Validation and Feasibility of the Medication Acceptability Questionnaire to Investigate Tablet and Liquid Alendronic Acid with Older Hospital Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Questionnaire Testing and Refinement
2.3. Study Sample and Setting
2.3.1. Eligibility
- Deemed by the healthcare team as unable to provide written, informed consent
- Unable to self-administer medication during in-patient stay or unlikely to self-administer post discharge
- Dysphagia (Functional Oral Intake Scale score <7) [20]
2.3.2. Sample Size
2.4. Recruitment and MAQ Administration
2.5. Statistical Analysis and Psychometric Testing
3. Results
3.1. Questionnaire Testing and Refinement
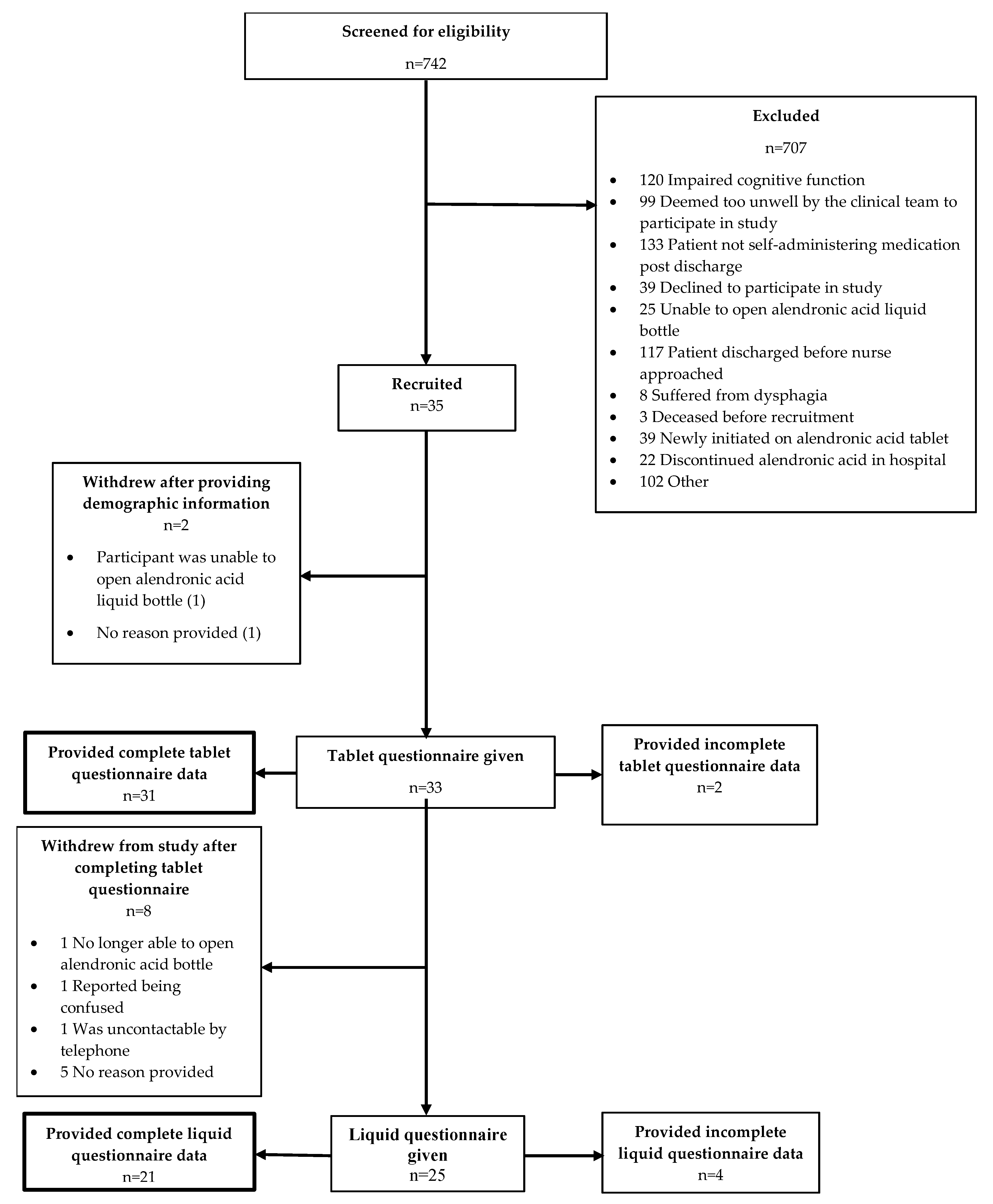
3.2. Recruitment
3.3. Questionnaire Responses and Psychometric Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinicola, V.; Giampà, E.; Di Bonito, M.; Ferretti, V.; Nuvoli, G.; Paoletti, F.; Piazzini, M.; Ranieri, M.; Tuveri, M.A. Results of a national multicentric study on compliance to treatment with various disphosphonate formulations in patients with postmenopausal osteoporosis. Minerva Endocrinol. 2015, 40, 187–193. [Google Scholar] [PubMed]
- Kanis, J.A.; Brazier, J.E.; Stevenson, M.; Calvert, N.W.; Lloyd Jones, M. Treatment of established osteoporosis: A systematic review and cost-utility analysis. Health Technol. Assess. 2002, 6, 1–146. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Gold, D.T.; Silverman, S.L.; Lewiecki, E.M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos. Int. 2007, 18, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Salter, C.; McDaid, L.; Bhattacharya, D.; Holland, R.; Marshall, T.; Howe, A. Abandoned Acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: A qualitative longitudinal study. PLoS ONE 2014, 9, e83552. [Google Scholar] [CrossRef] [PubMed]
- Tafaro, L.; Nati, G.; Leoni, E.; Baldini, R.; Cattaruzza, M.S.; Mei, M.; Falaschi, P. Adherence to anti-osteoporotic therapies: Role and determinants of “spot therapy”. Osteoporos. Int. 2013, 24, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Yood, R.A.; Emani, S.; Reed, J.I.; Lewis, B.E.; Charpentier, M.; Lydick, E. Compliance with pharmacologic therapy for osteoporosis. Osteoporos. Int. 2003, 14, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Rees, G.; Leong, O.; Crowston, J.G.; Lamoureux, E.L. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmology 2010, 117, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.; Zweidorff, O.K.; Hjelde, T.; Rødland, E.A. Problems when swallowing tablets. A questionnaire study from general practice. Tidsskr. Den Nor. Laegeforen. 1995, 115, 947–949. [Google Scholar]
- Rodenhuis, N.; De Smet, P.A.G.M.; Barends, D.M. The rationale of scored tablets as dosage form. Eur. J. Pharm. Sci. 2004, 21, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Golub, J.S.; Hapner, E.R.; Johns, M.M. Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia 2009, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Greenwood, C.; Ens, I.; Robertson, C.; Seidman-Carlson, R. Mealtime difficulties in a home for the aged: Not just dysphagia. Dysphagia 1997, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, R.; Bayne, J.R. Compliance with prescribed medication by elderly patients. Can. Med. Assoc. J. 1982, 127, 961. [Google Scholar] [PubMed]
- Hulka, B.S.; Kupper, L.L.; Cassel, J.C.; Efird, R.L.; Burdette, J.A. Medication use and misuse: Physician-patient discrepancies. J. Chronic Dis. 1975, 28, 7–21. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Medication Acceptability Questionnaire (MAQ). Available online: https://www.uea.ac.uk/pharmacy/research/maq (accessed on 17 May 2018).
- Payerne, E.; Bhattacharya, D. Identifying the Factors that Influence Patient Acceptability of Oral Medicines: A Systematic Review of Literature (CRD4201300). PROSPERO. 2013. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=4033 (accessed on 11 August 2018).
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available online: http://handbook-5-1.cochrane.org/ (accessed on 11 August 2018).
- Jääskeläinen, R. Think-aloud protocol. Handb. Transl. Stud. 2010, 1, 371–373. [Google Scholar]
- Willis, G.B. Cognitive Interviewing: A Tool for Improving Questionnaire Design; Sage Productions Ltd.: Thousand Oaks, CA, USA, 2005. [Google Scholar]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Massy-Westropp, N.M.; Gill, T.K.; Taylor, A.W.; Bohannon, R.W.; Hill, C.L. Hand grip strength: Age and gender stratified normative data in a population-based study. BMC Res. Notes 2011, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.G.; O’brien, A.G.; Nightingale, P.G. Cognitive assessment in elderly patients admitted to hospital: The relationship between the Abbreviated Mental Test and the Mini-Mental State Examination. Clin. Rehabil. 1999, 13, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; Venables, R.; Marriott, J.; Mills, T. The application of tribology in assessing texture perception of oral liquid medicines. Int. J. Pharm. 2015, 479, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.C.; Kriel, R.L.; Jones-Saete, C.M.; Ong, B.Y.; Jancik, J.T.; Remmel, R.P. Comparison of sprinkle versus syrup formulations of valproate for bioavailability, tolerance, and preference. J. Pediatr. 1992, 120, 634–638. [Google Scholar] [CrossRef]

| MAQ Domain | Internal Consistency (α) between Domain Item Scores | Criterion Validity Spearman’s Correlation Coefficient (p-Value) between Domain Subscale Score and Corresponding VAS |
|---|---|---|
| Convenience | 0.65 | 0.63 (<0.001) |
| Taste | 0.56 | 0.65 (<0.001) |
| Appearance | 0.71 | 0.58 (<0.001) |
| Efficacy | 0.81 | 0.71 (<0.001) |
| Tolerability | 0.91 | 0.37 (0.005) |
| Subscale Domain | Correlation Coefficient (p-Value) | |
|---|---|---|
| Tablet Global Acceptability VAS | Liquid Global Acceptability VAS | |
| Convenience | 0.40 (0.022) | 0.63 (0.001) |
| Taste | 0.41 (0.018) | 0.64 (0.001) |
| *Appearance | −0.17 (0.355) | 0.44 (0.031) |
| Efficacy | 0.21 (0.243) | 0.62 (0.002) |
| *Tolerability inverted | −0.06 (0.753) | 0.20 (0.361) |
| Tablet Global Acceptability VAS | Liquid Global Acceptability VAS | |||
|---|---|---|---|---|
| Subscale Domain | Regression Coefficient (95% CI) | p-Value | Regression Coefficient (95% CI) | p-Value |
| Convenience | −0.09 (−0.53, 0.34) | 0.675 | 0.50 (0.05, 0.94) | 0.032 |
| Taste | 1.21 (0.57, 1.86) | <0.001 | 0.63 (0.13, 1.13) | 0.017 |
| Appearance | −0.68 (−1.05, −0.3) | <0.001 | −0.05 (−0.56, 0.46) | 0.837 |
| Efficacy | 0.30 (−0.04, 0.63) | 0.077 | 0.53 (−0.25, 1.30) | 0.167 |
| Tolerability inverted | −0.01 (−0.28, 0.26) | 0.938 | −0.11 (−0.92, 0.70) | 0.769 |
| Constant | 10.74 (3.94, 17.54) | 0.002 | −12.77 (−26.66, 1.12) | 0.069 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, S.; Clark, A.; May, H.; Bhattacharya, D. Validation and Feasibility of the Medication Acceptability Questionnaire to Investigate Tablet and Liquid Alendronic Acid with Older Hospital Patients. Pharmacy 2018, 6, 84. https://doi.org/10.3390/pharmacy6030084
Scott S, Clark A, May H, Bhattacharya D. Validation and Feasibility of the Medication Acceptability Questionnaire to Investigate Tablet and Liquid Alendronic Acid with Older Hospital Patients. Pharmacy. 2018; 6(3):84. https://doi.org/10.3390/pharmacy6030084
Chicago/Turabian StyleScott, Sion, Allan Clark, Helen May, and Debi Bhattacharya. 2018. "Validation and Feasibility of the Medication Acceptability Questionnaire to Investigate Tablet and Liquid Alendronic Acid with Older Hospital Patients" Pharmacy 6, no. 3: 84. https://doi.org/10.3390/pharmacy6030084





