaro, S.; Di Lorenzo, G.; Tomaino, C.; Bramanti, P. Walking to your right music: A randomized controlled trial on the novel use of treadmill plus music in Parkinson’s disease. J. Neuroeng. Rehabil. 2019, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Sacks, O. Awakenings; Summit Books: New York, NY, USA, 1973. [Google Scholar]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015, 5, 1185. [Google Scholar] [CrossRef] [Green Version]
- Braunlich, K.; Seger, C.; Thaut, M. Rhythmic auditory cues shape neural network recruitment in Parkinson’s disease during repetitive motor behavior. Eur. J. Neurosci. 2019, 49, 849–858. [Google Scholar] [CrossRef]
- Tomaino, C.M. Clinical applications of music in neurologic rehabilitation. Music and Memory: Accessing Residual Function; Tomaino, C.M., Ed.; MMB Music, Inc.: St. Louis, MO, USA, 1998; pp. 19–27. [Google Scholar]
- Assaneo, M.F.; Poeppel, D. The coupling Between auditory and Motor Cortices is Rate-restricted: Evidence for and Intrinsic Speech Motor Rhythm. Sci. Adv. 2018, 4, EAA03842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaGasse, A.B.; Thaut, M.H. The neurobiological foundation of neurologic music therapy. J. Music. Med. 2013, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Altenmüller, E.; Marco-Pallares, J.; Munte, T.F.; Schneider, S. Neural reorganization underlies improvement in stroke –induced motor dysfunction by music-supported therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Gross, J.; Thut, G. A new unifying account of the roles of neuronal entrainment. Curr. Biol. 2019, 29, R890–R905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Large, E.W.; Snyder, J.S. Pulse and meter as neural resonance. Ann. N. Y. Acad. Sci. 2009, 1169, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Large, E.W.; Herrera, J.A.; Velasco, M.J. Neural networks for beat perception in musical rhythm. Front. Syst. Neurosci. 2015, 9, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapeton, J.I.; Haque, R.; Wittig, J.H., Jr.; Inati, S.K.; Zaghloul, K.A. Large-scale communication in the human brain is rhythmically modulated through alpha coherence. Curr. Biol. 2019, 29, 2801–2811. [Google Scholar] [CrossRef]
- Bartel, L.; Mosabbir, A. Possible mechanisms for the effects of sound vibration on human health. Healthcare 2021, 9, 597. [Google Scholar] [CrossRef]
- Marr, D. A theory of cerebellar cortex. J. Physiol. 1968, 202, 437–470. [Google Scholar] [CrossRef]
- Chauvigné, L.A.; Gitau, K.M.; Brown, S. The neural basis of audiomotor entrainment: An ALE meta-analysis. Front. Hum. Neurosci. 2014, 8, 776. [Google Scholar] [CrossRef] [Green Version]
- Evers, S.; Tölgyesi, B. Music and the cerebellum. Adv. Exp. Med. Biol. 2022, 1378, 195–212. [Google Scholar] [CrossRef]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef]
- McLachlan, N.M.; Wilson, S.J. The contribution of brainstem and cerebellar pathways to auditory recognition. Front. Psychol. 2017, 8, 265. [Google Scholar] [CrossRef]
- Yamazaki, T.; Lennon, W. Revisiting a theory of Cerebral Cortex. Neurosci. Res. 2019, 148, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.J.; Grahn, J.A. Music brain and movement. In The Rutledge Companion to Music Cognitions; Ashley, R., Timmers, R., Eds.; Rutledge Press: New York, NY, USA, 2017; pp. 63–73. [Google Scholar]
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Martinez, M.J.; Parsons, L.M. The neural basis of human dance. Cereb. Cortex 2006, 16, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.L.; Levitin, D.J. The neurochemistry of music. Trends Cogn. Sci. 2013, 17, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozaradan, S.; Schwartze, M.; Obermeier, C.; Kotz, S.A. Specific contributions of basal ganglia and cerebellum to the neural tracking of rhythm. Cortex 2017, 95, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Sacks, O.; Tomaino, S.M. Music and neurological disorder. Int. J. Arts Med. 1991, 1, 7–9. [Google Scholar]
- Simmons-Stern, N.R.; Budson, A.E.; Ally, B.A. Music as a memory enhancer in patients with Alzheimer’s disease. Neuropsychologia 2010, 48, 3164–3167. [Google Scholar] [CrossRef] [Green Version]
- Magee, W.; Clark, I.; Tamplin, J.; Bradt, J. Music interventions for acquired brain injury. Cochrane Database Syst. Rev. 2017, 1, CD006787. [Google Scholar] [CrossRef]
- Thaut, M.H.; Schleiffers, S.; Davis, W.B. Analysis of EMG activity in biceps and triceps muscle in a gross motor task under the influence of auditory rhythm. J. Music. Ther. 1991, 28, 64–88. [Google Scholar] [CrossRef]
- Thaut, M.H.; Gardiner, J.C.; Holmberg, D.; Horwitz, J.; Kent, L.; Andrews, G.; Donelan, B.; McIntosh, G.R. Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Ann. N. Y. Acad. Sci. 2009, 1169, 406–416. [Google Scholar] [CrossRef]
- Tomaino, C.M. Effective music therapy techniques in the treatment of non-fluent aphasia. Ann. N. Y. Acad. Sci. 2012, 1252, 312. [Google Scholar] [CrossRef] [PubMed]
- Särkämo, T.; Tervaniemi, M.; Laitinen, S. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008, 131, 866–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.E.; Churchill, N.; Leggieri, M.; Vuong, V.; Tau, M.; Fornazzari, L.R.; Thaut, M.H.; Schweizer, T.A. Long-known music exposure effects on brain imaging and cognition in early-stage cognitive decline: A pilot study. J. Alzheimer’s Dis. 2021, 84, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Brett, M. Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex 2009, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nombela, C.; Hughes, L.; Owen, A.; Grahn, J. Into the Groove: Can rhythm influence Parkinson’s Disease. Neurosci. Biobehav. Rev. 2013, 37, 2564–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Munte, T.; Rodriguez-Fornells, A.; Sailer, M.; Altenmüller, E. Music-supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music Percept. 2010, 27, 271–280. Available online: http://www.jstor.org/stable/10.1525/mp.2010.27.4.271 (accessed on 10 May 2021). [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaino, C.M. Auditory Cueing of Pre-Learned Skills and Role of Subcortical Information Processing to Maximize Rehabilitative Outcomes Bridging Science and Music-Based Interventions. Healthcare 2022, 10, 2207. https://doi.org/10.3390/healthcare10112207
Tomaino CM. Auditory Cueing of Pre-Learned Skills and Role of Subcortical Information Processing to Maximize Rehabilitative Outcomes Bridging Science and Music-Based Interventions. Healthcare. 2022; 10(11):2207. https://doi.org/10.3390/healthcare10112207
Chicago/Turabian StyleTomaino, Concetta M. 2022. "Auditory Cueing of Pre-Learned Skills and Role of Subcortical Information Processing to Maximize Rehabilitative Outcomes Bridging Science and Music-Based Interventions" Healthcare 10, no. 11: 2207. https://doi.org/10.3390/healthcare10112207




