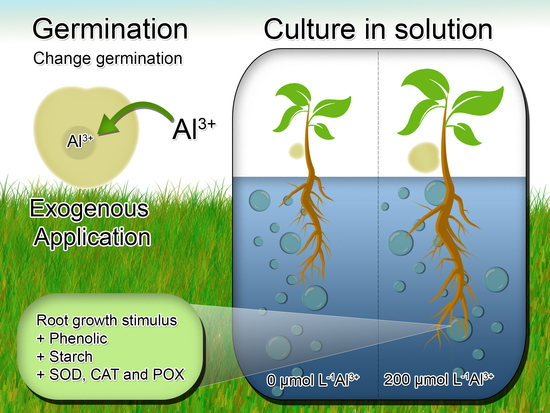
Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material, Growth Conditions and Al Treatments
2.2. Germination Test
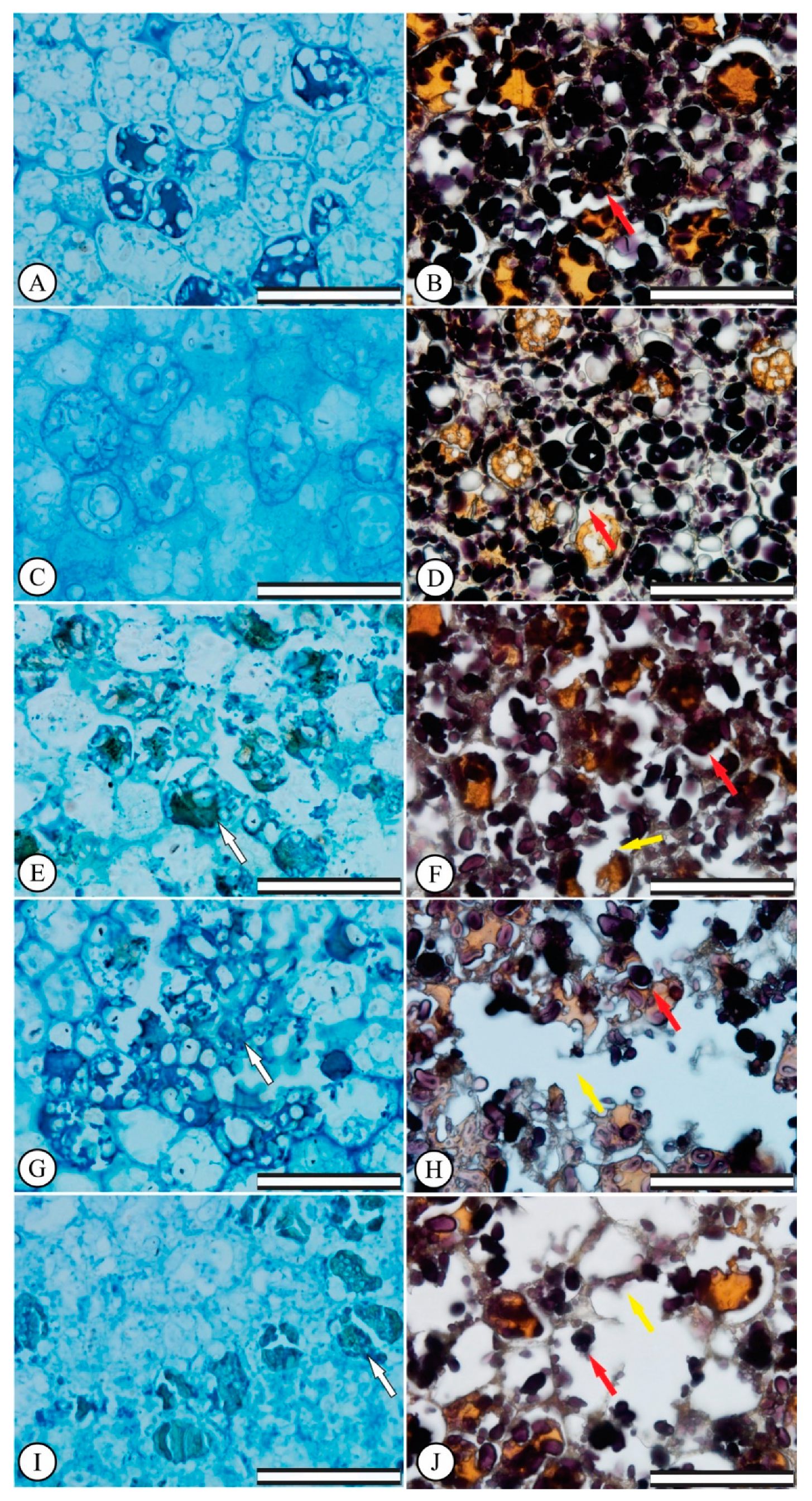
2.3. Morphoanatomical Seed Characterization
2.4. Hydroponic Young Plant Growth
2.4.1. Visible Root and Leaf Symptoms
2.4.2. Root Growth Measurements
2.4.3. Chlorophyll a Fluorescence
2.4.4. Gas Exchange
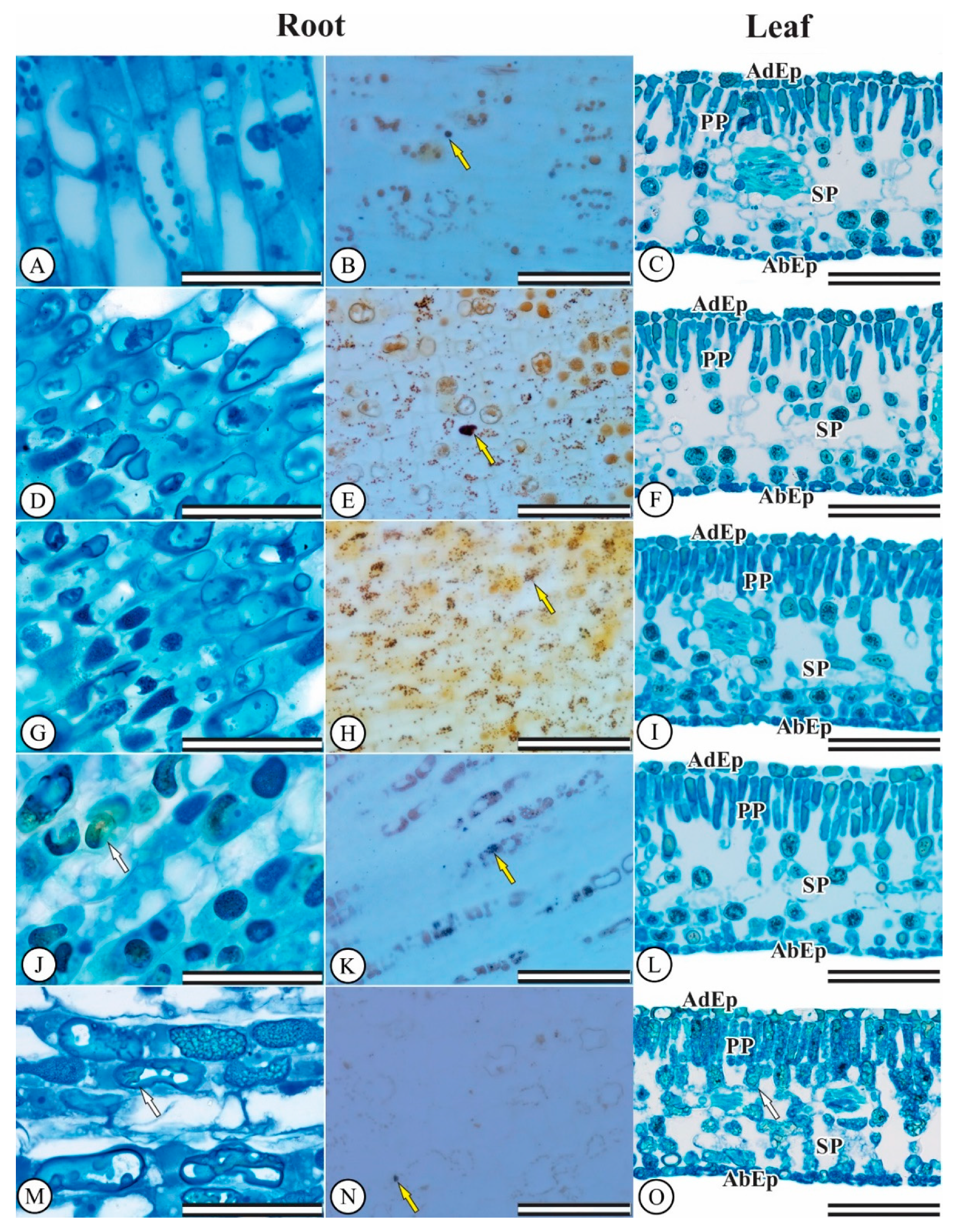
2.4.5. Morphoanatomical Root and Leaf Characterization
2.5. Al Content Quantification
2.6. Hydroponic culture: antioxidant enzyme activity
2.7. Statistical Analyses
3. Results
3.1. Germination
3.2. Germination: Anatomical Seed Changes
3.3. Hydroponic Culture: Visible Morphological Symptoms
3.4. Hydroponic Culture: Anatomical Seedling Changes
3.5. Hydroponic Culture: Chlorophyll a Fluorescence and Gas Exchanges
3.6. Hydroponic Culture: Antioxidant Enzyme Activity
3.7. Al Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Sperisen, C. Aluminum exclusion and aluminum tolerance in woody plants. Front. Plant Sci. 2013, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminium. J. Exp. Bot. 1997, 48, 1115–1124. [Google Scholar] [CrossRef] [Green Version]
- Kinraide, T.B. Identity of the rhizotoxic aluminium species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Azom, A. Aluminum Dross Recycling-A New Technology for Recycling Aluminum Waste Products. 2003. Available online: https://www.azom.com/article.aspx?ArticleID=2150 (accessed on 13 October 2018).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mousavi, S.R.; Balali-Mood, M.; Riahi-Zanjani, B.; Yousefzadeh, H.; Sadeghi, M. Concentrations of mercury, lead, chromium, cadmium, arsenic and aluminum in irrigation water wells and wastewaters used for agriculture in Mashhad, northeastern Iran. Int. J. Occup. Med. Environ. Health 2013, 4, 80–86. [Google Scholar]
- Mandour, R.A.; Azab, Y.A. The Prospective Toxic Effects of Some Heavy Metals Overload in Surface Drinking Water of Dakahlia Governorate, Egypt. Int. J. Occup. Environ. Med. 2011, 2, 245–253. [Google Scholar] [PubMed]
- Tahara, K.; Norisada, M.; Yamanoshita, T.; Kojima, K. Role of aluminum-binding ligands in aluminum resistance of Eucalyptus camaldulensis and Melaleuca cajuputi. Plant Soil 2008, 302, 175–187. [Google Scholar] [CrossRef]
- Jesus, D.S.; Martins, F.M.; Neto, A.D.A. Structural changes in leaves and roots are anatomical markers of aluminum sensitivity in sunflower. Pesqui. Agropecu. Trop. 2016, 46, 383–390. [Google Scholar] [CrossRef]
- Mihailovic, N.; Drazic, G.; Vucinic, Z. Effects of aluminium on photosynthetic performance in Al-sensitive and Al-tolerant maize inbred lines. Photosynthetica 2008, 46, 476–480. [Google Scholar] [CrossRef]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. BioMetals 2012, 25, 1141–1154. [Google Scholar] [CrossRef]
- Jansen, S.; Broadley, M.R.; Robbrecht, E.; Smets, E. Aluminum hyperaccumulation in Angiosperms: A review of its phylogenetic significance. Bot. Rev. 2002, 68, 235–269. [Google Scholar] [CrossRef]
- Jansen, S.; Watanabe, T.; Smets, E. Aluminium accumulation in leaves of 127 species in Melastomataceae, with comments on the Order Myrtales. Ann. Bot. 2002, 90, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.M.; Barros, L.M.G.; Echevarria, G.F.; Amaral, L.I.V.; Cotta, M.G.; Rossatto, D.R.; Haridasan, M.; Franco, A.C. Al-hyperaccumulator Vochysiaceae from the Brazilian Cerrado store aluminum in their chloroplasts without apparent damage. Environ. Exp. Bot. 2011, 70, 37–42. [Google Scholar] [CrossRef]
- Haridasan, M. Nutritional adaptations of native plants of the Cerrado biome in acid soils. Braz. J. Plant Physiol. 2008, 20, 183–195. [Google Scholar] [CrossRef]
- Malta, P.G.; Silva, S.A.; Ribeiro, C.; Campos, N.V.; Azevedo, A.A. Rudgea viburnoides (Rubiaceae) overcomes the low soil Fertility of the Brazilian Cerrado and hyperaccumulates aluminum in cell walls and chloroplasts. Plant Soil 2016, 408, 369–384. [Google Scholar] [CrossRef]
- Haridasan, M. Aluminium accumulation by some cerrado native species of central, Brazil. Plant Soil 1982, 65, 265–273. [Google Scholar] [CrossRef]
- Schmitt, M.; Watanabe, T.; Jansen, S. The effects of aluminium on plant growth in a temperate and deciduous aluminium accumulating species. AoB Plants 2016, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, H.; Xu, J.; Chun, X.; Ni, D. Effects of aluminium on ultrastructure and antioxidant activity in leaves of tea plant. Acta Phys. Plant 2011, 33, 973–978. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Vasconcelos Filho, S.C.; Rodrigues, D.A.; Rodrigues, C.L.; Sales, J.F.; Vital, R.G. Influence of aluminum on root growth and of anatomy Stenocalyx dysentericus (DC.) O. Berg. Afr. J. Biotechnol. 2016, 15, 1193–1200. [Google Scholar] [CrossRef]
- Ranal, M.A.; Mendes-Rodrigues, C.; Teixeira, W.F.; Oliveira, A.P.; Romero, R. Seed germination of Microlicia fasciculata, an apomictic and aluminium accumulator species: Unexpected intraspecific variability in a restricted Neotropical savanna área. Flora 2016, 220, 8–16. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Huang, W.L.; Yang, L.T.; Huang, Z.R.; Lai, N.W.; Chen, L.S. Aluminum-responsive genes revealed by RNA-Seq and related physiological responses in leaves of two Citrus species with contrasting aluminum tolerance. Ecotoxicol. Environ. Saf. 2018, 158, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.A.; Pereira, E.B.C.; Pereira, A.V.; Ribeiro, J.F.R. Cagaita: Biologia e Manejo; Embrapa Cerrados: Brasília, Brazil, 2003; p. 80. [Google Scholar]
- Martinotto, C.; Paiva, R.; Soares, F.P.; Santos, B.R.; Nogueira, R.C. Cagaiteira (Eugenia dysenterica DC.); Boletim Técnico n° 78; Editora UFLA: Lavras, Brazil, 2008; p. 21. [Google Scholar]
- Vieira Neto, R.D.; da Silva Junior, J.F.; da Ledo, A.S. Fruticultura Tropical: Espécies Regionais e Exóticas; dos Santos-Serejo, J.A., Dantas, J.L.L., Sampaio, C.V., da Silva Coelho, Y., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; pp. 323–338. [Google Scholar]
- Matsumoto, H. Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 2000, 200, 1–46. [Google Scholar] [PubMed]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling use in electron microscopy. J. Cell Biol. 1962, 27, 137–138. [Google Scholar]
- Karnovsky, M.J.A. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. Front. Plant Sci. 1965, 4, 1–12. [Google Scholar]
- O’Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; WH Freeman: San Francisco, CA, USA, 1962; p. 408. [Google Scholar]
- Tolrà, R.; Vogel-Mikus, K.; HAjiboland, R.; Klump, P.; Pongrac, P.; KAulich, B.; Gianconcelli, A.; Babin, V.; Barceló, J.; Regvar, M.; et al. Localization of aluminium in tea (Camellia sinensis) leaves using low energy X-ray fluorescence spectro-microscopy. J. Plant Res. 2011, 124, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.A.; Vasconcelos-Filho, S.C.; Rodrigues, C.L.; Rodrigues, D.A.; Silva, G.P.; Sales, J.F.; Nascimento, K.J.T.; Teles, E.M.G.; Rehn, L.S. Aluminum influence on Hancornia speciosa seedling emergence, nutriente accumulation, growth and root anatomy. Flora 2017, 236, 9–14. [Google Scholar] [CrossRef]
- Vasconcelos, S.S.; Rossielo, R.O.P.; Jacob-Neto, J. Parâmetros morfológicos para estabelecer tolerância diferencial à toxicidade de alumínio em cultivares de arroz. Pesqui. Agropecu. Bras. 2002, 37, 357–363. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence e a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional de Plantas: Princípio e Aplicações, 2nd ed.; Associação Brasileira Para Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1997. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay aplicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Havir, E.A.; Mchale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotylus of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senencence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehley, A.C. Assay of catalases and peroxidases. Method Enzymol. 1955, 2, 764–775. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—Dye binding. Anal. Biochem. 1976, 2, 248–254. [Google Scholar] [CrossRef]
- Yamashita, O.M.; Guimarães, S.C. Germination of Conyza canadensis and C. bonariensis seeds under presence of aluminum in the substrate. Cienc Rural 2011, 41, 599–601. [Google Scholar] [CrossRef]
- Koszo, C.R.R.; Rinaldi, M.C.S.; Barbedo, C.J. Germinação de sementes de Erythrina speciosa Andr., Eugenia brasiliensis Lam. e Cucumis sativus L. em meio ácido. Hoehnea 2007, 34, 271–282. [Google Scholar] [CrossRef]
- Wang, S.; Ren, X.; Huang, B.; Wang, G.; Zhou, P.; Na, Y. Aluminium-induced reduction of plant growth in alfalfa (Medicago sativa) is mediated by interrupting auxin transport and accumulation in roots. Sci. Rep. 2016, 6, 30079. [Google Scholar] [CrossRef] [PubMed]
- Påhlsson, A.B. Influence of aluminium on biomass, nutrients, soluble carbohydrates and phenols in beech (Fagus sylvatica). Physiol. Plantarum. 1990, 78, 79–84. [Google Scholar] [CrossRef]
- Čiamporová, M. Morphological and structural responses of plant roots to aluminum at organ, tissue, and cellular levels. Biol Plant. 2002, 45, 161–171. [Google Scholar] [CrossRef]
- Alvarez, I.; Reynaldo, O.S.I.; Testillano, P.; Risueno, M.C.; Arias, M. Morphological and cellular changes in rice roots (Oryza sativa L.) caused by Al stress. Bot. Stud. 2012, 53, 67–73. [Google Scholar]
- Tolrà, R.; Barceló, J.; Poschenrieder, C. Constitutive and aluminium-induced patterns of phenolic compounds in two maize varieties differing in aluminium tolerance. J. Inorg. Biochem. 2009, 103, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M. Effects of aluminum exposures on growth, photosynthetic efficiency, lipid peroxidation, antioxidant enzymes and artemisinin content of Artemisia annua L. J. Phytol. 2010, 8, 23–37. [Google Scholar]
- Hajiboland, R.; Bahrami-Rad, S.; Barcelo, J.; Poschenrieder, C. Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis). J. Plant Nutr. Soil Sci. 2013, 176, 616–625. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.B.; Mazzanti, C.M.A.; Gonçalves, J.F.; Cargnelutti, D.; Tabaldi, L.A.; Becker, A.G.; Calgaroto, N.S.; Farias, J.G.; Battisti, V.; Bohrer, D.; et al. Aluminum-induced oxidative stress in cucumber. Plant Physiol. Biochem. 2010, 48, 683–689. [Google Scholar] [CrossRef]
- Ghanati, F.; Morita, A.; Yokota, H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 2005, 276, 133–141. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Naeem, M.; Idress, M.; Moinuddin; Silva, J.A.T.; Ram, M. Exogenous nitric oxide donor protects Artemisia annua from oxidative stress generated by boron and aluminium toxicity. Ecotoxicol. Environ. Saf. 2012, 80, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010, 68, 1–13. [Google Scholar] [CrossRef]

| Al3+ Concentration (Al2(SO4)3 | GRI | Germination (%) |
|---|---|---|
| 0 μmol L−1 | 1.21 ± 0.09 | 80 ± 3.65 |
| 200 μmol L−1 | 1.14 ± 0.06 | 75 ** ± 2.52 |
| 400 μmol L−1 | 1.08 ± 0.03 | 64 ** ± 1.63 |
| 600 μmol L−1 | 0.87 ** ± 0.05 | 61 ** ± 3.79 |
| 800 μmol L−1 | 0.85 ** ± 0.09 | 56 ** ± 2.83 |
| F | ** | ** |
| CV% | 6.39 | 4.29 |
| Al3+ Concentration (Al2(SO4)3 | RL | TRG% | RRE% |
|---|---|---|---|
| 0 μmol L−1 | 4.05 ± 0.24 | 18.92 ± 0.28 | 100.00 ± 0.00 |
| 200 μmol L−1 | 5.51 * ± 0.35 | 29.87 ** ± 1.15 | 130.22 ** ± 5.09 |
| 400 μmol L−1 | 4.17 ± 0.37 | 19.52 ± 0.69 | 103.57 ± 4.17 |
| 600 μmol L−1 | 4.23 ± 0.31 | 24.30 ** ± 0.94 | 109.73 ± 4.23 |
| 800 μmol L−1 | 4.74 ± 0.36 | 25.42 ** ± 1.51 | 85.06 ± 4.74 |
| F | * | ** | ** |
| CV% | 16.21 | 9.51 | 11.53 |
| Chlorophyll a Fluorescence Traits | ||||
| Al3+ Concentration (Al2(SO4)3 | Fv/Fm | ΔF/Fm′ | ETR | NPQ |
| 0 | 0.74 ± 0.024 | 0.56 ± 0.01 | 245.24 ± 8.02 | 0.72 ± 0.05 |
| 200 | 0.68 ± 0.040 | 0.65 ** ± 0.02 | 275.43 ± 13.37 | 0.52 ± 0.18 |
| 400 | 0.62 ± 0.060 | 0.60 ± 0.3 | 272.82 ± 20.58 | 0.65 ± 0.28 |
| 600 | 0.71 ± 0.040 | 0.63 ± 0.00 | 282.73 ± 9.95 | 0.64 ± 0.23 |
| 800 | 0.71 ± 0.027 | 0.51 ± 0.01 | 245.10 ± 17.21 | 1.01 ± 0.27 |
| F | NS | ** | NS | NS |
| CV (%) | 13.15 | 8.24 | 12.33 | 68.82 |
| Gas Exchange Traits | ||||
| Al3+ Concentration (Al2(SO4)3 | A | gs | E | Ci/Ca |
| 0 | 9.12 ± 0.62 | 0.14 ± 0.02 | 1.39 ± 0.21 | 0.73 ± 0.021 |
| 200 | 7.55 ± 0.99 | 0.18 ± 0.03 | 1.72 ± 0.24 | 0.78 ± 0.020 |
| 400 | 7.23 ± 1.00 | 0.14 ± 0.03 | 1.37 ± 0.24 | 0.73 ± 0.053 |
| 600 | 7.98 ± 0.58 | 0.16 ± 0.04 | 1.49 ± 0.34 | 0.70 ± 0.015 |
| 800 | 7.67 ± 0.98 | 0.15 ± 0.04 | 1.36 ± 0.28 | 0.70 ± 0.039 |
| F | NS | NS | NS | NS |
| CV (%) | 24.20 | 47.56 | 40.27 | 10.12 |
| Al3+ Concentration (Al2(SO4)3 | SOD | CAT | POX |
|---|---|---|---|
| 0 μmol L−1 | 43.02 ± 1.85 | 25.58 ± 2.55 | 0.52 ± 0.25 |
| 200 μmol L−1 | 58.79 ± 3.28 | 74.74 ** ± 3.61 | 3.03 ** ± 0.27 |
| 400 μmol L−1 | 40.31 ± 3.27 | 57.48 ** ± 9.80 | 3.13 ** ± 0.29 |
| 600 μmol L−1 | 30.37 ± 7.09 | 15.97 ± 0.85 | 2.33 ** ± 0.32 |
| 800 μmol L−1 | 62.50 * ± 6.53 | 30.32 ± 4.41 | 2.26 ** ± 0.09 |
| F | * | ** | ** |
| CV% | 30.39 | 59.12 | 46.30 |
| Al-Bark from Adult Trees (mg kg−1) | Al-Leaves from Adult Trees (mg kg−1) | ||
| Plant 1 | 1188.25 | 588.05 | |
| Plant 2 | 1027.25 | 543.80 | |
| Plant 3 | 1080.95 | 549.02 | |
| Plant 4 | 1433.32 | 515.23 | |
| Plant 5 | 1356.87 | 567.21 | |
| Al3+ Concentration (Al2(SO4)3 | Al-Seeds Germination Test | Al-root Hydroponic Cultivation | Al-Leaves Hydroponic Cultivation |
| 0 μmol L−1 | 111.07 ± 6.81 | 521.04 ± 13.66 | 140.94 ± 1.96 |
| 200 μmol L−1 | 123.33 ± 4.85 | 1984.23 ** ± 15.78 | 195.14 ** ± 2.84 |
| 400 μmol L−1 | 130.08 ± 14.13 | 2146.13 ** ± 60.40 | 210.10 ** ± 5.21 |
| 600 μmol L−1 | 132.60 ± 9.58 | 2300.67 ** ± 54.13 | 228.39 ** ± 10.20 |
| 800 μmol L−1 | 180.82 ** ± 12.95 | 2332.46 ** ± 37.70 | 239.62 ** ± 2.47 |
| F | ** | ** | ** |
| CV% | 21.31 | 37.91 | 17.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida Rodrigues, A.; Carvalho Vasconcelos Filho, S.; Müller, C.; Almeida Rodrigues, D.; de Fátima Sales, J.; Zuchi, J.; Carlos Costa, A.; Lino Rodrigues, C.; Alves da Silva, A.; Pereira Barbosa, D. Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth. Plants 2019, 8, 317. https://doi.org/10.3390/plants8090317
Almeida Rodrigues A, Carvalho Vasconcelos Filho S, Müller C, Almeida Rodrigues D, de Fátima Sales J, Zuchi J, Carlos Costa A, Lino Rodrigues C, Alves da Silva A, Pereira Barbosa D. Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth. Plants. 2019; 8(9):317. https://doi.org/10.3390/plants8090317
Chicago/Turabian StyleAlmeida Rodrigues, Arthur, Sebastião Carvalho Vasconcelos Filho, Caroline Müller, Douglas Almeida Rodrigues, Juliana de Fátima Sales, Jacson Zuchi, Alan Carlos Costa, Cássia Lino Rodrigues, Adinan Alves da Silva, and Danilo Pereira Barbosa. 2019. "Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth" Plants 8, no. 9: 317. https://doi.org/10.3390/plants8090317
APA StyleAlmeida Rodrigues, A., Carvalho Vasconcelos Filho, S., Müller, C., Almeida Rodrigues, D., de Fátima Sales, J., Zuchi, J., Carlos Costa, A., Lino Rodrigues, C., Alves da Silva, A., & Pereira Barbosa, D. (2019). Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth. Plants, 8(9), 317. https://doi.org/10.3390/plants8090317