1. Introduction
Malaria is a leading cause of mortality and morbidity among the under-five and the pregnant women in Sub Saharan Africa [
1]. These groups are at high risk due to weakened and immature immunity respectively. The need for value of money calls for the cost effective analysis of malaria interventions. This will also contribute to effective ways of controlling the spread of malaria in Kenya. Malaria transmission is highly variable across Kenya because of the different transmission intensities driven by climate and temperature. Kenya has four malaria epidemiological zones; the endemic areas, the seasonal malaria transmission, the malaria epidemic prone areas and the low risk malaria areas [
2,
3].
The current reduction in the number of malaria related cases are due to the scale up efforts of the current malaria interventions in Kenya namely use of long-lasting insecticide-treated bed nets (LLINs), indoor residual spraying (IRS), chemoprevention for most vulnerable such as intermittent preventive treatment for pregnant women (IPTp), confirmation of malaria diagnostics through rapid diagnostics tests (RDTs) and microscopy for every suspected case (case management) and timely treatment with artemisinin-based combination therapies (ACTs) [
1,
3]. As scale up effort increases, it is important to understand how these interventions can be optimally allocated alongside one another and on a large scale for different transmission settings. In addition, there are few guidelines about how best to deploy scarce resources for malaria control.
Cost effectiveness analysis has become an important tool in understanding the dynamics of disease transmission and in decision making processes regarding intervention programs for disease control [
4,
5]. Cost effectiveness analysis is carried out to inform decision makers on how to determine where to allocate resources for malaria interventions especially when they are limited. The analysis compares the costs and health effects of an intervention to assess the extent to which it can be regarded as providing value for money and the choice of the technique depends on the nature of the benefits specified. Cost-effectiveness analysis is often used in the field of health services, where it may be inappropriate to monetize health effect. The most commonly used outcome measure is quality-adjusted life years (QALY) [
6].
The incremental cost-effectiveness ratio (ICER) has become the common measure for cost effectiveness analysis and is calculated in order to achieve the goal of comparing the costs and the effectiveness of the intervention strategies. Ridrogues
et al. [
7] conducted cost effective analysis using ICER but for TB. Okosun
et al. [
8] conducted cost effective analysis using ICER for three malaria intervention strategies and not for different transmission settings. He further did not consider the cost effective intervention strategies for the at risk group
i.e., the pregnant and the under five children. Stukey
et al. [
5] modeled the cost effectiveness of malaria control interventions in the highlands of western Kenya using simulation modeling in OpenMalaria modeling platform but did not consider the effect of IPTp and those at risk population. White
et al. [
4] conducted a systematic review costs and cost-effectiveness of malaria control interventions but not for different transmission setting and for the at risk groups. Hansena
et al. [
9] conducted cost effectiveness analysis of three health interventions for the pregnant women but for low transmission settings. There is no cost effectiveness analysis done for the optimal control strategies for different malaria transmission settings in Kenya considering the at risks age groups.
IPTp is one of the WHO recommended prevention therapy for the pregnant women. IPTp has been shown to be effective in reducing maternal and infant mortality that are related to malaria for the most at risk group for malaria [
10,
11,
12,
13]. No cost effectiveness analysis has been done for the IPTp. In this study, we carry out cost effective analysis of one or all possible combinations of malaria control strategies for different transmission settings.
2. Model Formulation
The ordinary differential equations that describe the interactions between the human and mosquito population is formulated and described by Otieno
et al. [
14]. A deterministic malaria transmission dynamics model with intervention strategies for the most at risk groups for malaria (under five children and the pregnant women) is formulated and analyzed to investigate the optimal malaria control strategies on the transmission dynamics among the pregnant women and children under five years of age.
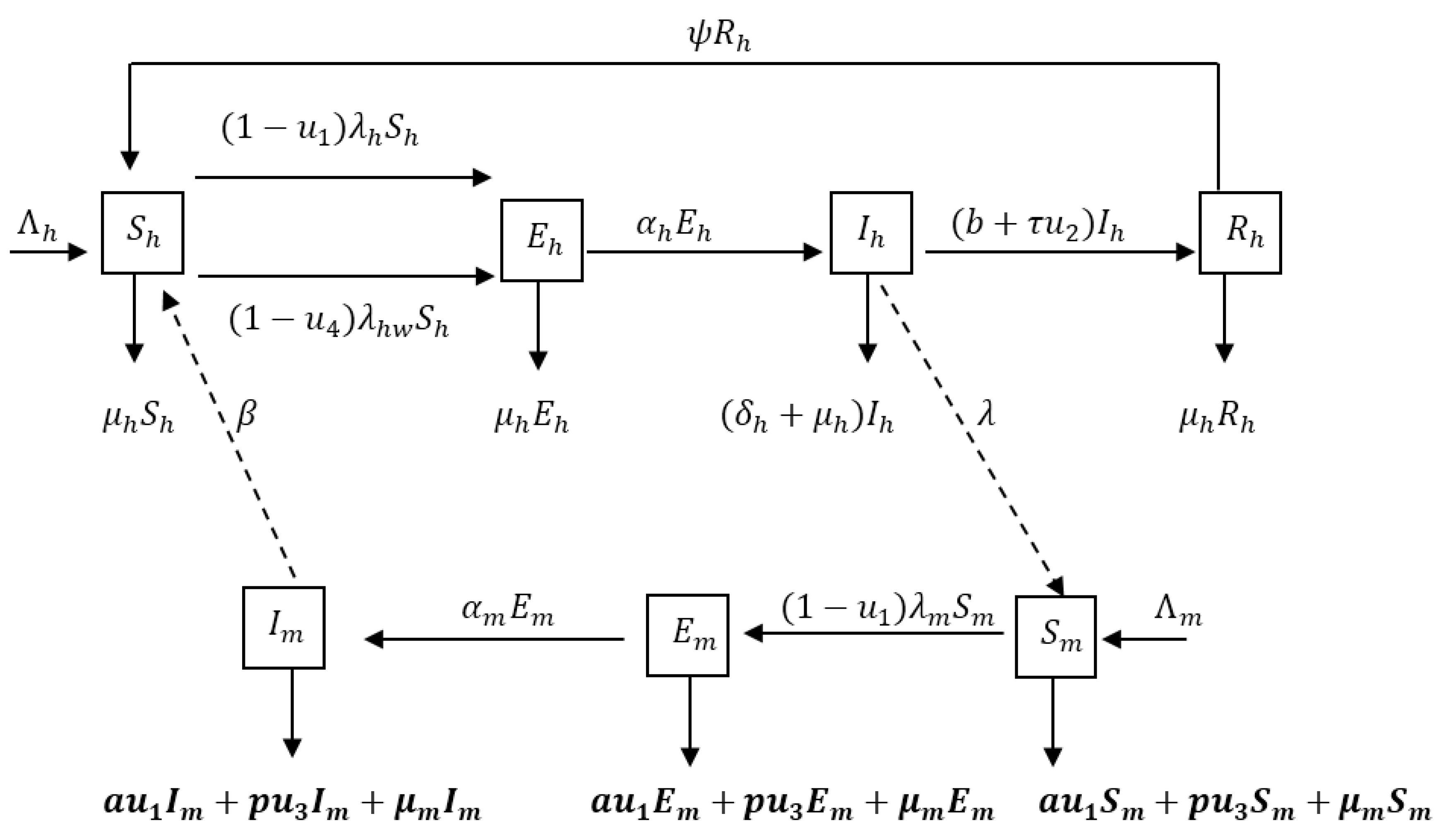
The population under study is subdivided into compartments according to the individual’s disease status. We consider a seven-dimensional model, which consists of population of Susceptible , Exposed humans , Infected humans , Recovered humans , Susceptible mosquitoes , Exposed mosquitoes and Infected mosquitoes . The total population sizes at time for humans and mosquitoes are denoted by and respectively. We employ the SEIRS type model for humans to describe a disease with temporary immunity on recovery from infection. Mosquitoes are assumed not to recover from the parasites so the mosquito population can be described by the SEI model. In the model we incorporate four time dependent control measures simultaneously: (i) the use of treated bednets ; (ii) treatment of infective humans ; (iii) spray of insecticides and (iv) treatment to protect pregnant women and their new born children: intermittent preventive treatment (IPTp) for pregnant women . represents the number of individuals not yet infected with the malaria parasite at time , represents individuals who are infected but not yet infectious, is the class representing infected with malaria and are capable of transmitting the disease to susceptible mosquitoes and represents the class of individuals who have temporarily recovered from the disease.
Figure 1 describes the dynamics of malaria in human and mosquito populations together with interventions.
The susceptible pregnant and under five humans () are recruited at the rate, . They either die from natural causes (at a rate ) or move to the exposed class () by acquiring malaria through contact with infectious mosquitoes at a rate or , where is the transmission probability per bite, is the per capita biting rate of mosquitoes, is the contact rate of vector per human per unit time, is the preventive measure using ITNs, is the preventive measure using IPTp, is the infectious mosquitoes at time , is the total number of individuals (pregnant and under 5) and is the total number of pregnant women. Susceptible class is divided into whole population (under five years and pregnant women) being exposed and the population for the pregnant women being exposed. Exposed individuals move to the infectious class after the development of clinical symptoms at the rate . Infectious individuals are assumed to recover at a rate , where is the rate of spontaneous recovery, is the control on treatment of infected individuals and is the efficacy of treatment. Infectious individuals who do not recover die at a rate . Individuals infected with malaria suffer a disease induced death at rate of , and natural death . Infected individuals then progress to partially immune group where upon recovery the partially immune individual losses immunity at the rate and becomes susceptible again.
Susceptible mosquitoes () are recruited at the rate and acquire malaria infection (following contact with humans infected with malaria) at the rate . They either die from natural causes (at a rate ) or move to the exposed class by acquiring malaria through contacts with infected humans at a rate , where is the probability for a vector to get infected after biting an infectious human and are individuals infected by malaria at time . The mosquito population is reduced, due to the use of insecticides spray, at a rate , where represents the control due to IRS and represents the efficacy of IRS. Mosquito population is also reduced as a result of natural death () and at the rate , where represents the control due to ITNs and is the efficacy due to ITNs. Newly infected mosquitoes are moved into the exposed class () at a rate and progresses to the class of symptomatic mosquitoes ().
The model is extended to formulate the optimal control problem from where cost effectiveness analysis of the optimal control strategies will be done. Following Otieno
et al. [
14] the state variables of the model are represented and described in
Table 1.
Table 2 describes and shows parameters of the model.
Table 3 describes and represents malaria prevention and control strategies practiced in Kenya.
Other assumptions of the model:
Population for human and mosquito is constant (no immigrants)
No recovery for infected mosquitoes
Mosquitoes do not die to disease infection
All parameters in the model are non-negative
Putting the above formulations and assumptions together as described by Otieno
et al. [
14] gives the following system of non-linear differential equations describing the dynamics of malaria in human and mosquito populations together with interventions.
The malaria dynamics model with intervention strategies being practiced in four different transmission settings in Kenya as described by Otieno
et al. [
14] gives the following system of differential equations
with initial conditions:
is the percapita incidence rate among mosquitoes (force of infection for susceptible mosquitoes), and is the force of infection for susceptible humans (pregnant and under 5), is the force of infection for susceptible pregnant humans.
The total population sizes are for the human is and for mosquito is and their differential equations are given by and respectively.
Mathematical Analysis of the Malaria Model with Intervention Strategies
The basic properties and analysis of the formulated malaria model with control strategies through mathematical analysis of the model is described and done by Otieno
et al. [
14].
The feasible solutions set for the Model (1) given by
is positively-invariant and the hence Model (1) is biologically, epidemiologically meaningful and mathematically well-posed in the domain
.
Following Otieno
et al. [
14], System (1) has always a disease free equilibrium given by
Basic Reproduction Number
The matrices
and
for the new infection terms and the remaining transfer terms at disease free equilibrium [
15], respectively, are given by
and
Following Otieno
et al. [
14], it follows that the basic reproduction of Model (1), denoted by
, is given by
Stability Analysis of Disease Free Equilibrium Point
Local Stability of Disease Free Equilibrium Point
Theorem 1. The disease free equilibrium point for System (1) is locally asymptotically stable if and unstable if .
Proof. Following Otieno
et al. [
14], the Jacobian matrix
of the malaria Model (1) at the disease-free equilibrium point is given by
The eigenvalues of the Jacobian matrix are the solutions of the characteristic equation
Expanding the determinant into a characteristic equation we have
Thus, applying to the Routh-Hurwitz criteria [
16] to the Polynomial Equation (5), we have that all determinants of the Hurwitz matrices are positive. Hence all the eigenvalues of the Jacobian have negative real part, implying that the DFE point is stable
.
Conversely, if it implies that , and since the remaining coefficients () of the polynomial are positive then all the roots of this polynomial cannot have negative real parts. Hence, the DFE point is unstable ().
Sensitivity Analysis
Sensitivity analysis to assess the relative impact of each of parameters of the basic reproductive number was done and described by Otieno
et al. [
14]. The normalized forward sensitivity index of the reproduction number with respect to these parameters given in
Table 4 is computed. The index measures the relative change in a variable with respect to relative changes in parameters.
Definition. Following Chitnis et al. [17], the normalized forward sensitivity index of a variable, , that depends on a parameter, , is defined as: .
Following Otieno
et al. [
14], the sensitivity index of the model parameters are given by
Sensitivity indices for the control parameters are given by
Sensitivity indices are calculated using parameters in
Table 5.
Through sensitivity analysis as shown by Otieno
et al. [
14], it is observed that the most sensitive parameters to
across all the settings was the mosquito’s natural death rate,
, and mosquito biting rate,
,this was followed by the by the mosquito contact rate with humans,
, probability of mosquito getting infected,
, the probability of humans getting infected,
, and the recruitment rate of mosquitoes and humans (see
Table 4). In the next section, we start by performing an economic evaluation of the intervention strategies then use optimal control to study the optimality of these interventions. Cost-effectiveness analysis of the optimal malaria control strategies is done to determine the most cost-effective as one or combination of the four intervention strategies namely, treatment effort of infected individuals, ITNs, IRS and IPTp. Cost-effectiveness analysis is undertaken using ICER in order to assess the extent to which the intervention strategies are beneficial and cost effective. The aim is to maximizing the level of benefits (health effects) relative to the level of resources available as shown by Okosun
et al. [
8].
3. Economic Evaluation
The economic evaluation of all four intervention techniques is evaluated in which effectiveness and cost-effectiveness of the interventions are investigated in order to minimize or eradicate malaria disease in the area under study. The following cost objective function is used [
18]
subject to the system of differential equation (1), where
denotes the per unit cost of ITNs (
);
denotes the per unit cost of treating an individual with malaria (
),
represents the per cunit area cost of IRS effort (
) and spraying houses and
represents the use of IPTp among the pregnant women (
). The discount rate of 3%–5% has been exponentially considered with a parameter
. The Lagrangian of the cost objective function is
Then the Hamiltonian equation with Lagrangian, state variables and adjoint variables is
The developed corresponding Hamiltonian equation is given by:
where
and
denote the marginal value/ shadow prices linked to their corresponding classes. The
where
represent the changes in the objective value of an optimal solution of an optimization problem by relaxing the constraint by one unit [
18]. These can be calculated by using Pontryagin’s Maximum Principle:
Hence using the Hamiltonian Equation (8) gives
Each intervention strategy is assessed by developing the Hamiltonian equation thereafter the economic tool will be employed.
3.1. Economic Evaluation of ITNs
The prevention parameter for the ITNs is denoted by
. The Hamiltonian equation,
, is differentiated with respect to
to obtain
in which
is the total marginal benefit due to the use of ITNs while
is the marginal cost of acquiring the ITNs. The equivalency of the marginal cost and marginal benefit leads one to achieve the optimal policy.
The third part of equation (10), shows that if this is achieved then the total marginal benefit of using ITNs is more than the total marginal cost; hence the gain of optimal malaria prevention. Then we can conclude that the susceptible and exposed individuals should best (effectively) use this prevention strategy in order to fight the epidemic. On the other hand, few susceptible and exposed individuals will use ITNs if the marginal cost is more than the marginal benefit. The effective use of this strategy will lead to achieve the optimal policy which says that increasing the use of ITNs increases the number of susceptible humans and uninfected mosquitoes.
3.2. Economic Evaluation of Treatment Effort of Infected Individuals
Here the control parameter for treatment of infectious individuals is given by
. The Hamiltonian equation,
, Equation (8) is differentiated with respect to
, giving
in which
is the marginal cost and
is the marginal benefit of treating infectious individuals. Hence,
The optimal policy is to ensure that the marginal costs for being treated is equal to the marginal benefit for the individuals being treated. Therefore, from Equation (11) all infected individuals must look for full treatment if the marginal benefit, , must be greater than the marginal cost, , for being treated. Otherwise, only few infected individuals will look for treatment.
3.3. Economic Evaluation of IRS
Insecticide residual spraying (IRS) prevention parameter in the Hamiltonian equation,
, Equation (8) is
. Then differentiating
with respect to
gives
where
is the marginal cost for IRS and
is the marginal benefit for using the sprayed houses. Furthermore, it can be deduced that the optimal policy for a sprayed house is given by
The spraying of insecticides against mosquitoes is optimal for malaria disease control if the marginal cost , is less than the marginal benefit, ).
3.4. Economic Evaluation of IPTp
Intermittent Preventive Treatment (IPTp) prevention parameter in the Hamiltonian equation,
, Equation (8) is
. Then differentiating
with respect to
gives
in which
is the total marginal benefit due to the use of IPTp while
is the marginal cost of acquiring the IPTp. The equivalency of the marginal cost and marginal benefit leads one to achieve the optimal policy.
4. Analysis of Optimal Control
We consider the objective function
subject to
And the total cost at time
is given by
where
are desired positive weights on the benefits of preventing infection and exposure plus total mosquito population. Here, we assume that there is no linear relationship between the coverage of these interventions and their corresponding costs, hence we choose a quadratic cost on the controls in keeping with what is in other literature on cost of control of epidemics [
8,
19,
20,
21]. Our goal with the given objective function is to minimize the number of infected humans, exposed humans and total mosquito population while minimizing the cost of control
and
. We seek an optimal control
and
such that
Where is the set of measurable functions defined from onto .
The necessary conditions that an optimal control must satisfy come from the Pontryagin’s Maximum Principle [
18]. This consists in minimizing, with respect to
/
Forming the Hamiltonian from the objective function (14) subject to Equations (1) and (15)
where
and
are the adjoint variables or co-state variables given by the following system:
By applying Pontryagin’s Maximum Principle [
18] and the existence result for the optimal control from Fleming and Rishel [
22], we obtain
Proposition 1. The optimal control that minimizes over is given by
where
and
are the adjoint variables or co-state variables satisfying Equation (18) and the following transversality conditions:
Proof. From Fleming and Rishel [
22], the existence of an optimal control is a consequence of the convexity of the integrand of
with respect to
,
a priori boundedness of the state variables, and the Lipschitz property of the state system with respect to the state variables. The differential equations governing the adjoint variables are obtained by differentiation of the Hamiltonian function, evaluated at the optimal control. Then the adjoint system can be written as,
Due to the a priori boundedness of the solutions of both the state and adjoint equations and the resulting Lipschitz structure of these equations, we obtain the uniqueness of the optimality Systems ((18)–(20)) for small .
The restriction on the length of time interval
is common in control problems [
8,
21,
23], and it guarantees the uniqueness of the optimality system.
By standard control arguments involving the bounds on the controls, we conclude that
Following Otieno
et al. [
24], the optimal control is obtained by solving the optimality Systems ((18)–(20)). An iterative scheme is used for solving the optimality system. We start by solving the state equations with a guess for the controls over the simulated time using fourth order Runge-Kutta scheme. Because of the transversality conditions (20), the adjoint equations are solved by the backward fourth order Runge-Kutta scheme using the current iterations solutions of the state equation. Then the controls are updated by using a convex combination of the previous controls and the value from the characterizations (19). This process is repeated and iterations stopped if the values of the unknowns at the previous iterations are very close to the ones at the present iterations as also described by Lenhart and Workman [
25].
Parameter values from
Table 5 are used for the numerical simulation
6. Numerical Results and Discussion
The parameters in the Model (1) were estimated using clinical malaria data and demographics statistics of Kenya. Those that were not available were obtained from literature published by researchers in malaria endemic countries which have similar environmental conditions compare to Kenya.
Table 5 provides a summary of the estimated values of all parameters as described by Otieno
et al. [
23] in addition to some from the literature. Data was collected from the literature, Division of Malaria Control (DOMC), Kenya National Bureau of Statistics, Malaria Indicator Survey for Kenya, Demographic Health Survey (DHS) for Kenya, WHO websites and hospital records (from Kisumu, Kisii, Chuka (Tharake-Nithi) and Nyeri counties representing the four different transmission settings/epidemiological zones in Kenya).
In addition the effect of the different intervention strategies are estimated as:
,
,
,
. The initial state variables are constant across all the epidemiological zones and are chosen as
,
,
,
,
,
and
. The numerical simulations are done in R statistical Computing platform [
31].
6.1. Numerical Simulations of the Economic Evaluations of the Malaria Model
A case of the endemic epidemiological zone is used for the illustrative purpose. Numerical simulations showing the impact of the shadow prices (marginal value/cost) and marginal benefits by evaluating the shadow prices at the start of the malaria epidemic and as a function of the numbers of recovered or protected at the time of outbreak (susceptible human beings).
The marginal cost and effect of the intervention strategies are simulated for the Endemic transmission setting and the results are shown in
Figure 2.
Simulations are also done for the epidemic, seasonal and low risks epidemiological zones.
Across all the four malaria transmission settings, it is observed that the marginal value (shadow price) of is much less damaging than the marginal values of and . The shadow price on the susceptible humans are increasing overtime while the shadow prices of exposed starts dropping at days and shadow prices on infected starts dropping at days. In addition, across all the four malaria transmission settings, shows that the shadow price on starts at higher positive values, increases and stabilizes at higher prices closer to the total susceptible population. As more individuals recover from the disease the cost of the disease is still higher. It is also observed that across all the four malaria transmission settings shows that the marginal benefit of use of treatment is much smaller than the marginal benefit of IPTp, ITNs and IRS in that order. Smaller amounts of treatment is needed compared to IPTp, ITNs and IRS in that order to be able to eliminate the disease.
6.2. Numerical Simulation of the Optimal Malaria Control Strategies and Cost-Effectiveness Analysis
Numerical simulations are further done to show the infections averted and the cost associated with the infections averted by the intervention strategies for the four different transmission settings. Rankings of the number infections averted (effectiveness) is then done so that ICER can be applied.
For the different transmission settings we compute the optimal solution for the 15 strategies and their associated effectiveness (infections averted) which is the difference between the numbers of infections when there is no intervention and when there are interventions. The strategies were classified as follows: ITN only (Strategy A), treatment only (Strategy B), IRS only (Strategy C), IPTp only (Strategy D), treatment and ITNs (Strategy E), ITNs and IRS (Strategy F), ITNs and IPTp (Strategy G), treatment and IRS (Strategy H), treatment and IPTp (Strategy I), IPTp and IRS (Strategy J), ITNs, treatment and IRS (Strategy K), ITNs, treatment and IPTp (Strategy L), ITNs, IRS and IPTp (Strategy M), IRS, treatment and IPTp (Strategy N), ITNs, treatment, IRS and IPTp (Strategy O). Based on the model simulation results, the strategies practiced in Kenya for different epidemiological settings were ranked in the order of increasing effectiveness.
The infections averted and cost of the intervention used is used to determine the cost-effectiveness of different combinations of the four intervention strategies using the ICER. We determined the total cost of the combined intervention strategies and the infections averted for different transmission settings.
The ICER for every two competing strategies for each epidemiological scenario is calculated and this shows the cost effectiveness for each strategy. The cost-effectiveness calculations are further verified using the computation of incremental cost-effectiveness ratios in table form for each epidemiological zone in order to have a complete overview of the outcome.
The
Table 6 below summarizes the ranking of simulation results on the effectiveness (infections averted) and the total costs by the different strategies for endemic scenario in Kenya.
The ICER for every two competing strategies was calculated and the results are presented in
Table 7.
Alternatives that are more expensive and less ineffective are excluded (A, F, D, J, N, O and H). These are the strategies that have higher ICER when compared. Having excluded strategy A, F, D, J, N, O and H, ICERs are recalculated for the remaining strategies (C, G, M, I, L, B, H and K) and are shown in
Table 8.
The dominated strategies (G, I, L and K) are then excluded and the ICERs are recalculated again (
Table 9). These are the strategies that have higher ICER when compared.
In
Table 9 the most cost effective quadrant will be strategy M and strategy B and in deciding between them the size of the available budget must be brought to bear. Strategy M is the combination of ITNs, IRS and IPTp while strategy B is the use of treatment only.
Repeating the same procedure for the remaining epidemiological zones (epidemic, seasonal and low), the findings shows that for the endemic regions the combination of ITNs , IRS , and IPTp is the most cost-effective of all the combined strategies developed in this study for malaria disease control and prevention; for the epidemic prone areas is the combination of the treatment , and IRS ; for seasonal areas is the combination ITNs plus treatment ; and for the low risk areas is the use of treatment only. The result confirms the role which the four intervention strategies are playing in order to eradicate or minimize the spreading of the malaria disease.
7. Discussion
This paper conducted cost effective analysis of one or all possible combinations of malaria control strategies for different transmission settings in order to assess the extent to which the intervention strategies are beneficial and cost effective. For the four different transmission settings the optimal control model simulation was done for the 15 strategies and their associated effectiveness
, which is the difference between the numbers of infections when there is no intervention and when there are interventions, was used to rank the effectiveness of the interventions [
7,
8]. The marginal costs and benefits have also been investigated. ICER was then used to compare the health and economic benefits of the intervention strategies. Numerical simulation compared the marginal value and marginal effect for the four intervention strategies [
8] across the four different transmission settings.
The findings of the study shows that for the endemic regions the combination of ITNs, IRS, and IPTp was the most cost-effective of all the combined strategies developed in this study for malaria disease control and prevention. This findings are different from the findings of Okosun
et al. [
8], who found that the combination of the spray of insecticides and treatment of infective individuals were the cost effective strategies. This may be due to the fact that in our study we considered the at most risk groups while in the Okusun
et al. [
8] they considered whole population. The findings shows that preventive measures tends to have a greater health benefit in a cost effective or economical manner in minimizing malaria transmission for the most at risk groups. Stuckey
et al. [
5] showed that increasing coverage of vector control interventions (preventive strategies) had a larger simulated impact compared to adding treatment measures.
Our results shows that for the epidemic prone areas the cost effective strategy was the combination of the treatment and IRS which agrees with Okosun
et al. [
8]. This is because the combination of the preventive and treatment actions tend to be more effective in the reduction of parasitic prevalence to below 1% [
32]. This is due to the fact that infected mosquito population is reduced by IRS and the infected human population is reduced via the treatment.
For seasonal areas, the findings of this study showed that the combination ITNs and treatment would be the most cost effective intervention strategy to reduce malaria transmission among the under-five and the pregnant women. This is slightly different with the findings of Griffin
et al. [
30] who found that for the high seasonal transmission settings the use of LLITNs, IRS and treatment would help reduce the transmission of malaria.
The results showed that for the low risk areas is the use of treatment only. These findings were different from Hansen
et al. [
9] who found that the most cost effective strategy was the use of ITNs alone in Uganda low transmission settings.
The result confirms the role which the four intervention strategies are playing in order to eradicate or minimize the spreading of the malaria disease among the at risk groups. The policy implications of these findings is that different transmission settings require different interventions that are health beneficial and cost effective. The results can guide decision makers in making more informed and evidence-based choices on the health resources being allocated. These findings may help inform the development of guidelines for prevention of malaria among the under-five and the pregnant women in different transmission settings in Kenya as well as in other African countries.
These findings were based on the use of secondary data, a more designed study may be needed to ascertain the findings of these studies. Including other possible positive externalities would improve the cost-effectiveness of interventions strategies.