Interactions between the Ionic Liquid and the ZrO2 Support in Supported Ionic Liquid Membranes for CO2 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Chemicals and Materials
2.2. Synthesis of 1-Butyl-3-Methylimidazolium Tetrafluoroborate ([C4mim][BF4])
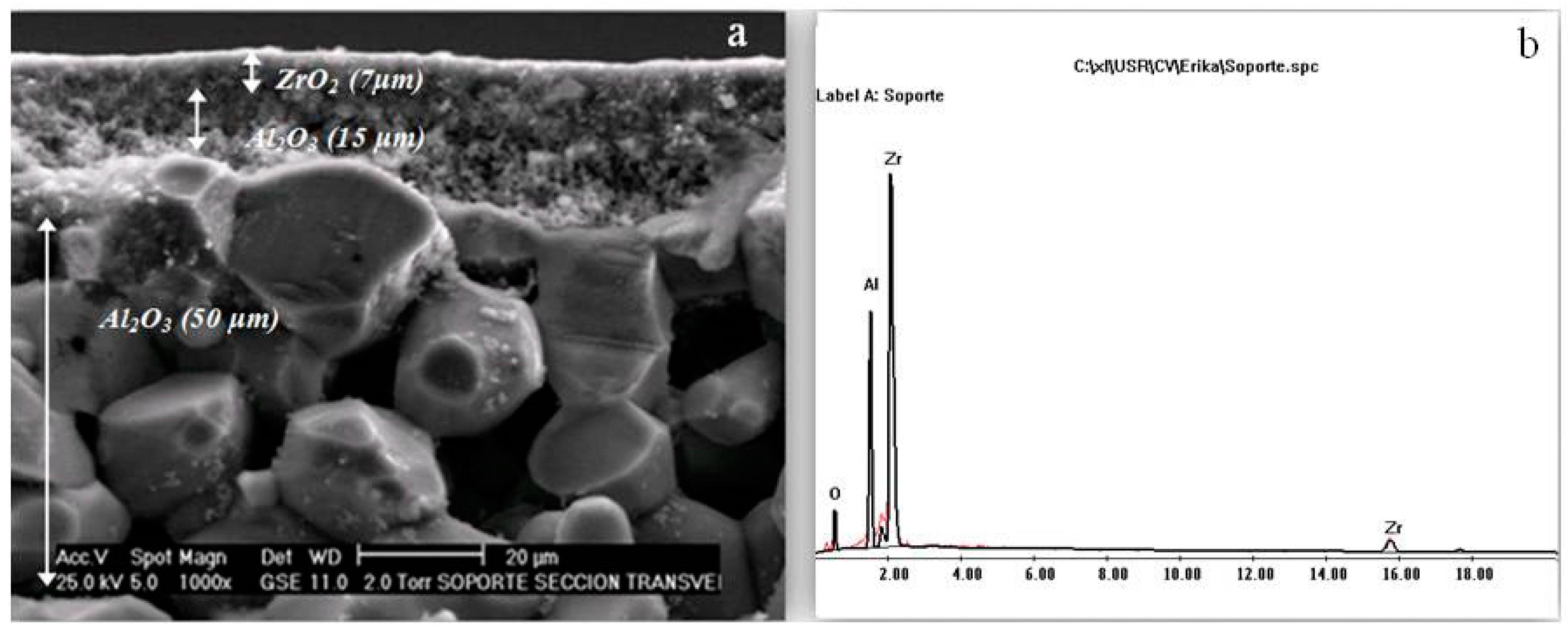
2.3. ZrO2-Al2O3 Support
2.4. Preparation of Supported Ionic Liquid Membranes
2.5. Estimation of the Trans-Membrane Pressure
2.6. Ionic Liquid and SILM Characterization
2.7. Evaluation of SILMs and Single Gas Permeances of CO2 and N2
3. Results and Discussion
3.1. SEM/EDX Characterization of the ZrO2 Support
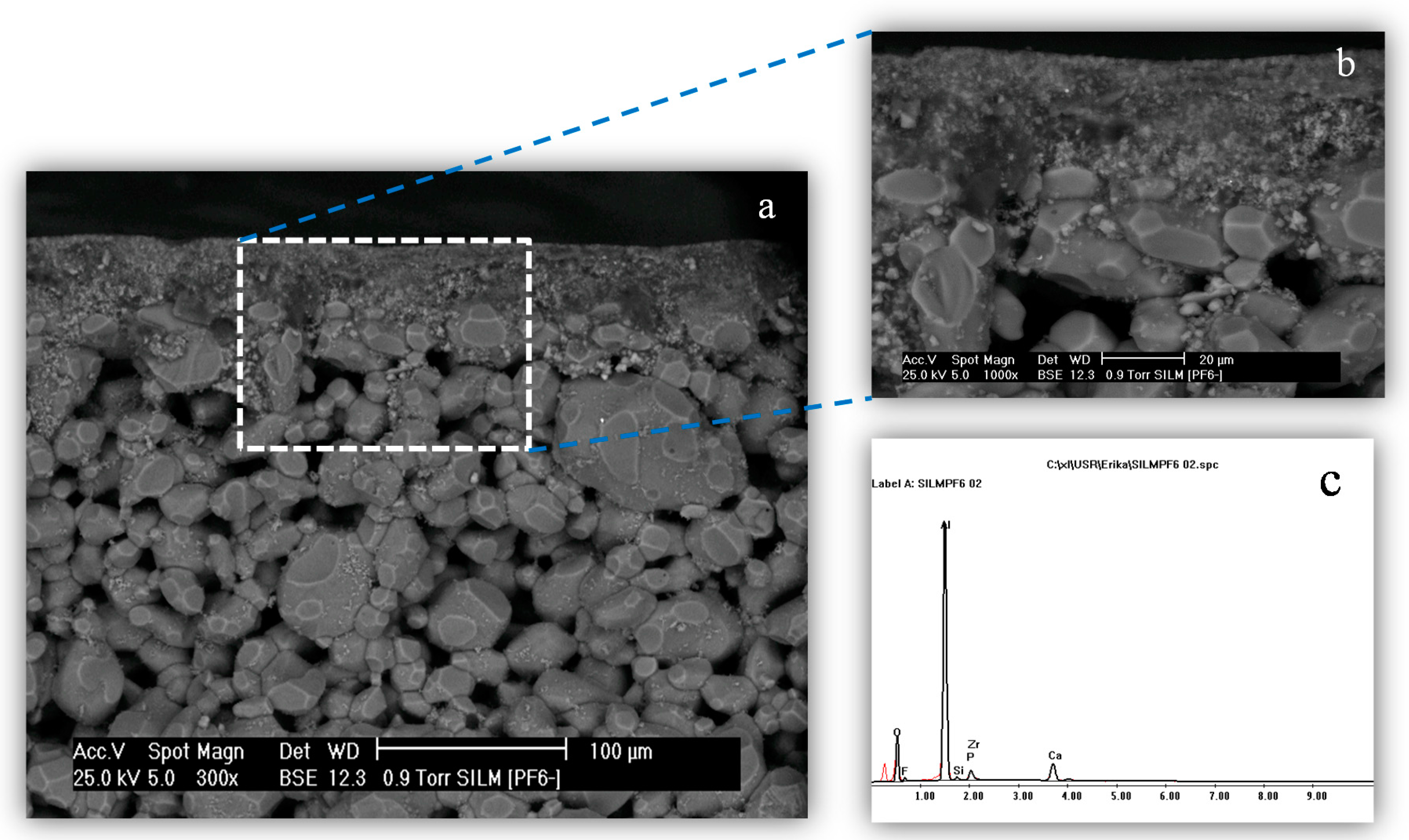
3.2. Study of the Interaction of ILs with the ZrO2 Support
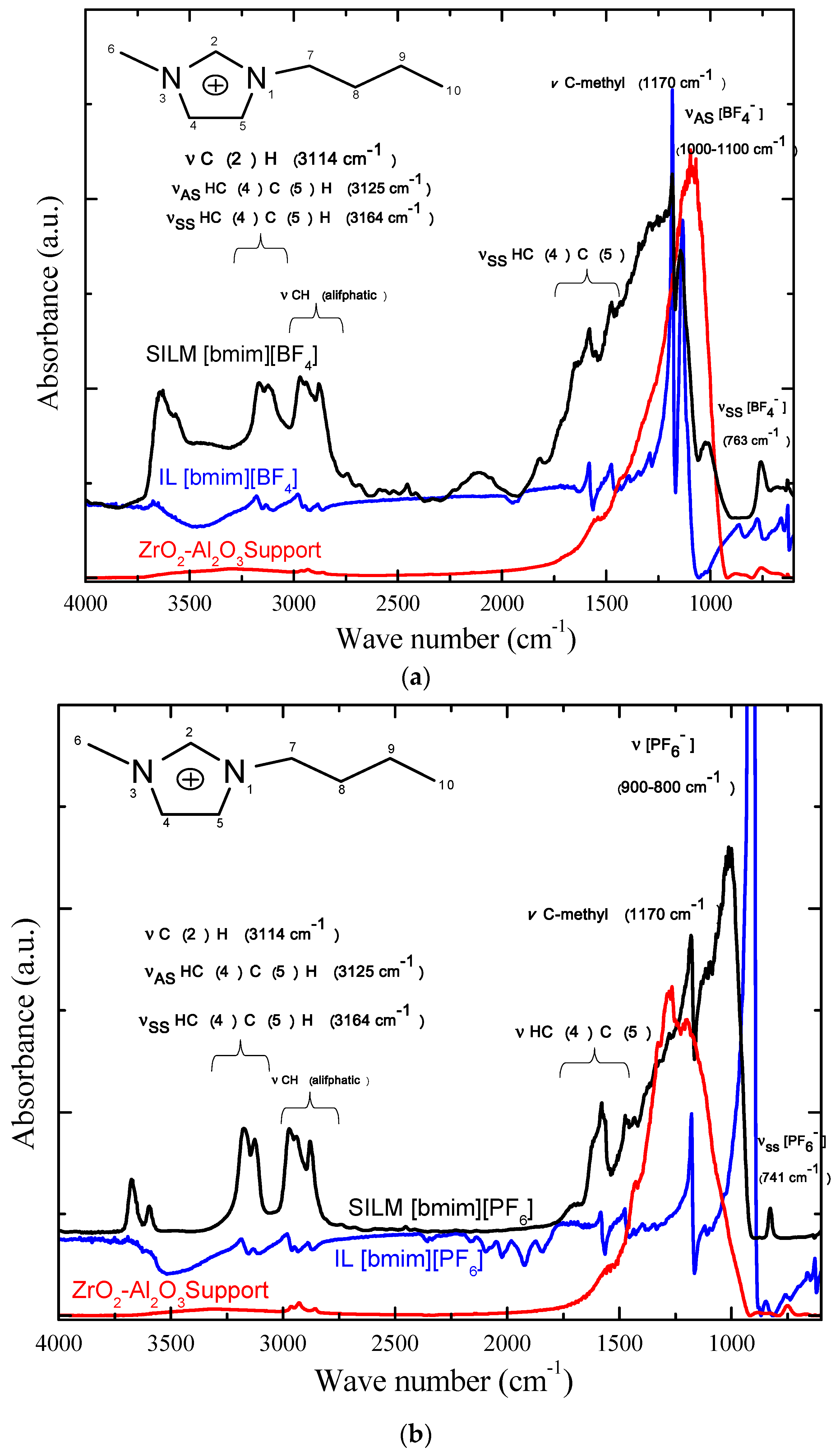
3.3. FTIR (DR)
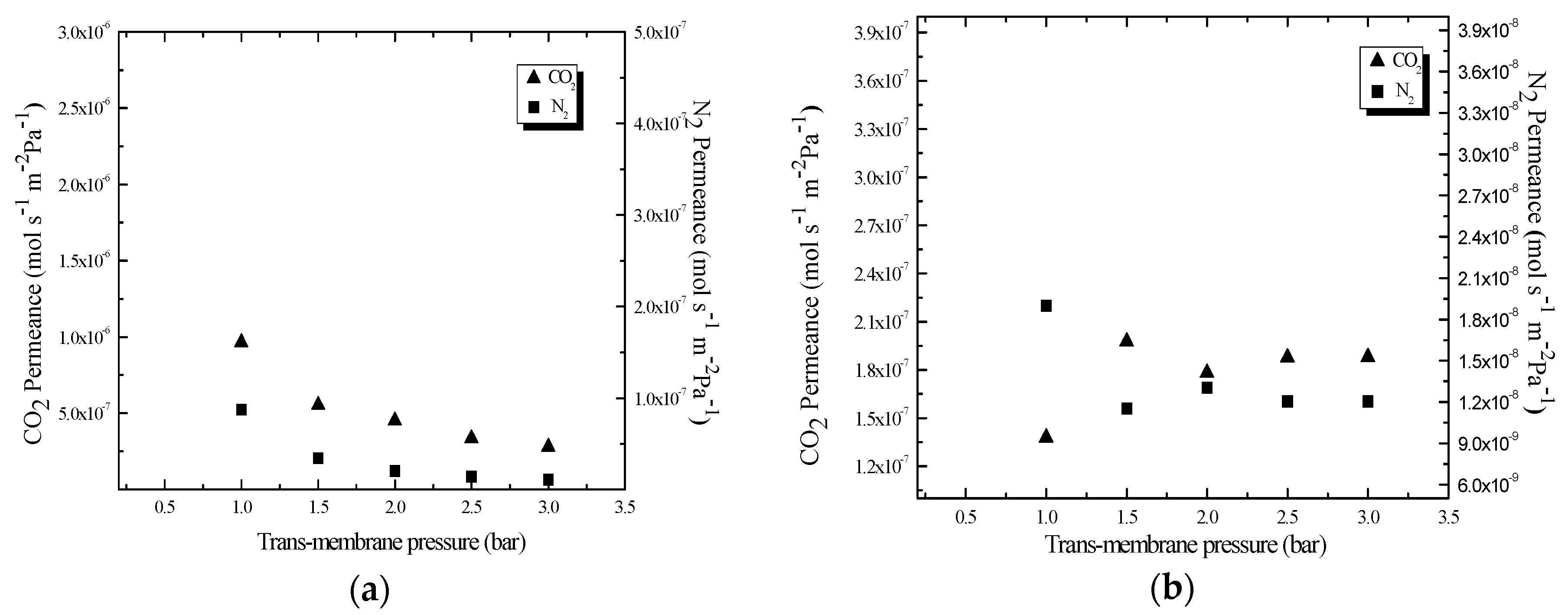
3.4. CO2 Separation Performance
3.5. Effects of ILs Anion Species on Permeance
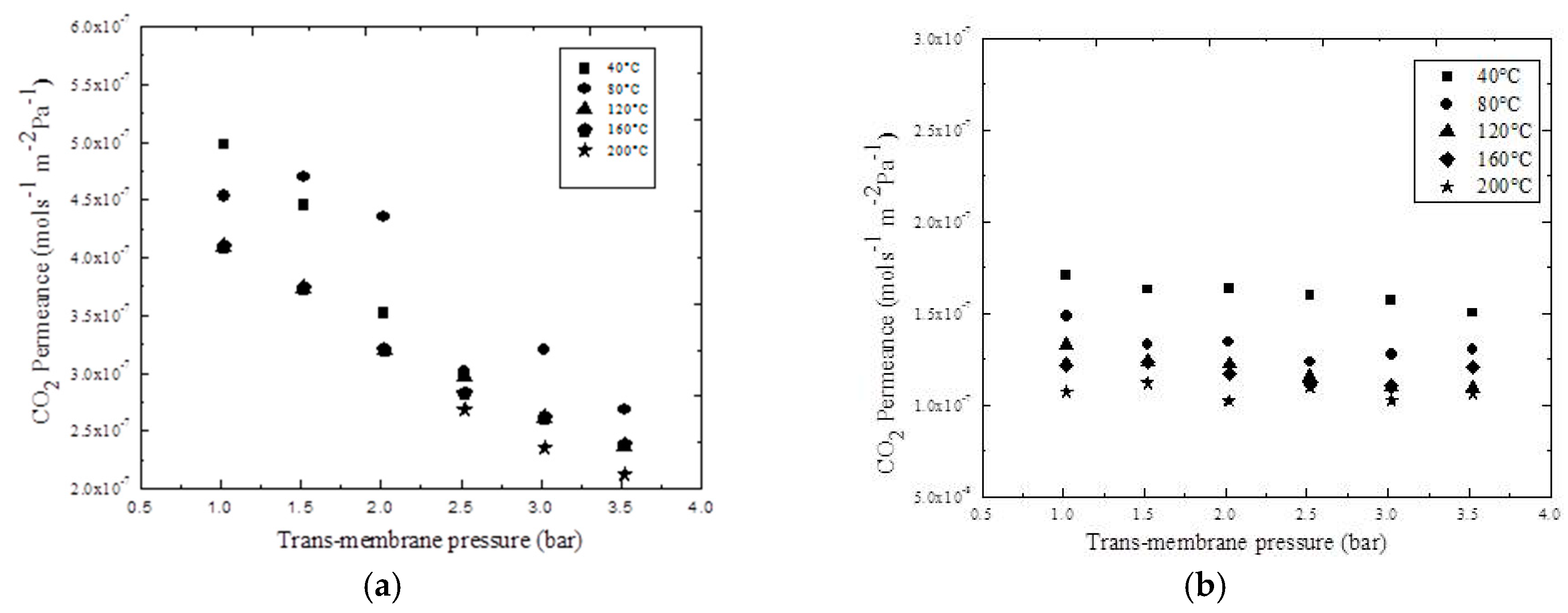
3.6. Effects of Temperature on the Permeance in SILMs
3.7. CO2/N2 Ideal Selectivity for Different SILMs
3.8. Separation Factor
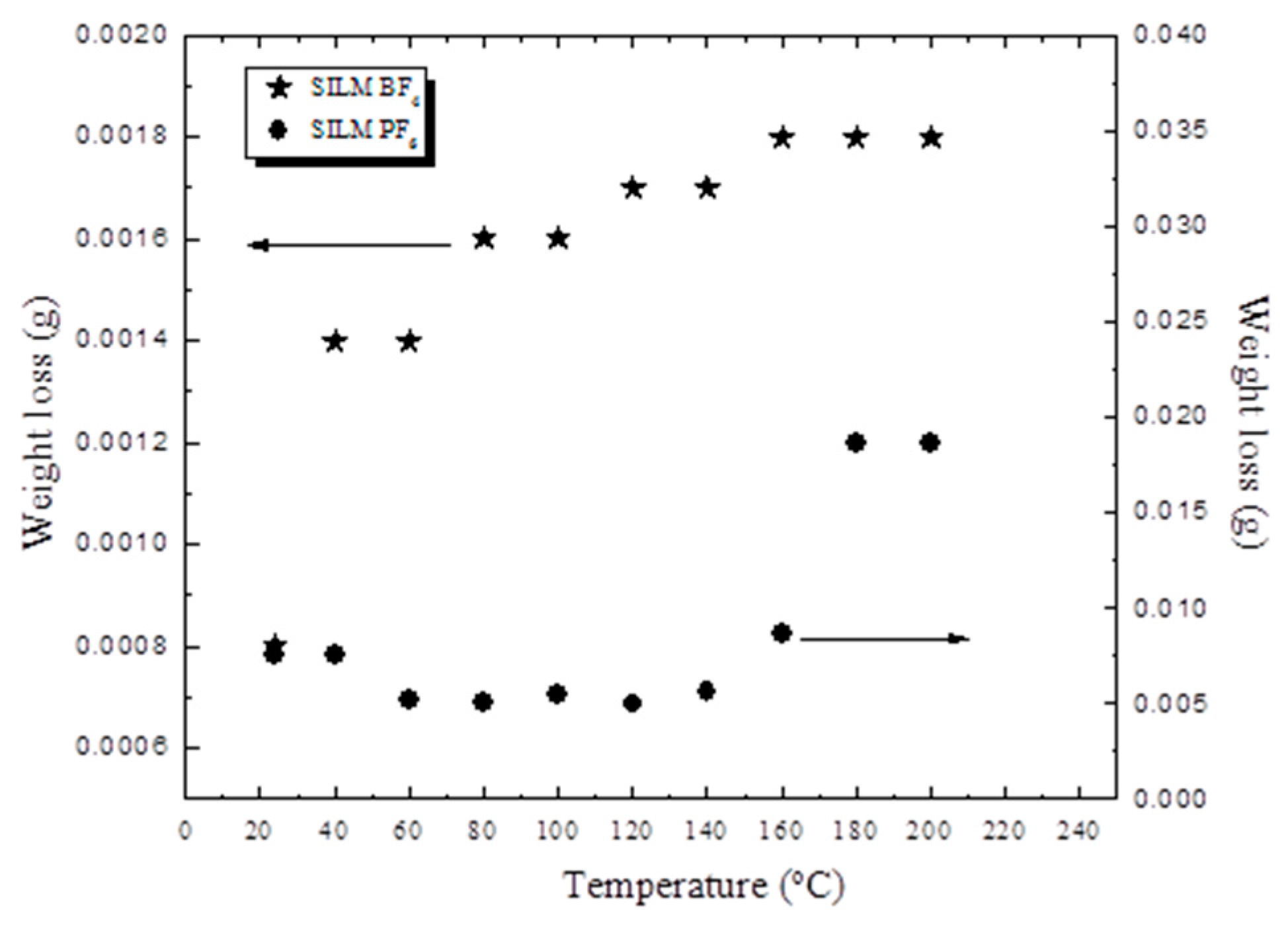
3.9. Loss of Ionic Liquids after CO2 Separation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, W.; He, G.; Nie, F.; Zhang, L.; Feng, H.; Liu, H. Membrane liquid loss mechanism of supported ionic liquid membrane for gas separation. J. Membr. Sci. 2013, 411, 73–80. [Google Scholar] [CrossRef]
- Zhao, L.; Riensche, E.; Menzer, R.; Blum, L.; Stolten, D. A parametric study of CO2/N2 gas separation membrane processes for post-combustion capture. J. Membr. Sci. 2008, 325, 284–294. [Google Scholar] [CrossRef]
- Department of the New Energy and Industrial Technology Development Organization (NEDO). Ceramic membrane to combat global warming. Membr. Technol. 1997, 92, 11–12. [Google Scholar]
- Albo, J.; Yoshioka, T.; Tsuru, T. Porous Al2O3/TiO2 tubes in combination with 1-ethyl-3-methylimidazolium acetate ionic liquid for CO2/N2 separation. Sep. Purif. Technol. 2014, 122, 440–448. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Fabrication of zirconia composite membrane by in-situ hydrothermal technique and its application in separation of methyl orange. Ecotoxicol. Environ. Saf. 2015, 121, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.P.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, L.; Coppel, Y.; Favier, I.; Teuma, E.; Serp, P. Imidazolium-based ionic liquids immobilized on solid supports: Effect on the structure and thermostability. Dalton Trans. 2010, 39, 7565–7568. [Google Scholar] [CrossRef] [PubMed]
- Blath, J.; Christ, M.; Deubler, N.; Hirth, T.; Schiestel, T. Gas solubilities in room temperature ionic liquids—Correlation between RTiL-molar mass and Henry’s law constant. Chem. Eng. J. 2011, 172, 167–176. [Google Scholar] [CrossRef]
- Kanakubo, M.; Aizawa, T.; Nanjo, H.; Kameda, Y.; Amo, Y.; Usuki, T. Liquid structures of 1-butyl-3-methylimidazolium tetrafluoroborate and carbon dioxide mixtures by X-ray diffraction measurements. Fluid Phase Equilibria 2010, 297, 183–186. [Google Scholar] [CrossRef]
- Kerlé, D.; Ludwig, R.; Geiger, A.; Paschek, D. Temperature dependence of the solubility of carbon dioxide in imidazolium-based ionic liquids. J. Phys. Chem. B 2009, 113, 12727–12735. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.L.; Brennecke, J.F. Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, T.K.; Bara, J.E.; Gabriel, C.; Noble, R.D.; Gin, D.L. Interpretation of CO2 solubility and selectivity in nitrile-funcionalized room-temperature ionic liquids using a group contribution approach. Ind. Eng. Chem. Res. 2008, 47, 7005–7012. [Google Scholar] [CrossRef]
- Ruth, E.; Baltus, R.M.; Benjamin, H.; Culbertson, H.L.; David, W.; DePaoli, S.D.; Douglas, C. Examination of the potential of ionic liquids for gas separations. Sep. Sci. Technol. 2005, 40, 525–541. [Google Scholar]
- Scovazzo, P.; Kieft, J.; Finan, D.A.; Koval, C.; Noble, R. Gas separations using non-hexafluorophosphate [PF6]‒ anion supported ionic liquid membranes. J. Membr. Sci. 2004, 238, 57–63. [Google Scholar] [CrossRef]
- Hernández, F.J.; De los Ríos, A.P.; Tomás, F.; Palacios, J.M.; Víllora, G. Preparation of supported ionic liquid membranes: Influence of the ionic liquid immobilization method on their operational stability. J. Membr. Sci. 2009, 341, 172–177. [Google Scholar] [CrossRef]
- Fortunato, R.; González, M.J.; Kubasiewicz, M.; Luque, S.; Alvarez, J.R.; Alfonso, C.A.M.; Coelhoso, I.M.; Crespo, J.G. Liquid membranes using ionic liquids: The influence of water on solute transport. J. Membr. Sci. 2005, 249, 153–162. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F. Ionic liquids in supported liquid membrane technology. Chem. Eng. J. 2011, 171, 242–254. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, A.P. Applying a QSPR correlation to the prediction of surface tensions of ionic liquids. Fluid Phase Equilibria 2008, 265, 57–65. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Fernandes, A.M.; Marrucho, I.M.; Queimada, A.J.; Coutinho, J.A.P. Surface tensions of imidazolium based ionic liquids: Anion, cation, temperature and water effect. J. Colloid Interface Sci. 2007, 314, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2011, 58, 2885–2899. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Suárez, P.A.Z.; Souza, R. Preparation of 1-butyl-3-methyl imidazolium-based room temperature ionic liquids. Org. Synth. 2004, 10, 184. [Google Scholar]
- Aki, S.N.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Kreiter, R.; Overbeek, J.P.; Correia, L.A.; Vente, F.J. Pressure resistance of thin ionic liquid membranes using tailored ceramic supports. J. Membr. Sci. 2011, 370, 175–178. [Google Scholar] [CrossRef]
- Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting. Available online: http://www.astm.org/Standards/D1434 (accessed on 5 August 2015).
- Koros, W.J.; Ma, Y.H.; Shimidzut, T. Terminology for membrane and membrane processes. J. Membr. Sci. 1996, 120, 149–159. [Google Scholar]
- Kazarian, S.G.; Briscoe, B.J.; Welton, T. Combining ionic liquids and supercritical fluids: In situ ATR-IR study of CO2 dissolved in two ionic liquids at high pressures. Chem. Commun. 2000, 2047–2048. [Google Scholar] [CrossRef]
- Baiker, A.; Jutz, F.; Andanson, J.M. Investigation of binary and ternary systems of ionic liquids with water and/or supercritical CO2 by in situ attenuated total reflection infrared spectroscopy. J. Phys. Chem. B 2010, 114, 2111–2117. [Google Scholar]
- Jeon, Y.; Sung, J.; Seo, C.; Lim, H.; Cheong, H.; Kang, M.; Moon, B.; Ouchi, Y.; Kim, D. Structures of ionic liquids with different anions studied by infrared vibration spectroscopy. J. Phys. Chem. B 2008, 112, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, R.J. Influence of the ionic liquid/gas surface on ionic liquid chemistry. Phys. Chem. Chem. Phys. 2012, 14, 5071–5089. [Google Scholar] [CrossRef] [PubMed]
- Barghi, S.H.; Adibi, M.; Rashtchian, D. An experimental study on permeability, diffusivity, and selectivity of CO2 and CH4 through [bmim][PF6] ionic liquid supported on an alumina membrane: Investigation of temperature fluctuations effects. J. Membr. Sci. 2010, 362, 346–352. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Zhao, Y.; Ho, W.S. CO2 Capture and H2 purification: Prospects for CO2-selective membrane processes. AIChE J. 2013, 59, 1033–1045. [Google Scholar] [CrossRef]
- Kim, D.; Baek, H.; Hong, S.; Lee, H. Study on inmobilized liquid membrane using ionic liquid and PVDF hollow fiber as a support for CO2/N2 separation. J. Membr. Sci. 2011, 372, 346–354. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; Ríos, A.P.; Hernándes, F.J.; Segado, S.S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Iarikov, D.D.; Hacarlioglu, P.; Oyama, S.T. Supported room temperature ionic liquid membranes for CO2/CH4 separation. Chem. Eng. J. 2011, 166, 401–406. [Google Scholar] [CrossRef]
- Jian, Y.Y.; Zhou, Z.; Jiao, Z.; Li, L.; Wu, Y.T.; Zhang, Z.B. SO2 gas separation using supported ionic liquid membranes. J. Phys. Chem. B 2007, 111, 5058–5061. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, Y.; Wang, W.; Li, L.; Zhou, Z.; Zhang, Z. Permeability and selectivity of sulfur dioxide and carbon dioxide in supported ionic liquid Membranes. Sep. Sci. Technol. 2009, 17, 596–601. [Google Scholar] [CrossRef]
- Larachi, F.; Siaj, M.; Rahman, M.H. Ionic liquids for CO2 capture-development and progress. Chem. Eng. Process. Process Intensif. 2010, 49, 313–322. [Google Scholar]
- Luis, P.; Neves, L.A.; Afonso, C.A.M.; Coelhoso, I.M.; Crespo, J.G.; Garea, A.; Irabien, A. Facilitated transport of CO2 and SO2 through supported ionic liquid membranes (SILMs). Desalination 2009, 245, 485–493. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Marginn, E.J. Why is CO2 so soluble in imidazolium-based ionic liquids. J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Jindaratsamee, P.; Shimoyama, Y.; Morizaki, H.; Ito, A. Effects of temperature and anion species on CO2 permeability and CO2/N2 separation coefficient through ionic liquid membranes. J. Chem. Thermodyn. 2011, 43, 311–314. [Google Scholar] [CrossRef]
- Joan, F.; Brennecke, E.J. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar]
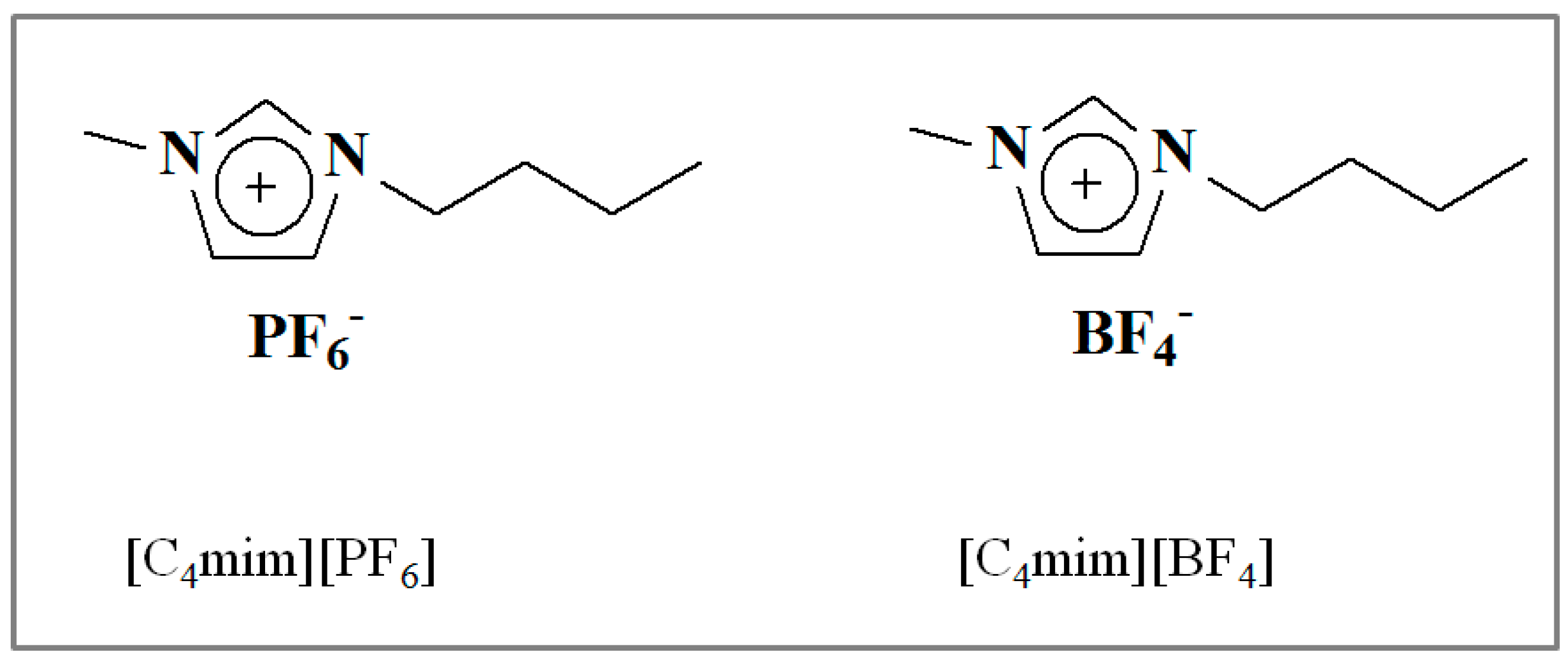



| Ionic Liquid | Short Name | Molecular Weight (g·mol−1) | Density (g·mL−1) 20 °C | Melting Point (°C) | Viscosity (cP) | Surface Tension mN·m−1 |
|---|---|---|---|---|---|---|
| 1-butyl-3-methyl imidazolium tetrafluoroborate | [bmim][BF4] | 226.02 | 1.064 | −71 °C | 34 | 44.81 |
| 1-butyl-3-methyl imidazolium hexafluorophosphate | [bmim][PF6] | 284.18 | 1.38 | 12 °C | 450 | 43.52 |
| SILM | Thickness (μm) Estimated through Equation (1) | Thickness (μm) Estimated by SEM |
|---|---|---|
| [bmim][BF4] | 25.64 | 25 |
| [bmim][PF6] | 72.02 | 65 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fuentes, C.E.; Pergher, S.B.; Gutiérrez-Arzaluz, M.; Mugica-Álvarez, V.; Terrés, E.; Torres-Rodríguez, M. Interactions between the Ionic Liquid and the ZrO2 Support in Supported Ionic Liquid Membranes for CO2 Separation. Technologies 2016, 4, 32. https://doi.org/10.3390/technologies4040032
Sánchez-Fuentes CE, Pergher SB, Gutiérrez-Arzaluz M, Mugica-Álvarez V, Terrés E, Torres-Rodríguez M. Interactions between the Ionic Liquid and the ZrO2 Support in Supported Ionic Liquid Membranes for CO2 Separation. Technologies. 2016; 4(4):32. https://doi.org/10.3390/technologies4040032
Chicago/Turabian StyleSánchez-Fuentes, Cinthia E., Sibele B. Pergher, Mirella Gutiérrez-Arzaluz, Violeta Mugica-Álvarez, Eduardo Terrés, and Miguel Torres-Rodríguez. 2016. "Interactions between the Ionic Liquid and the ZrO2 Support in Supported Ionic Liquid Membranes for CO2 Separation" Technologies 4, no. 4: 32. https://doi.org/10.3390/technologies4040032








