Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia Xanthochymus Hook
Abstract
:1. Introduction
2. Results
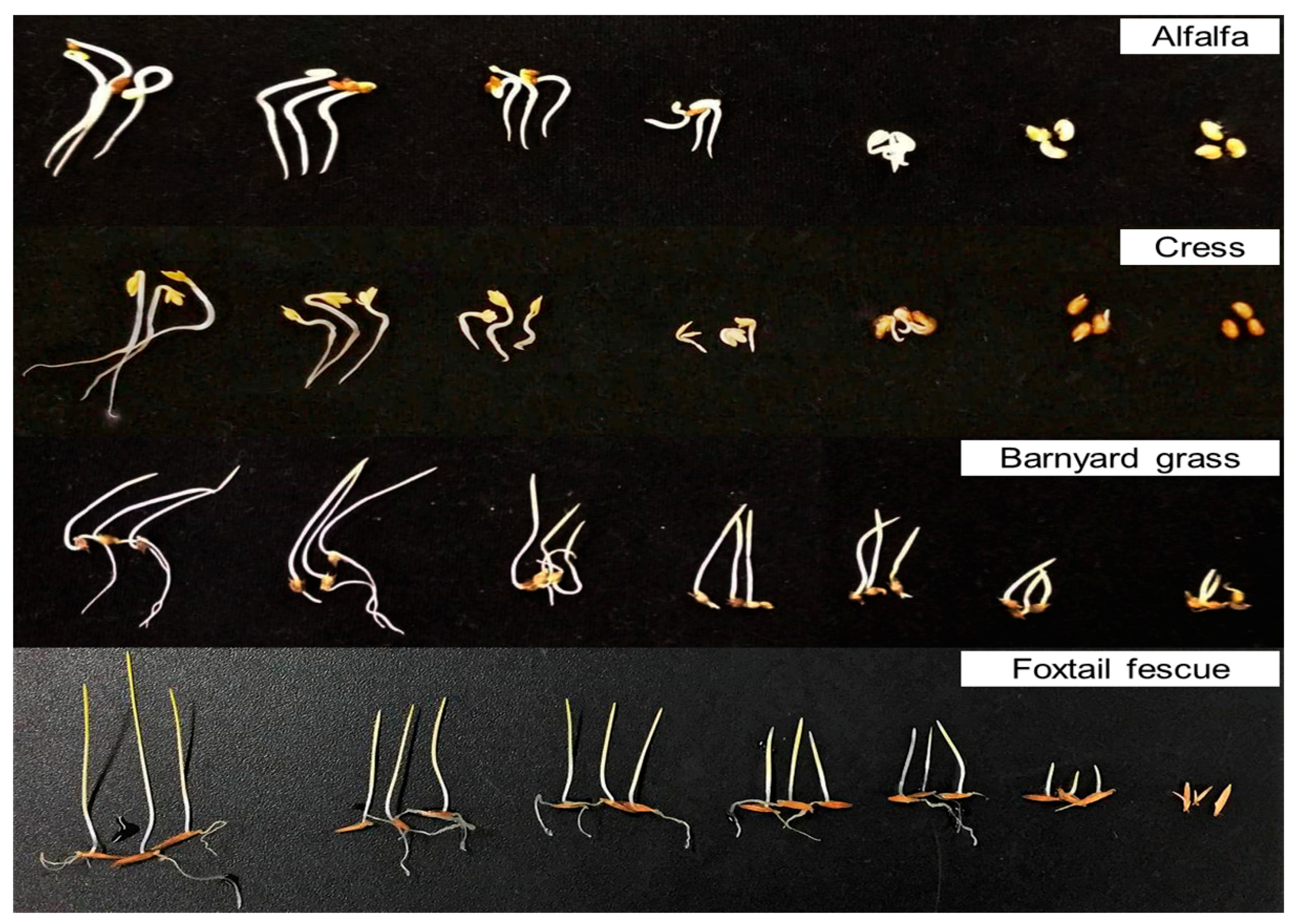
2.1. Allelopathic Activity of Garcinia Xanthochymus Leaf Extracts
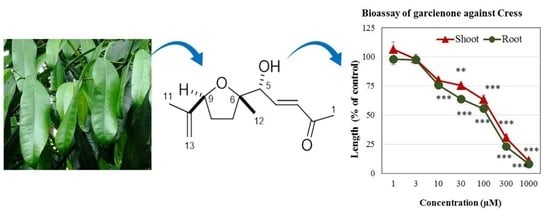
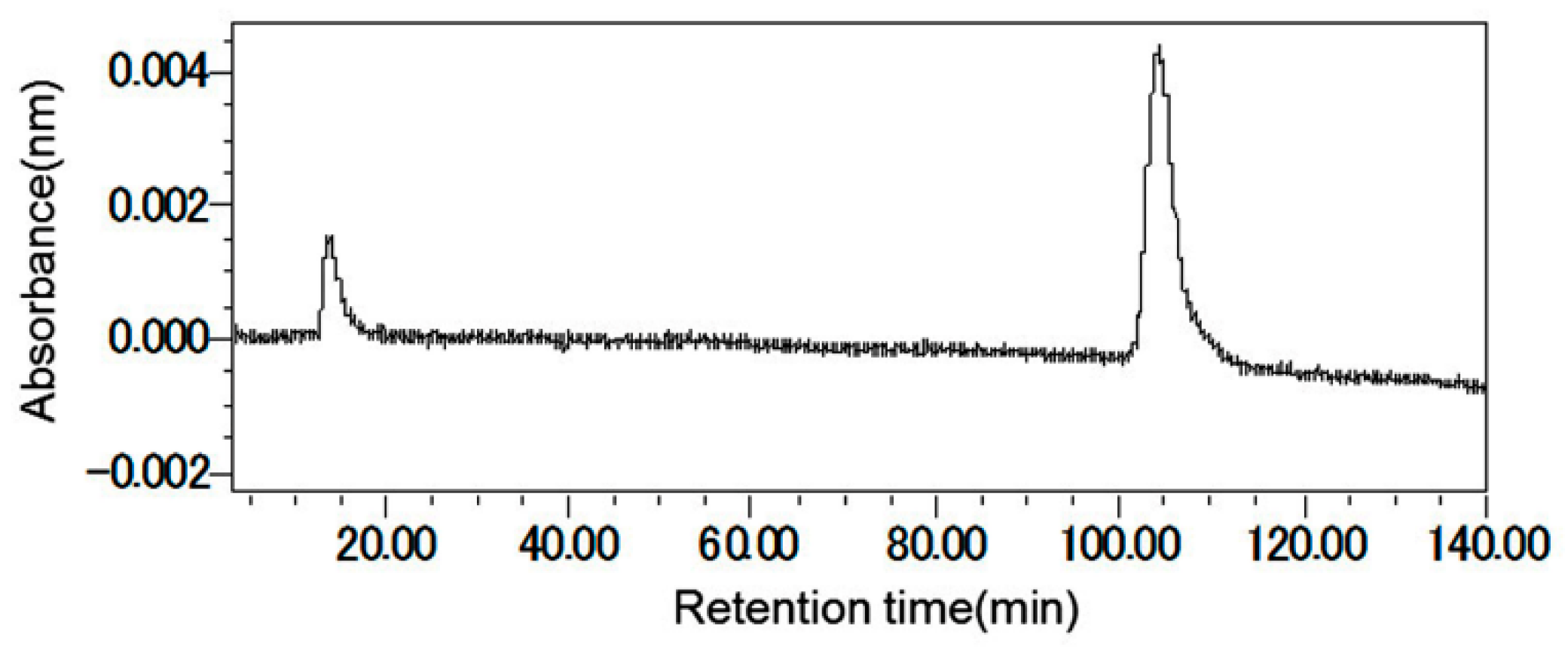
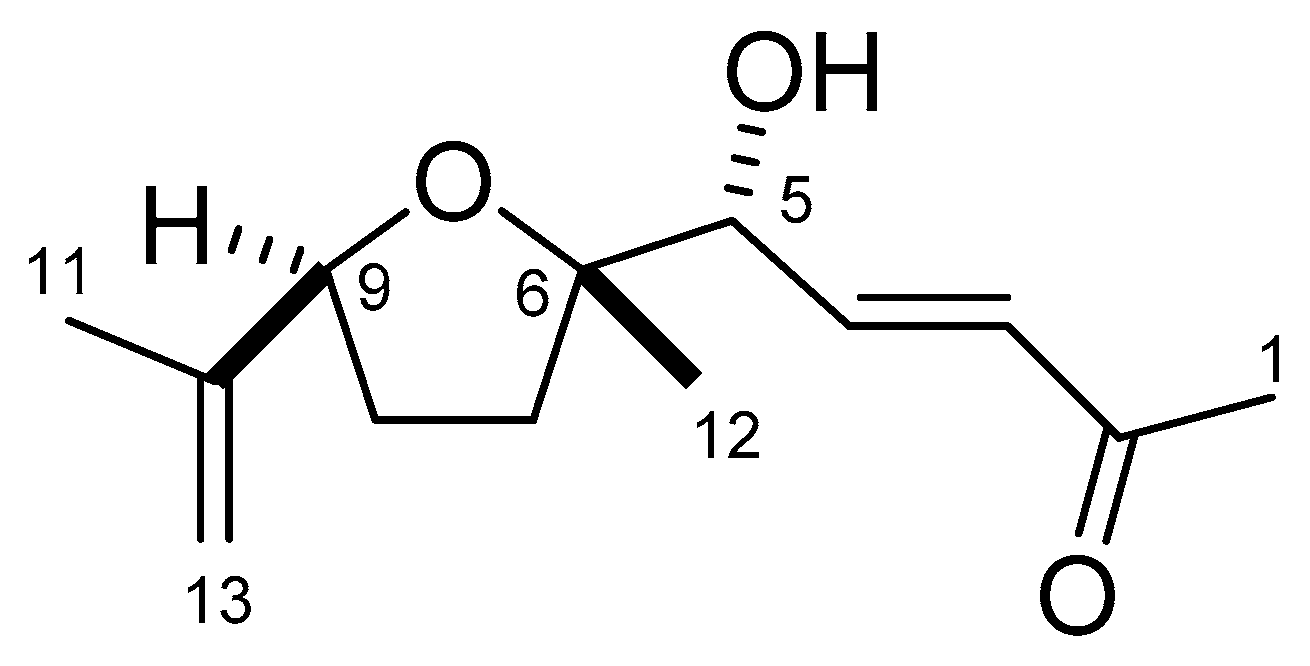
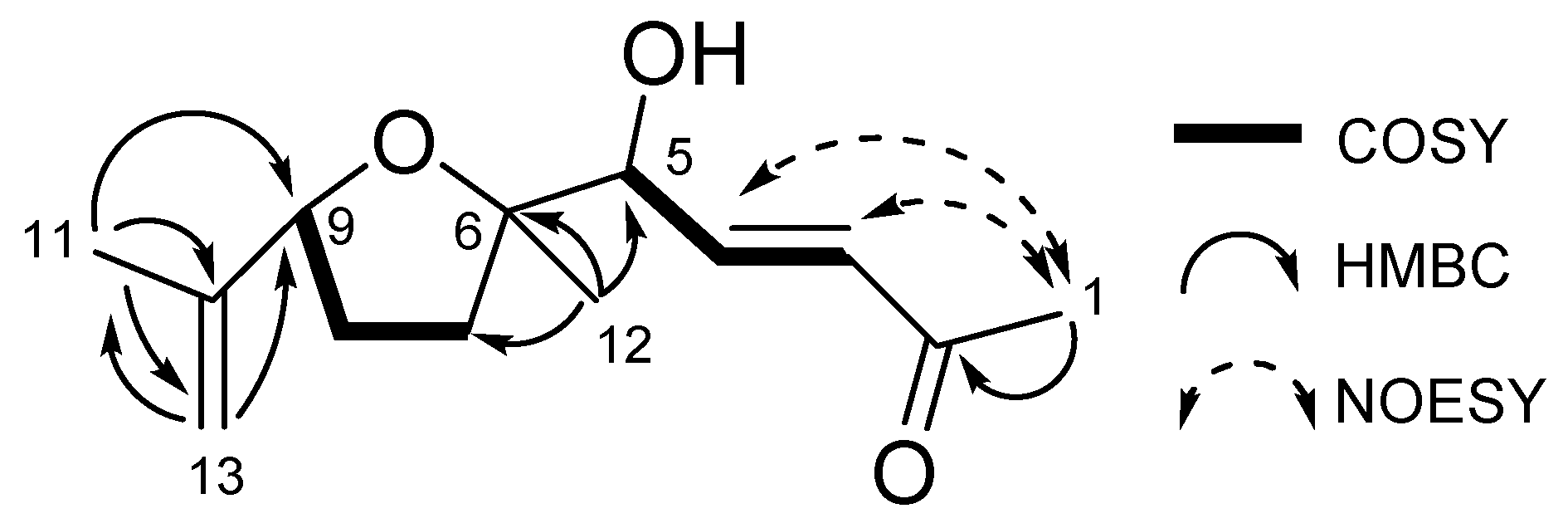
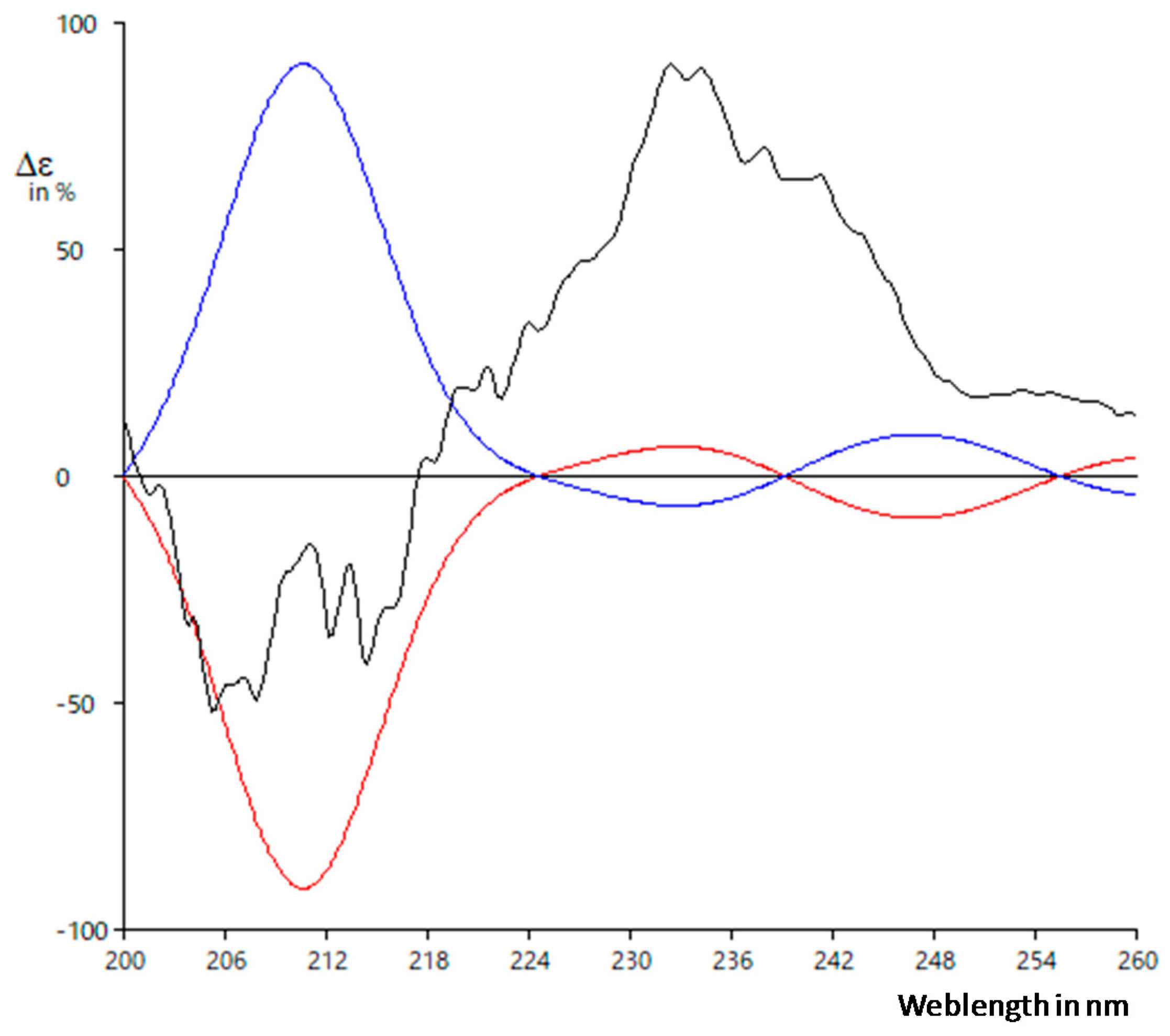
2.2. Structure Determination of Active Substance
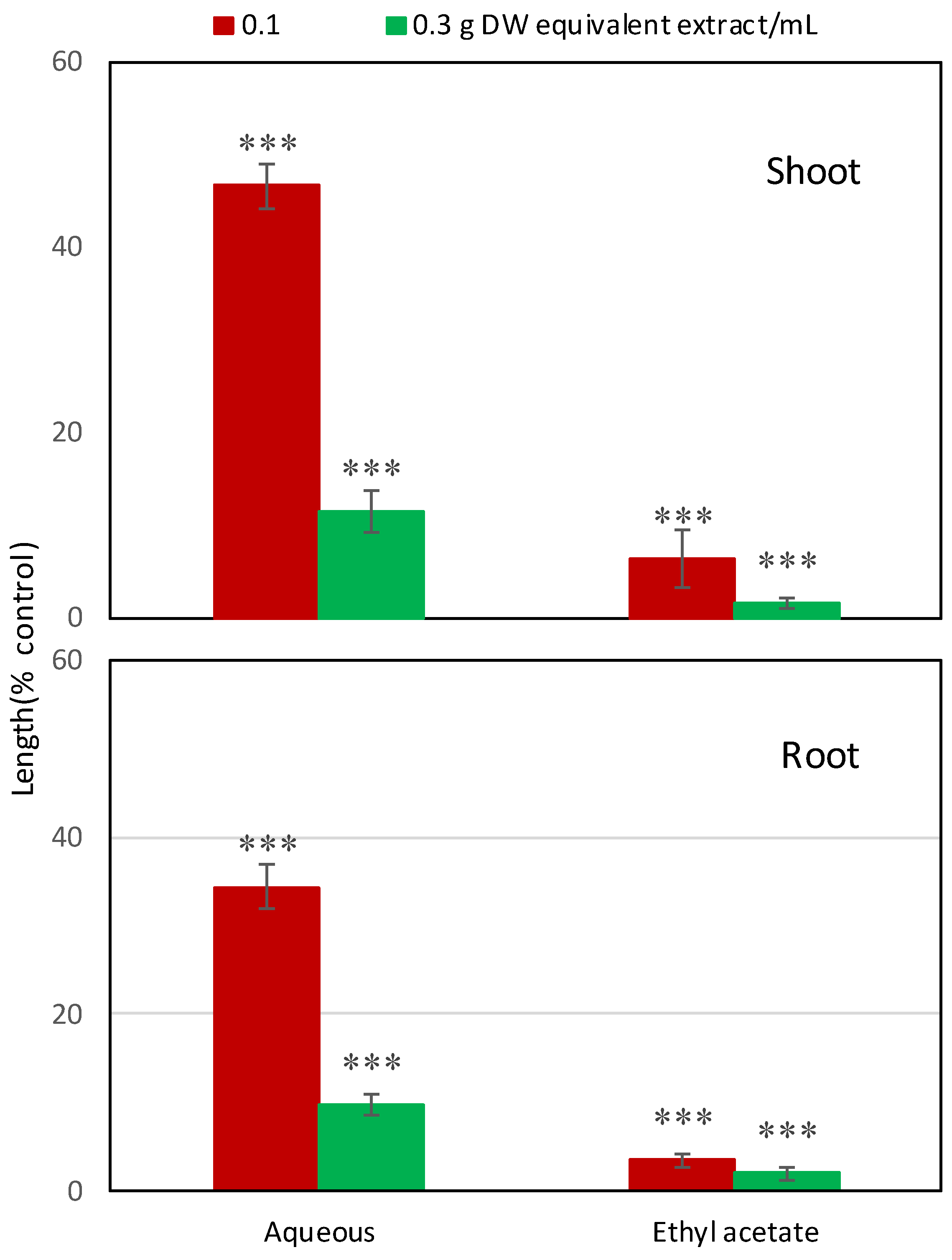
2.3. Biological Activity of Garcienone
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Bioassay
4.4. Purification of the Active Substance
4.5. Characterization of the Compound
4.5.1. NMR Chemical Shifts Calculation Methods
4.5.2. ECD Calculation Methods
4.6. Spectral Data
4.7. Bioassay of the Isolated Compound
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jabran:, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Feng, G.; Chen, M.; Ye, H.C.; Zhang, Z.K.; Li, H.; Chen, L.L.; Zhang, J. Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; El-Wakeel, M.A. Comparison of safe weed control methods with chemical herbicide in potato field. Bull. Natl. Res. Cent. 2019, 43, 16. [Google Scholar] [CrossRef]
- Kleinowski, A.M.; Ribeiro, G.A.; Milech, C.; Braga, E.J.B. Potential allelopathic and antibacterial activity from Alternanthera philoxeroides. Hoehnea 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Adetunji, C.; Oloke, J.; Kumar, A.; Swaranjit, S.; Akpor, B. Synergetic effect of rhamnolipid from Pseudomonas aeruginosa C1501 and phytotoxic metabolite from Lasiodiplodia pseudotheobromae C1136 on Amaranthus hybridus L. and Echinochloa crus-galli weeds. Environ. Sci. Pollut. Res. 2017, 24, 13700–13709. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Hakeem, K.R.; Qureshi, M.I. Weed control through herbicide-loaded nanoparticles. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 507–527. [Google Scholar]
- Yang, X.F.; Lei, K.; Kong, C.H.; Xu, X.H. Effect of allelochemical tricin and its related benzothiazine derivative on photosynthetic performance of herbicide-resistant barnyard grass. Pestic. Biochem. Physiol. 2017, 143, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crops Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Watson, S.B.; Asolkar, R.N.; Boddy, L.G. Sarmentine, a natural herbicide from Piper species with multiple herbicide mechanisms of action. Front. Plant Sci. 2015, 6, 222. [Google Scholar] [CrossRef]
- Valli, M.; Dos Santos, R.N.; Figueira, L.D.; Nakajima, C.H.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. Development of a natural products database from the biodiversity of Brazil. J. Nat. Prod. 2013, 76, 439–444. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghnaya, A.; Hamrouni, L.; Amri, I.; Ahoues, H.; Hanana, M.; Romane, A. Study of allelopathic effects of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat. Prod. Res. 2016, 30, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Appiah, K.; Mardani, H.; Omari, R.; Eziah, V.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Fujii, Y. Involvement of carnosic acid in the phytotoxicity of Rosmarinus officinalis leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Einhellig, F.A.; Leather, G.R. Potentials for exploiting allelopathy to enhance crop production. J. Chem. Ecol. 1988, 14, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.D. The Flora of British India; L. Reeve and Co.: London, UK, 1874; pp. 78–79. [Google Scholar]
- Joseph, K.S.; Dandin, V.S.; Murthy Hosakatte, N. Chemistry and biological activity of Garcinia xanthochymus: A review. Biol. Act. Prod. Nat. 2016, 6, 173–194. [Google Scholar]
- Chen, Y.; Gan, F.; Jin, S.; Liu, H.; Wu, S.; Yang, W.; Yang, G. Adamantyl derivatives and rearranged benzophenones from Garcinia xanthochymus fruits. RSC Adv. 2017, 7, 17289–17296. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Bussmann, R.W.; Hart, R.H.; Li, P.; Bai, Y.; Long, C. Garcinia in Southern China: Ethnobotany, management, and niche modeling. Econ. Bot. 2016, 70, 416–430. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Trinh, B.T.; Tran, T.B.; Nguyen, L.T.T.; Jäger, A.K.; Nguyen, L.H.D. Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 2017, 27, 3301–3304. [Google Scholar] [CrossRef]
- Manohar, S.H.; Naik, P.M.; Patil, L.M.; Karikatti, S.I.; Murthy, H.N. Chemical composition of Garcinia xanthochymus seeds, seed oil, and evaluation of its antimicrobial and antioxidant activity. J. Herbs Spices Med. Plants 2014, 20, 148–155. [Google Scholar] [CrossRef]
- Koodkaew, I.; Senaphan, C.; Sengseang, N.; Suwanwong, S. Characterization of phytochemical profile and phytotoxic activity of Mimosa pigra L. Agric. Nat. Res. 2018, 52, 162–168. [Google Scholar] [CrossRef]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant. Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic property and an allelopathic substance in Eleocharis atropurpurea (Retz.). Theor. Exp. Plant Physiol. 2018, 30, 347–355. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning the stereochemistry of pairs of diastereoisomers using GIAO NMR shift calculation. J. Org. Chem. 2009, 74, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Goodman, J.M. Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: The DP4 probability. J. Am. Chem. Soc. 2010, 132, 12946–12959. [Google Scholar] [CrossRef] [PubMed]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2011, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 2014, 9, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic compounds in Rumex maritimus. Plant Biosyst. 2018, 152, 804–809. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Okuda, N. Involvement of an autotoxic compound in asparagus decline. J. Plant Physiol. 2018, 224, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tominaga, T.; Ohno, O.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Plant growth inhibitory activity and active substances with allelopathic potential of cogongrass (Imperata cylindrica) rhizome. Weed Biol. Manag. 2018, 18, 92–98. [Google Scholar] [CrossRef]
- Okada, S.; Iwasaki, A.; Kataoka, I.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of kiwifruit leaves and isolation of a phytotoxic substance. Sci. Hortic. 2019, 250, 243–248. [Google Scholar] [CrossRef]
- Schrödinger Release 2017-3: MacroModel; Schrödinger, LLC: New York, NY, USA, 2017; Available online: https://www.schrodinger.com/macromodel (accessed on 1 April 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A. 03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Robertson, L.P.; Duffy, S.; Wang, Y.; Wang, D.; Avery, V.M.; Carroll, A.R. Pimentelamines A–C, indole alkaloids isolated from the leaves of the Australian tree Flindersia pimenteliana. J. Nat. Prod. 2017, 80, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J.; Nitsch-Velasquez, L.; Rudolph, M. Time-dependent density functional response theory for electronic chiroptical properties of chiral molecules. In Electronic and Magnetic Properties of Chiral Molecules and Supramolecular Architectures; Naaman, R., Beratan, D., Waldeck, D., Eds.; Springer: Berlin, Germany, 2010; pp. 1–98. [Google Scholar]
- Autschbach, J. Time-dependent density functional theory for calculating origin-independent optical rotation and rotatory strength tensors. Chem. Phys. Chem. 2011, 12, 3224–3235. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Spec Dis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
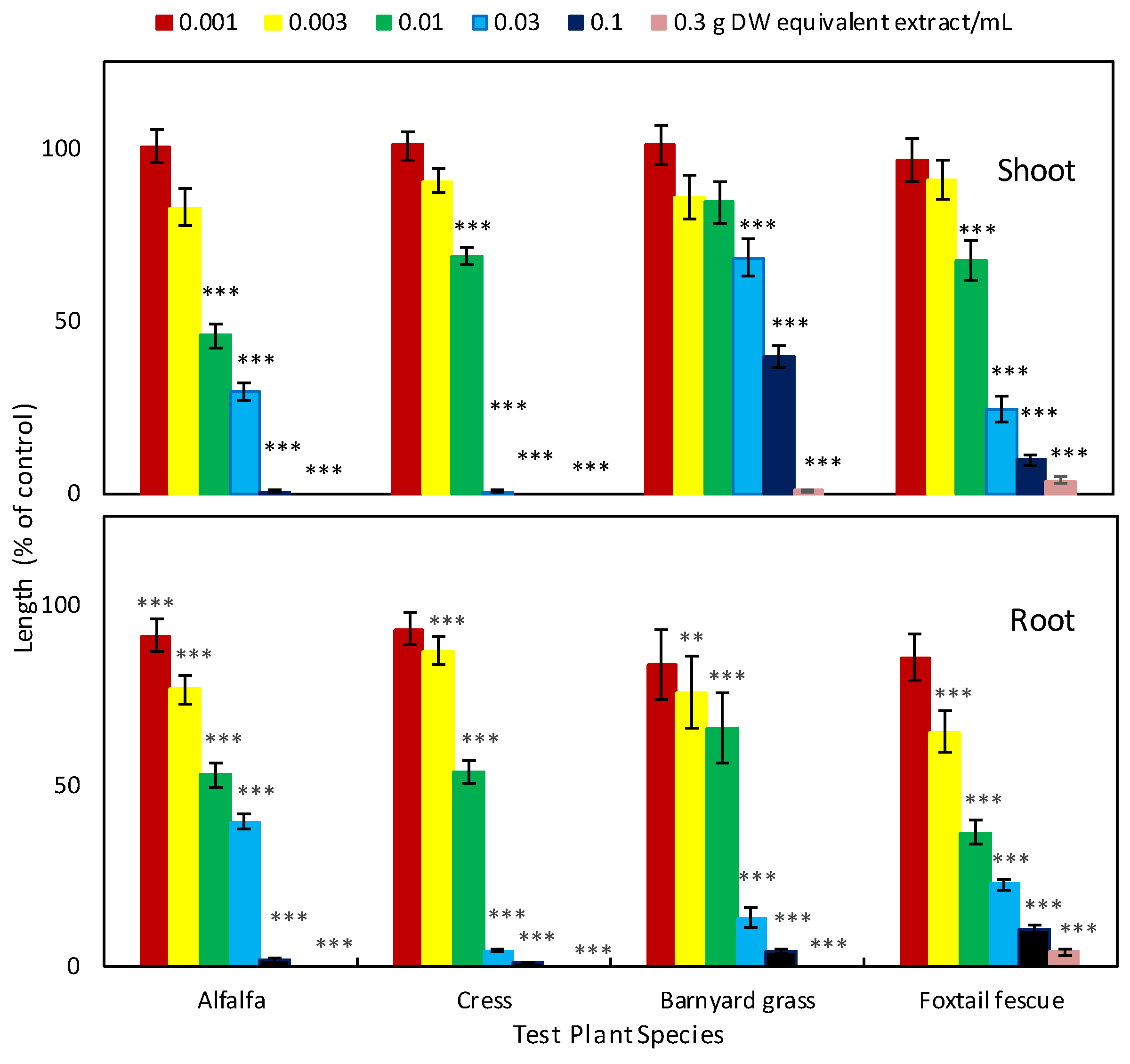


| Aqueous Methanol Extracts (mg Dry Weight Equivalent Extract/mL) | ||
|---|---|---|
| Test Plant Species | Shoot | Root |
| Alfalfa | 8.70 | 13.11 |
| Cress | 13.55 | 10.93 |
| Barnyard grass | 65.31 | 13.99 |
| Foxtail fescue | 15.67 | 5.75 |
| δC, Type b | δHa (J in Hz) | |
|---|---|---|
| 1 | 28.1, CH3 | 2.28, s |
| 2 | 198.3, C | |
| 3 | 130.9, CH | 6.45, dd (16.2, 1.8) |
| 4 | 143.8, CH | 6.76, dd (16.2, 4.8) |
| 5 | 76.3, CH | 4.30, m |
| 6 | 85.6, C | |
| 7a | 31.9, CH2 | 2.09, m |
| 7b | 1.53, m | |
| 8a | 31.3, CH2 | 2.06, m |
| 8b | 1.82, m | |
| 9 | 84.7, CH | 4.39, dd (10.7, 5.7) |
| 10 | 145.4, C | |
| 11 | 17.9, CH3 | 1.73, s |
| 12 | 24.6, CH3 | 1.26, s |
| 13a | 110.9, CH2 | 5.03, s |
| 13b | 4.84, s | |
| 5-OH | 2.65, d (1.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rob, M.M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia Xanthochymus Hook. Plants 2019, 8, 301. https://doi.org/10.3390/plants8090301
Rob MM, Iwasaki A, Suzuki R, Suenaga K, Kato-Noguchi H. Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia Xanthochymus Hook. Plants. 2019; 8(9):301. https://doi.org/10.3390/plants8090301
Chicago/Turabian StyleRob, Md. Mahfuzur, Arihiro Iwasaki, Ryota Suzuki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2019. "Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia Xanthochymus Hook" Plants 8, no. 9: 301. https://doi.org/10.3390/plants8090301
APA StyleRob, M. M., Iwasaki, A., Suzuki, R., Suenaga, K., & Kato-Noguchi, H. (2019). Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia Xanthochymus Hook. Plants, 8(9), 301. https://doi.org/10.3390/plants8090301