Stress Responses of Shade-Treated Tea Leaves to High Light Exposure after Removal of Shading
Abstract
:1. Introduction
2. Results
2.1. Long-Term Shading Treatment of Tea in Field Condition
2.2. Changes in Chl Content and Chl a/b Ratio
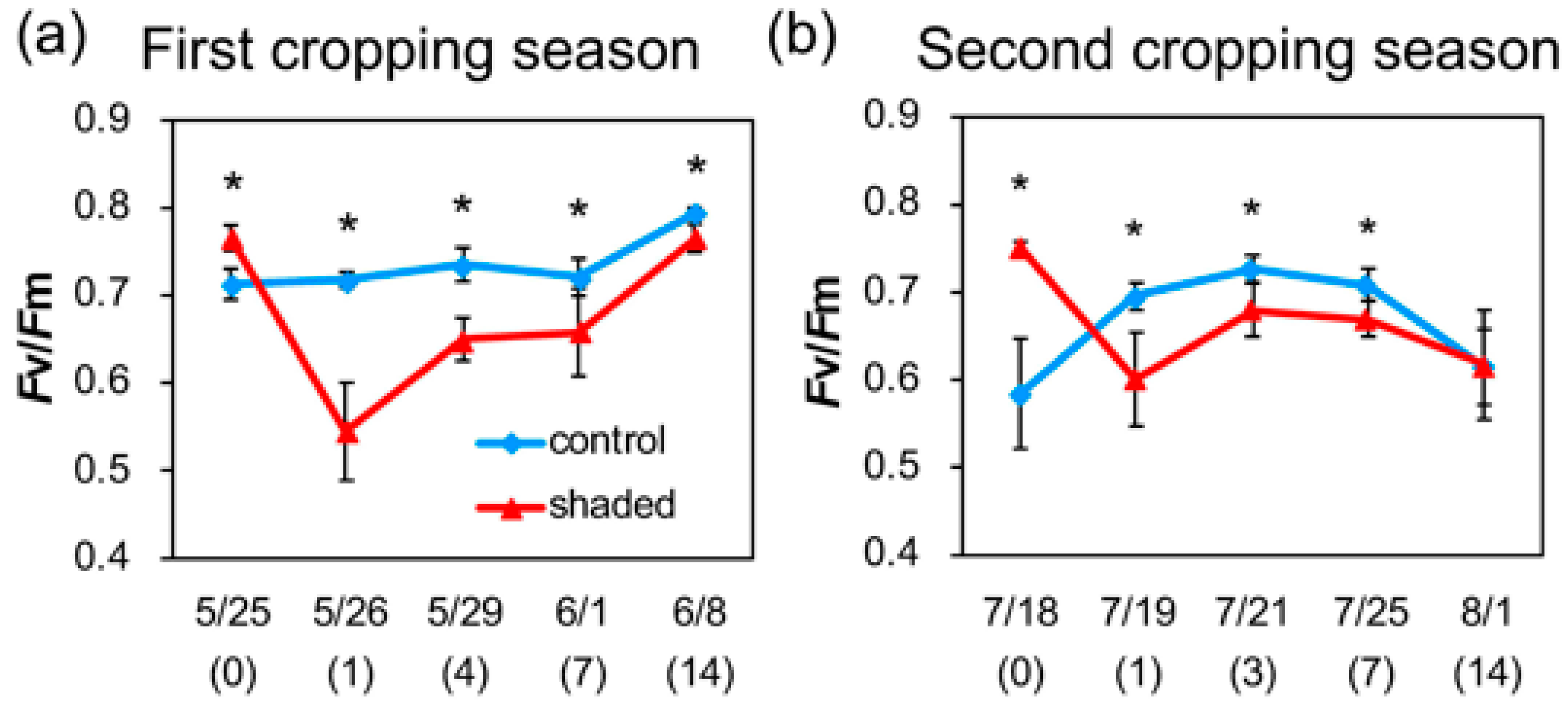
2.3. Changes in Chl Fluorescence
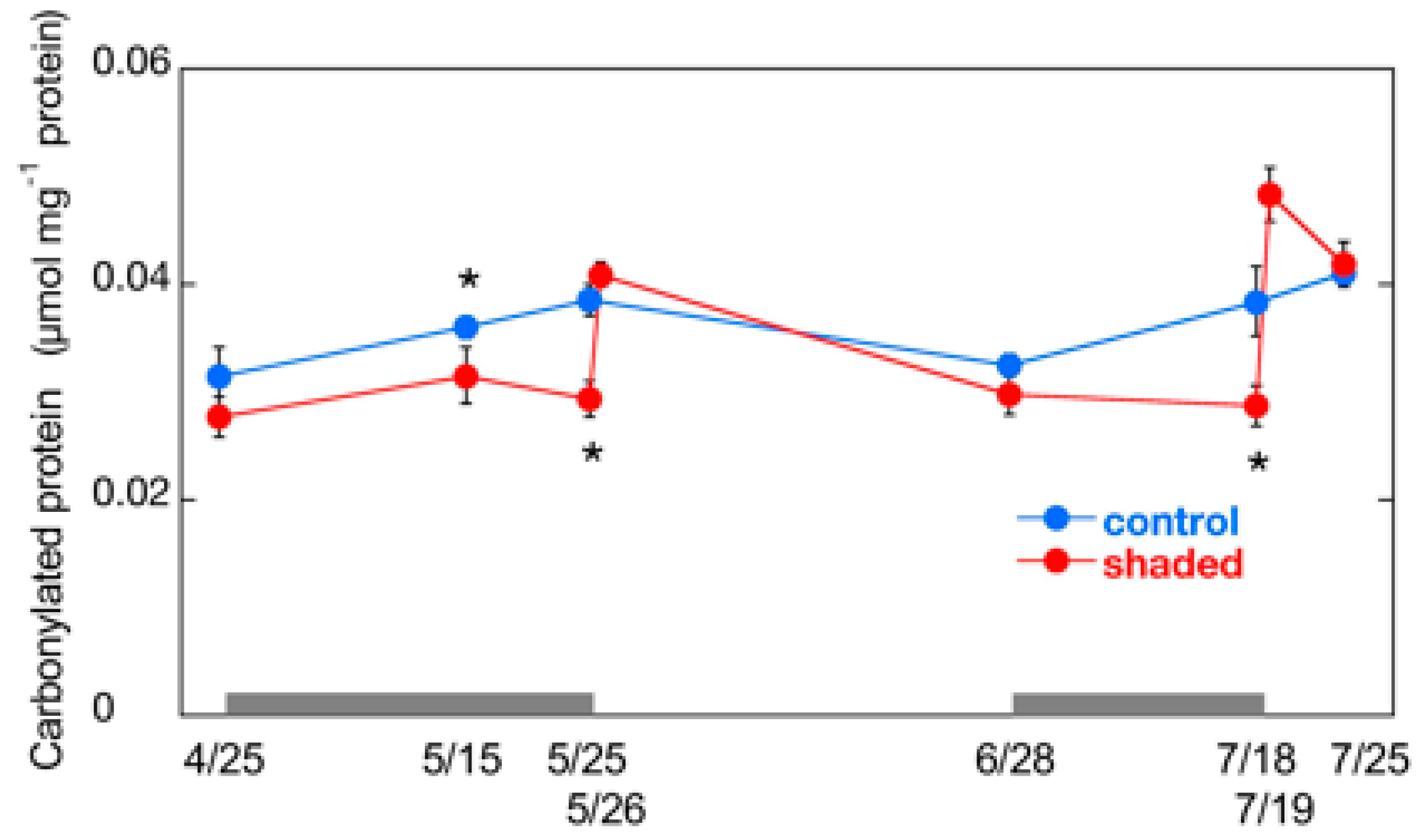
2.4. Changes in Carbonylated Protein Content
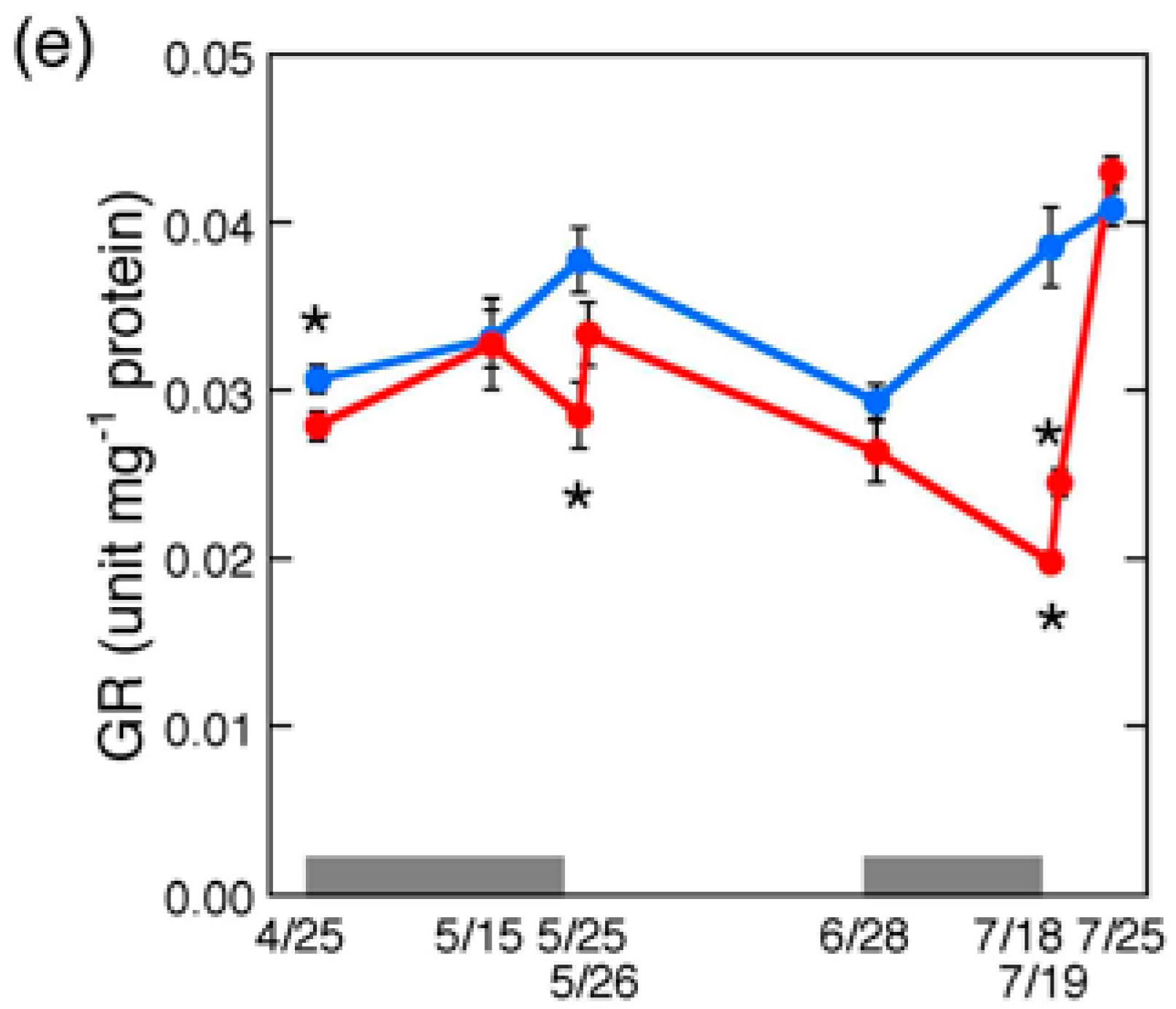
2.5. Changes in Antioxidative Enzyme Activities
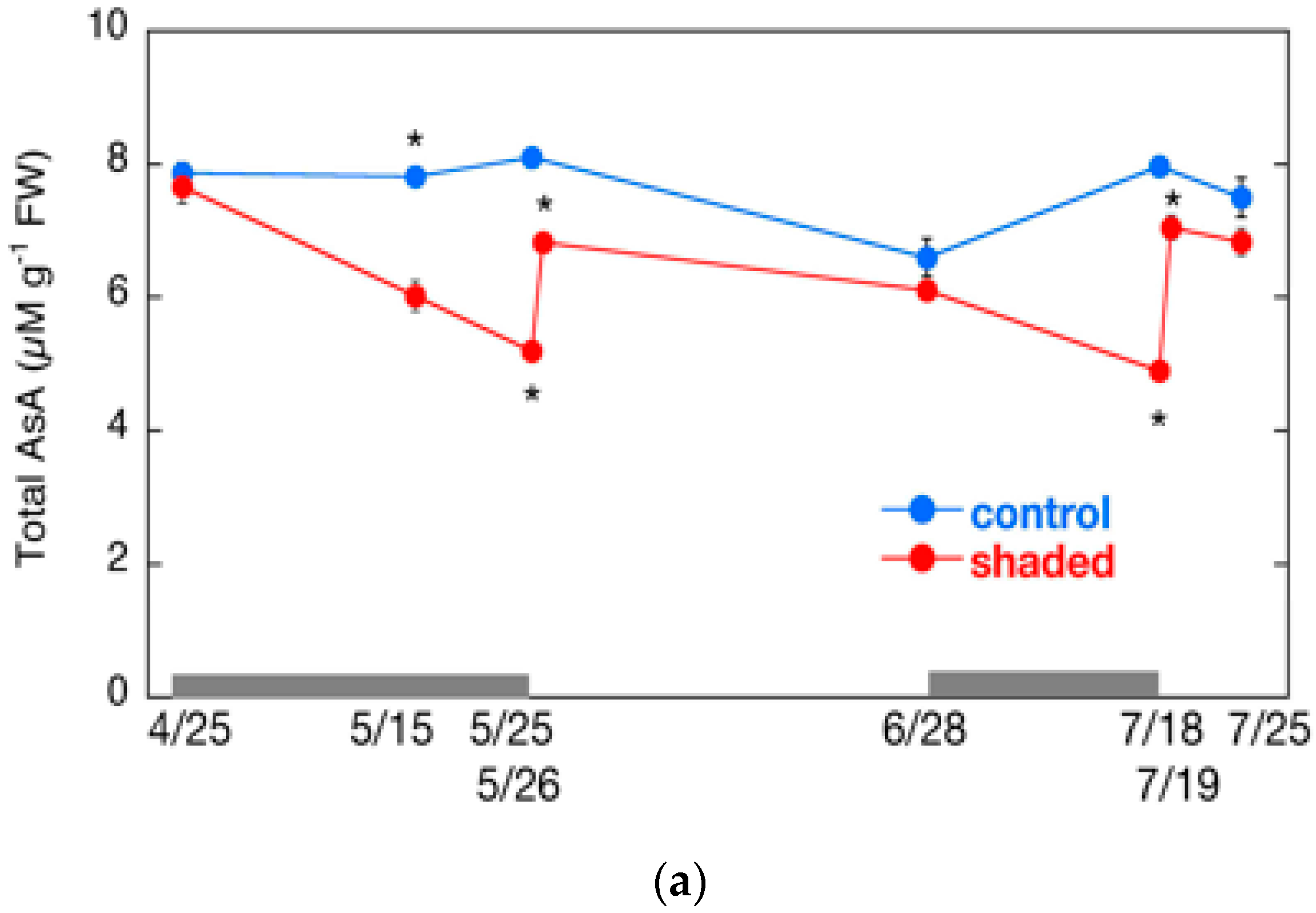
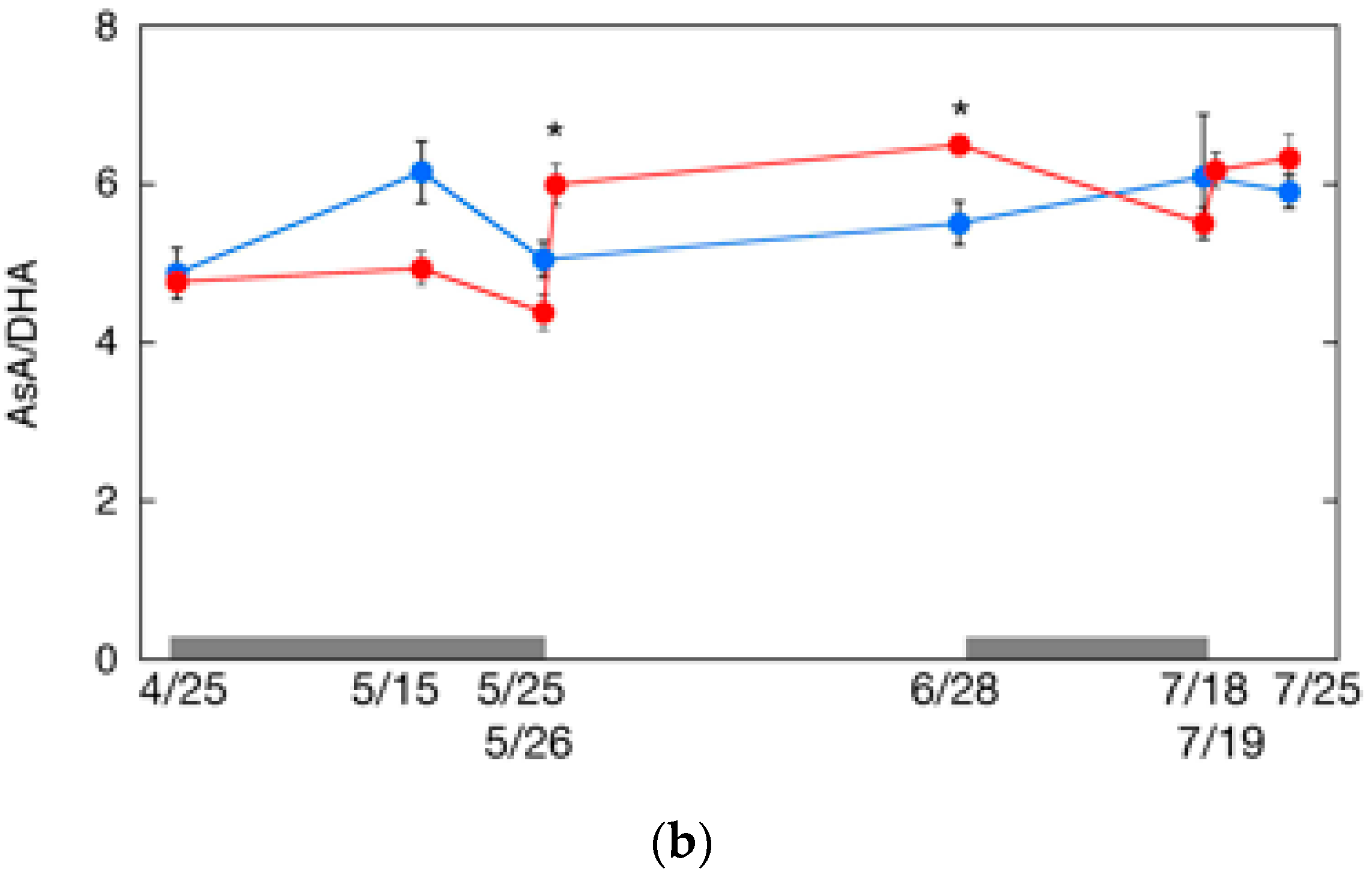
2.6. Changes in AsA Content and Its Redox Level
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Measurement of Chl Fluorescence
4.3. Measurement of Chl Contents
4.4. Spectrophotometric Determination of Carbonylated Proteins
4.5. Assay of Antioxidative Enzymes
4.6. The Determination of AsA Content
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekborg-Ott, K.H.; Taylor, A.; Armstrong, D.W. Varietal differences in the total and enantiomeric composition of theanine in tea. J. Agric. Food Chem. 1997, 45, 353–363. [Google Scholar] [CrossRef]
- McDowell, I.; Owuor, P. Taste of tea. New Sci. 1992, 133, 30–33. [Google Scholar]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; Rao, A.; Owen, G.N. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac. J. Clin. Nutr. 2008, 17, 167–168. [Google Scholar] [PubMed]
- Ashardiono, F.; Cassim, M. Adapting to climate change: Challenges for Uji tea cultivation. J SustaiN 2015, 3, 32–36. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L.; Shan, Y.; Liu, Y.; Tian, Y.; Xia, T. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, J.H.; Son, N.; Kim, S.-H.; Park, J.-D.; Jang, D.-J.; Jeong, Y.; Kim, H.-J. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.H.; Park, C.Y.; Kim, E.-H.; Park, J.S.; Hong, Y.-S. Diverse metabolite variations in tea (Camellia sinensis L.) leaves grown under various shade conditions revisited: A metabolomics study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; She, G.; Zhang, X.; Jordan, B.; Chen, Q.; Zhao, J.; Wan, X. Metabolite profiling and transcriptomic analyses reveal an essential role of UVR8-mediated signal transduction pathway in regulating flavonoid biosynthesis in tea plants (Camellia sinensis) in response to shading. BMC Plant Biol. 2018, 18, 233. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation: Changes in morphology, color, and chemical components of tea shoots due to shade. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Hayashi, K. Effect of the difference in the covering methods on growth of tea tree and the canopy surface temperature in summer based on thermal images. Tea Res. J. 2019, 127, 1–10, (in Japanese with English abstract). [Google Scholar]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Deutsch, J.C. Dehydroascorbic acid. J. Chromatogr. A 2000, 881, 299–307. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Vyas, D.; Kumar, S.; Ahuja, P.S. Tea (Camellia sinensis) clones with shorter periods of winter dormancy exhibit lower accumulation of reactive oxygen species. Tree Physiol. 2007, 27, 1253–1259. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant. 2009, 31, 261–269. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huang, W.; Wang, G.L.; Wu, Z.J.; Zhuang, J. Expression profile analysis of ascorbic acid-related genes in response to temperature stress in the tea plant, Camellia sinensis (L.) O. Kuntze. Genet. Mol. Res. 2016, 15, gmr15048756. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.-W.; Wu, Z.-J.; Wang, Y.-X.; Teng, R.-M.; Zhuang, J. Differentially expressed protein and gene analysis revealed the effects of temperature on changes in ascorbic acid metabolism in harvested tea leaves. Hortic. Res. 2018, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Chen, Z.; Sun, W.; Deng, T.; Chen, M. De novo sequencing of the leaf transcriptome reveals complex light-responsive regulatory networks in Camellia sinensis cv. Baijiguan. Front. Plant Sci. 2016, 7, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, T.-Y.; Anderson, J.M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth. Res. 1984, 5, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]
- van Rensen, J.J.S.; Curwiel, V.B. Multiple functions of photosystem II. Indian J. Biochem. Biophys. 2000, 37, 377–382. [Google Scholar]
- Tyystjärvi, E. Photoinhibition of photosystem II. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 300, pp. 243–303. ISBN 978-0-12-405210-9. [Google Scholar]
- Barman, T.S.; Baruah, U.; Saikia, J.K. Irradiance Influences tea leaf (Camellia sinensis L.) photosynthesis and transpiration. Photosynthetica 2008, 46, 618–621. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.-M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Sugimoto, Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 2010, 231, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Miyatake, F.; Hiraoka, E.; Tamoi, M. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 2009, 230, 639–648. [Google Scholar] [CrossRef]
- Winger, A.M.; Taylor, N.L.; Heazlewood, J.L.; Day, D.A.; Millar, A.H. The cytotoxic lipid peroxidation product 4-hydroxy-2-nonenal covalently modifies a selective range of proteins linked to respiratory function in plant mitochondria. J. Biol. Chem. 2007, 282, 37436–37447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweetlove, L.J.; Møller, I.M. Oxidation of proteins in plants—Mechanisms and consequences. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 52, pp. 1–23. [Google Scholar]
- Logan, B.A.; Barker, D.H.; Demmig-Adams, B.; Adams, W.W. Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant Cell Environ. 1996, 19, 1083–1090. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Yonemitsu, M.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Arabidopsis Phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 2008, 283, 28842–28851. [Google Scholar] [CrossRef] [Green Version]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef] [Green Version]
- Grieco, M.; Tikkanen, M.; Paakkarinen, V.; Kangasjärvi, S.; Aro, E.-M. Phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol. 2012, 160, 1896–1910. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Mekala, N.R.; Aro, E.-M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta 2014, 1837, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 Is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Ishizaki, K.; Hanawa, H.; Mabuchi, T.; Shimakawa, G.; Yamamoto, H.; Miyake, C. Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol. Plant. 2017, 161, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Rossel, J.B.; Wilson, I.W.; Pogson, B.J. Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 2002, 130, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.-S.; Crisp, P.A.; Estavillo, G.M.; Cole, B.; Hong, F.; Mockler, T.C.; Pogson, B.J.; Chory, J. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA 2013, 110, 14474–14479. [Google Scholar] [CrossRef] [Green Version]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Gollan, P.J.; Mekala, N.R.; Isojarvi, J.; Aro, E.-M. Light-harvesting mutants show differential gene expression upon shift to high light as a consequence of photosynthetic redox and reactive oxygen species metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130229. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidsae in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Romero-Puertas, M.C.; Palma, J.M.; Gomez, M.; Del Rio, L.A.; Sandalio, L.M. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 2002, 25, 677–686. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide dismutase assays. Methods Enzym. 1984, 105, 93–104. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hossain, M.A.; Asada, K. Monodehydroascorbate reductase from cucumbers a flavin adenine dinucleotide enzyme. J. Biol. Chem. 1985, 260, 12920–12926. [Google Scholar] [PubMed]
- Hossain, M.A.; Asada, K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 1984, 25, 85–92. [Google Scholar]
- Shigeoka, S.; Onishi, T.; Nakano, Y.; Kitaoka, S. Characterization and physiological function of glutathione reductase in Euglena gracilis z. Biochem. J. 1987, 242, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Amako, K.; Ushimaru, T.; Ishikawa, A.; Ogishi, Y.; Kishimoto, R.; Goda, K. Heterologous expression of dehydroascorbate reductase from rice and its application to determination of dehydroascorbate concentrations. J. Nutr. Sci. Vitaminol. 2006, 52, 89–95. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, S.; Takemoto, T.; Ogihara, A.; Suzuki, K.; Masumura, T.; Satoh, S.; Takano, K.; Mimura, Y.; Morita, S. Stress Responses of Shade-Treated Tea Leaves to High Light Exposure after Removal of Shading. Plants 2020, 9, 302. https://doi.org/10.3390/plants9030302
Sano S, Takemoto T, Ogihara A, Suzuki K, Masumura T, Satoh S, Takano K, Mimura Y, Morita S. Stress Responses of Shade-Treated Tea Leaves to High Light Exposure after Removal of Shading. Plants. 2020; 9(3):302. https://doi.org/10.3390/plants9030302
Chicago/Turabian StyleSano, Satoshi, Tetsuyuki Takemoto, Akira Ogihara, Kengo Suzuki, Takehiro Masumura, Shigeru Satoh, Kazufumi Takano, Yutaka Mimura, and Shigeto Morita. 2020. "Stress Responses of Shade-Treated Tea Leaves to High Light Exposure after Removal of Shading" Plants 9, no. 3: 302. https://doi.org/10.3390/plants9030302




