H2O2 Induces Association of RCA with the Thylakoid Membrane to Enhance Resistance of Oryza meyeriana to Xanthomonas oryzae pv. oryzae
Abstract
:1. Introduction
2. Results
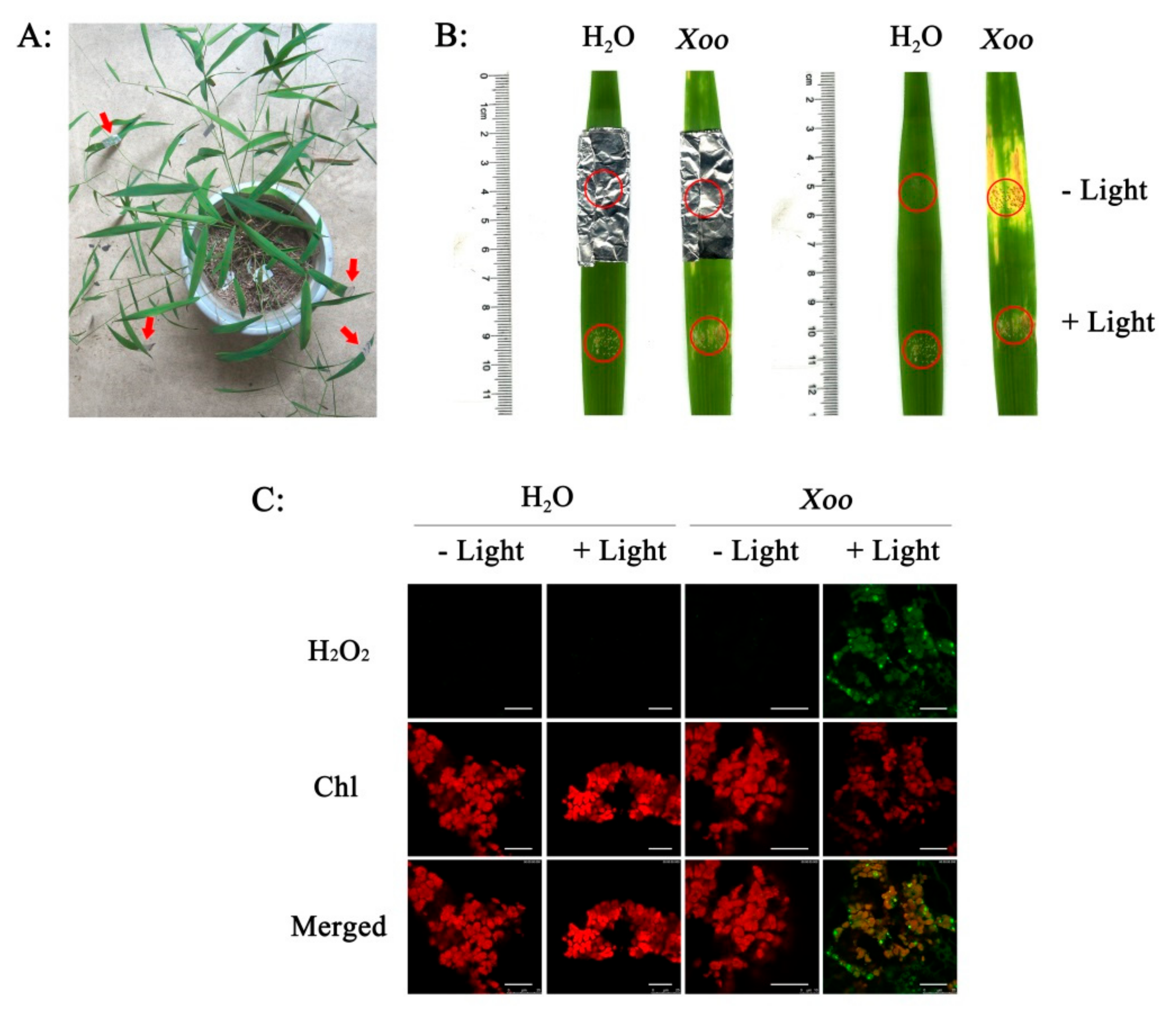
2.1. Resistance Analysis
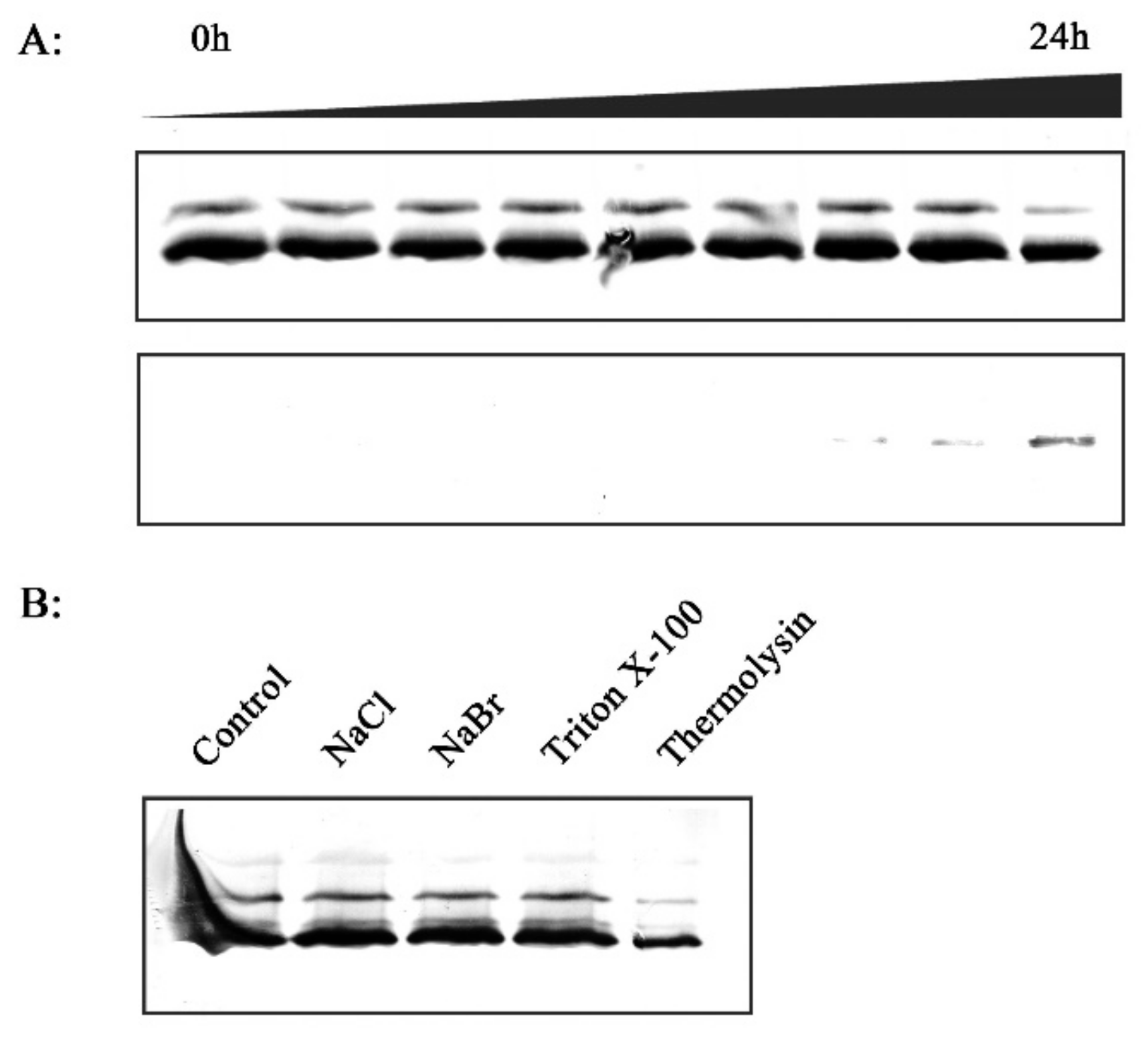
2.2. Effect of Xoo Inoculation on RCA Distribution
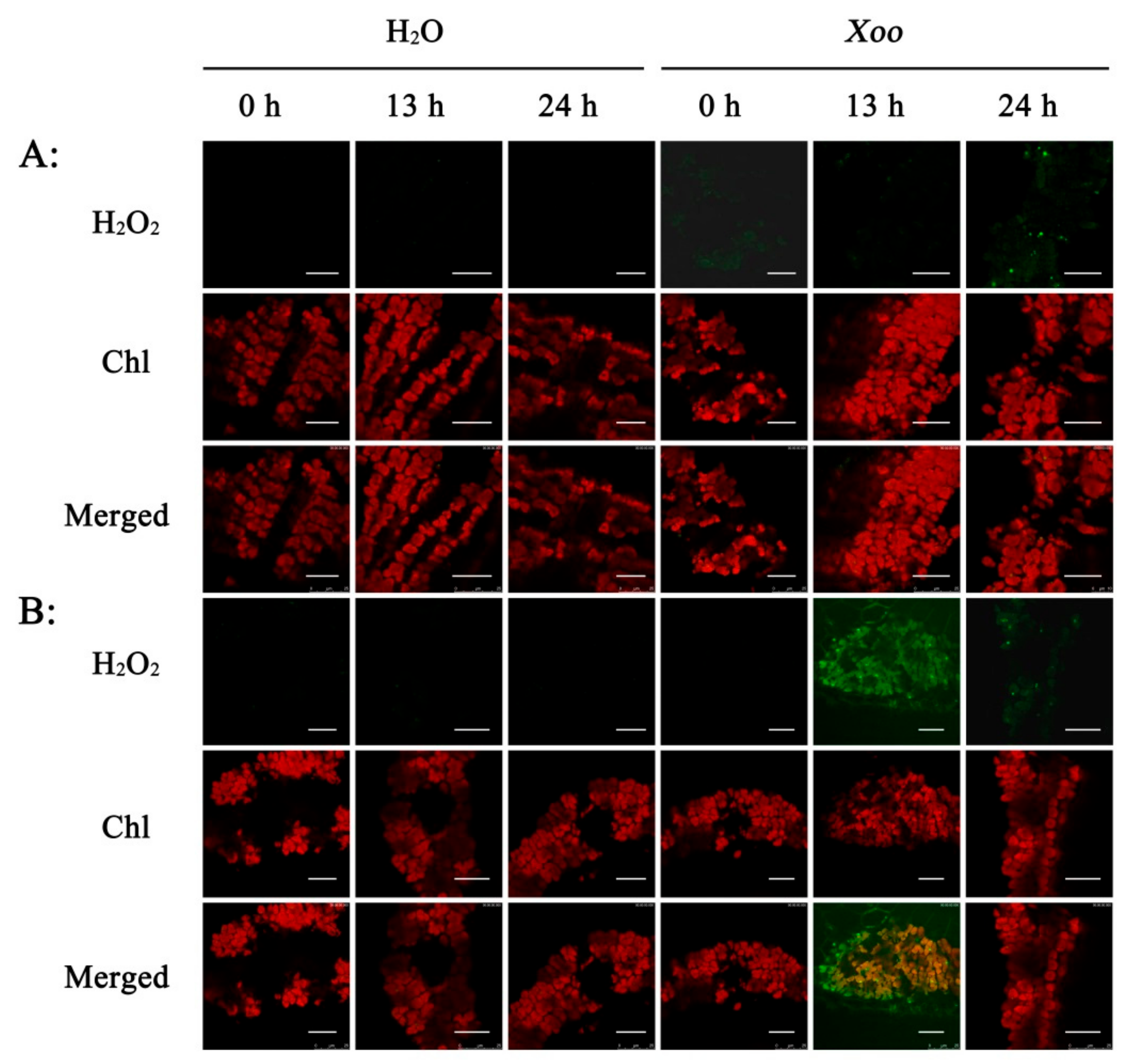
2.3. Effect of Xoo Inoculation on H2O2 Generation
2.4. Subcellular Localization of H2O2
2.5. Light-Dependent H2O2 Generation and Resistance in Wild Rice
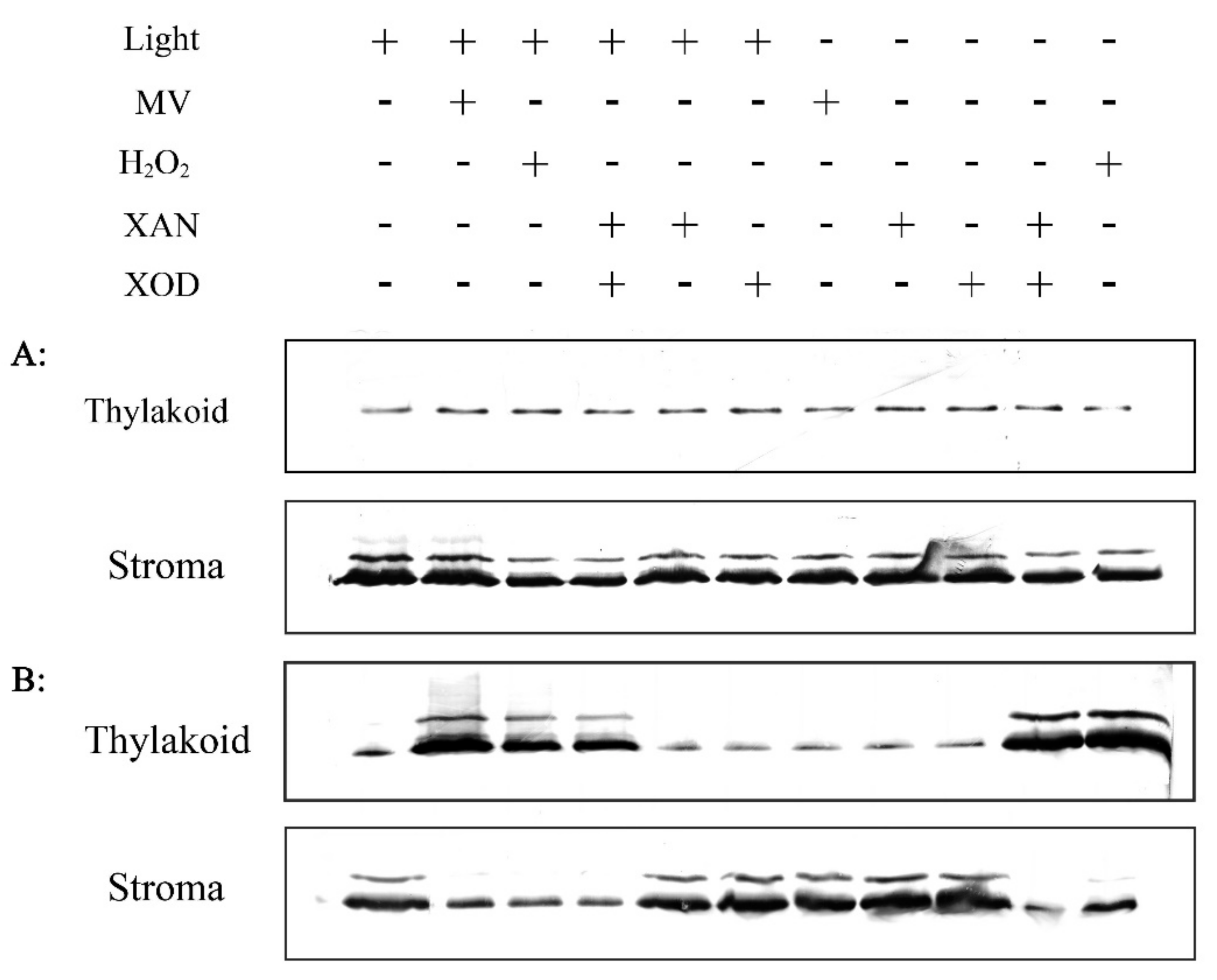
2.6. Effect of H2O2 on RCA Redistribution in Vitro
2.7. In Vitro Investigation of the Association between RCA and Thylakoid Membrane
3. Discussion
3.1. High Resistance of O. meyeriana to Xoo
3.2. H2O2 Generation in Chloroplasts of O. meyeriana Infected by Xoo
3.3. Chloroplast H2O2 Induced the Association of RCA with Thylakoid Membrane Following Infection of O. meyeriana with Xoo
3.4. Potential Novel Role of RCA in the Xoo Resistance of O. meyeriana
4. Materials and Methods
4.1. Plant Materials
4.2. Pathogen Inoculation and Resistance Identification
4.3. Measurements of Chlorophyll Fluorescence
4.4. Isolation and Sub-Fractionation of Chloroplasts
4.5. SDS-PAGE and Immunological Analyses
4.6. Determination of H2O2 in Leaf extracts
4.7. Subcellular Localization of H2O2
4.8. Salt Washing and Thermolysin Treatment of Isolated Thylakoids
4.9. Oxidative Treatment of Ruptured Chloroplasts
4.10. Shading Treatment of Wild Rice Leaves
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nino-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Genetics and Improvement of Resistance to Bacterial Blight in Rice, 1st ed.; Science Press: Beijing, China, 2007; pp. 1–4. [Google Scholar]
- Han, X.Y.; Yang, Y.; Wang, X.M.; Zhou, J.; Zhang, W.H.; Yu, C.L.; Cheng, C.; Cheng, Y.; Yan, C.Q.; Chen, J.P.; et al. Quantitative trait loci mapping for bacterial blight resistance in rice using bulked segregant analysis. Int. J. Mol. Sci. 2014, 15, 11847–11861. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, L.; Yan, C.Q.; Wang, X.M.; Yu, C.L.; Cheng, X.Y.; Cheng, Y.; Chen, J.P. Xylem secondary cell-wall thickening involved in defense responses of Oryza meyeriana to Xanthomonas oryzae pv. oryzae. Acta Phytopathol. Sin. 2012, 5, 505–514. [Google Scholar]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.A. Receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Yang, B.; Tian, D.; Wu, L.F.; Wang, D.J.; Sreekala, C.; Yang, F.; Chu, Z.Q.; Wang, G.L.; White, F.F.; et al. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 2005, 435, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Chen, L.T.; Zeng, C.Z.; Zhang, Q.Y.; Liu, P.Q.; Liu, Y.G.; Fan, Y.L.; Zhang, Q.; Zhao, K.J. Chromosome walking for fine mapping of Xa23 gene locus by using genomic libraries. Chinese J. Rice Sci. 2006, 20, 355–360. [Google Scholar]
- Tan, G.X.; Ren, X.; Weng, Q.M.; Shi, Z.Y.; Zhu, L.L.; He, G.C. Mapping of a new resistance gene to bacterial blight in rice line introgressed from Oryza officinalis. Acta Genet. Sin. 2004, 31, 724–729. [Google Scholar]
- Peng, S.Q.; Wei, Z.S.; Mao, C.X.; Luo, K.; Huang, H.Q.; Xiao, F.H. Identification of multiresistance of wild rice: Resource for high resistance to rice bacterial blight—Oryza meyeriana. Acta Agricul. Hunan. 1981, 5, 47–48. [Google Scholar]
- Peng, S.Q.; Wei, Z.S.; Mao, C.X.; Huang, H.Q.; Xiao, F.H.; Luo, K. Identification of multiresistance of O. meyeriana, O. officinalis and O. sativa f. spantanea growing in Yunan province. Acta Phytopathol. Sin. 1982, 12, 58–61. [Google Scholar]
- Zhang, Q.; Wang, C.; Shi, A. Evaluation of resistance to bacterial blight (Xanthomonas oryzae pv. oryzae) in wild rice species. Sci. Agricul. Sin. 1994, 27, 1–9. [Google Scholar]
- Yan, C.Q.; Qian, K.X.; Yan, Q.S.; Zhang, X.Q.; Xue, G.P.; Huangfu, W.G.; Wu, Y.F.; Zhao, Y.Z.; Xue, Z.Y.; Huang, J.; et al. Use of asymmetric somatic hybridization for transfer of the bacterial resistance trait from Oryza meyeriana L. to O. sativa L. ssp. japonica. Plant Cell Rep. 2004, 2, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.Q.; Qian, K.X.; Xue, G.P.; Wu, Z.C.; Chen, Y.L.; Yan, Q.S.; Zhang, X.Q.; Wu, P. Production of bacterial blight resistant lines from somatic hybridization between Oryza sativa L. and Oryza meyeriana L. J. Zhejiang Univ. Sci. 2004, 5, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, C.L.; Wang, X.M.; Yan, C.Q.; Cheng, Y.; Chen, J.P. Inoculation with Xanthomonas oryzae pv. oryzae induces thylakoid membrane association of Rubisco activase in Oryza meyeriana. J. Plant Physiol. 2011, 168, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Yang, Y.; Yan, C.Q.; Wang, X.M.; Yu, C.L.; Zhou, J.; Zhang, W.L.; Cheng, Y.; Cheng, X.Y.; Chen, J.P.; et al. Identification of quantitative trait loci for bacterial blight resistance derived from Oryza meyeriana and agronomic traits in recombinant inbred lines of Oryza sativa. J. Phytopathol. 2012, 160, 461–468. [Google Scholar] [CrossRef]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Bonfig, K.B.; Schreiber, U.; Gabler, A.; Roitsch, T.; Berger, S. Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 2006, 225, 1–12. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjarvi, J. ROS-talk-how the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef]
- Yokota, A.; Tsujimoto, N. Characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase carrying ribulose-1,5-bisphosphate at its regulatory sites and the mechanism of interaction of this form the enzyme with ribulose-1,5-bisphosphate carboxylase/oxygenase activase. Eur. J. Biochem. 1992, 204, 901–909. [Google Scholar] [CrossRef]
- Portis, A.R.J. Rubisco activase - Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Komatsu, S. Molecular cloning and characterization of cDNAs encoding two isoforms of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase in rice (Oryza sativa L.). J. Biochemy. 2000, 128, 383–389. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Keys, A.J.; Madgwick, P.J.; Carmo-Silva, A.E.; Andralojc, P.J. Rubisco regulation: A role for inhibitors. J. Exp. Bot. 2008, 59, 1569–1580. [Google Scholar] [CrossRef]
- Giri, A.P.; Wünsche, H.; Mitra, S. Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata. VII. changes in the plant’s proteome. Plant Physiol. 2006, 142, 1621–1641. [Google Scholar] [CrossRef]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; van de Loo, F.J.; Salvucci, M.E. The two forms of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol. 1997, 114, 439–444. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Badger, M.R.; von Caemmerer, S.; Andrews, T.J. Increased heat sensitivity of photosynthesis in tobacco plant with reduced Rubisco activase. Photosynth. Res. 2001, 67, 147–156. [Google Scholar] [CrossRef]
- Saibo, N.J.M.; Lourenco, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef]
- Hurt, E.; Hauska, G. A cytochrome b6/f complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur. J. Biochem. 1981, 17, 591–595. [Google Scholar] [CrossRef]
- Li, D.Y.; Liu, H.J.; Zhang, H.J.; Wang, X.; Song, F.M. OsBIRH1, a DEAD-box RNA helicase with functions in modulating defence responses against pathogen infection and oxidative stress. J. Exp. Bot. 2008, 59, 2133–2146. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef]
- Iwata, S.; Fukaya, Y.; Nakazawa, K.; Okuda, T. Effects of tannins on the oxidative damage of mouse ocular lens. I. Using the oxidative damage model induced by the xanthine-xanthine oxidase system. J. Ocul. Pharmacol. 1987, 3, 227–238. [Google Scholar] [CrossRef]
- Li, B.; HU, B.; Liao, D.F. Protective effect of gypenosides on free radical damage of isolated guinea pig papillary muscles. Chin. Pharmacol. Bull. 1993, 9, 287–290. [Google Scholar]
- Hussein, G.; Goto, H.; Oda, S.; Iguchi, T.; Sankawa, U.; Matsumoto, K.; Watanabe, H. Antihypertensive potential and mechanism of action of astaxanthin: II. Vascular reactivity and hemorheology in spontaneously hypertensive rats. Biol. Pharm. Bull. 2005, 28, 967–971. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wu, H.; Chen, H.; Liu, Y.L.; He, J.; Kang, H.Y.; Sun, Z.G.; Pan, G.; Wang, Q.; Hu, J.L.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotech. 2015, 33, 301–307. [Google Scholar] [CrossRef]
- Dong, Y.; Fang, X.P.; Yang, Y.; Xue, G.P.; Chen, X.; Zhang, W.L.; Wang, X.M.; Yu, C.L.; Zhou, L.; Mei, Q.; et al. Comparative proteomic analysis of susceptible and resistant rice plants during early infestation by small brown planthopper. Front. Plant Sci. 2017, 8, 1744. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 Links Apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained incre Torres ase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ren, D.T.; Pike, S.; Pallardy, S.; Gassmann, W.; Zhang, S.Q. Choroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007, 51, 941–954. [Google Scholar] [CrossRef]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplat stromules function during innate immunity. Develop. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Bolwell, G.P. Role of active oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 1999, 2, 287–294. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Bindschedler, L.V. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, C.Q.; Cao, B.H.; Xu, H.X.; Chen, J.P.; Jiang, D.A. Some photosynthetic responses to salinity resistance are transferred into the somatic hybrid descendants from the wild soybean Glycine cyrtoloba ACC547. Physiol. Plant. 2007, 129, 658–669. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.I.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Anderson, L.E.; Carol, A.A. Enzyme co-localization with Rubisco in pea leaf chloroplasts. Photosyn. Res. 2004, 82, 49–58. [Google Scholar] [CrossRef]
- Hong, J.; Jiang, D.A.; Weng, X.Y.; Wang, W.B.; Hu, D.W. Leaf anatomy, chloroplast ultrastructure, and cellular localisation of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBPCO) and RuBPCO activase in Amaranthus tricolor L. Photosyn. 2005, 43, 519–528. [Google Scholar] [CrossRef]
- Jin, S.H.; Hong, J.; Li, X.Q.; Jiang, D.A. Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves. Ann. Bot. 2006, 97, 739–744. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Mi, H.; Chen, G.Y.; Xu, D.Q. Reversible association of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase with the thylakoid membrane depends upon the ATP level and pH in rice without heat stress. J. Exp. Bot. 2010, 61, 2939–2950. [Google Scholar] [CrossRef] [Green Version]
- Rokka, A.; Zhang, L.; Aro, E.N. Rubisco activase: An enzyme with a temperature-dependent dual function? Plant J. 2001, 25, 463–471. [Google Scholar] [CrossRef]
- Yang, X.H.; Zheng, L.; Lu, C.M. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 2005, 138, 2299–2309. [Google Scholar] [CrossRef]
- Koh, E.; Carmieli, R.; Mor, A.; Fluhr, R. Singlet oxygen-induced membrane disruption and serpin-protease balance in vacuolar-driven cell death. Plant Physiol. 2016, 171, 1616–1625. [Google Scholar] [CrossRef]
- Dietz, K.; Turkan, I.; Krieger-Liszkay, A. redox-and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Bhuyan, M.; Hasanuzzaman, M.; Mahmud, J.A.; Hossain, M.S.; Bhuiyan, T.F.; Fujita, M. Unraveling morphophysiological and biochemical responses of Triticum aestivum L. to exreme pH: Coordinated actions of antioxidant defense and glyoxalase systems. Plants 2019, 8, 24. [Google Scholar] [CrossRef]
- Neuwald, A.F.; Aravind, L.; Spouge, J.L.; Koonin, E.V. AAA+: A class of chaperone-like ATPases associated with the assembly, operation and disassembly of protein complexes. Genome Res. 1999, 9, 27–43. [Google Scholar]
- Kauffman, H.E.; Reddy, A.P.K.; Hsieh, S.P.Y.; Merca, S.D. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Sanchez, A.C.; Ilag, L.L.; Yang, D.; Brar, D.S.; Ausubel, F.; Khush, G.S.; Yano, M.; Sasaki, T.; Li, Z.; Huang, N. Genetic and physical mapping of Xa13, a recessive bacterial blight resistance gene in rice. Theor. Appl. Genet. 1999, 98, 1022–1028. [Google Scholar] [CrossRef]
- Cline, K.; Andrews, J.; Mersey, B.; Newcomb, E.H.; Keegstra, K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc. Natl. Acad. Sci. USA 1981, 78, 3595–3599. [Google Scholar] [CrossRef] [Green Version]
- Gunasinghe, U.B.; Berger, P.H. Association of potato virus Y gene products with chloroplast in tobacco. Mol. Plant Microbe Interact. 1991, 4, 452–457. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant system in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Thordal- Christensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, B.; Qian, J.; Zou, B.; Hua, J. Disease resistance gene-induced growth inhibition is enhanced by rcd1 independent of defense activation in Arabidopsis. Plant Physiol. 2013, 161, 2005–2013. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Q.; Yang, Y.; Ye, S.; Liang, W.; Wang, X.; Zhou, J.; Yu, C.; Yan, C.; Chen, J. H2O2 Induces Association of RCA with the Thylakoid Membrane to Enhance Resistance of Oryza meyeriana to Xanthomonas oryzae pv. oryzae. Plants 2019, 8, 351. https://doi.org/10.3390/plants8090351
Mei Q, Yang Y, Ye S, Liang W, Wang X, Zhou J, Yu C, Yan C, Chen J. H2O2 Induces Association of RCA with the Thylakoid Membrane to Enhance Resistance of Oryza meyeriana to Xanthomonas oryzae pv. oryzae. Plants. 2019; 8(9):351. https://doi.org/10.3390/plants8090351
Chicago/Turabian StyleMei, Qiong, Yong Yang, Shenhai Ye, Weifang Liang, Xuming Wang, Jie Zhou, Chulang Yu, Chengqi Yan, and Jianping Chen. 2019. "H2O2 Induces Association of RCA with the Thylakoid Membrane to Enhance Resistance of Oryza meyeriana to Xanthomonas oryzae pv. oryzae" Plants 8, no. 9: 351. https://doi.org/10.3390/plants8090351
APA StyleMei, Q., Yang, Y., Ye, S., Liang, W., Wang, X., Zhou, J., Yu, C., Yan, C., & Chen, J. (2019). H2O2 Induces Association of RCA with the Thylakoid Membrane to Enhance Resistance of Oryza meyeriana to Xanthomonas oryzae pv. oryzae. Plants, 8(9), 351. https://doi.org/10.3390/plants8090351



