Climate, Life Form and Family Jointly Control Variation of Leaf Traits
Abstract
:1. Introduction
2. Results
2.1. Variation in the Leaf Traits of Plants
2.2. Trade-Off in the Leaf Traits of Plants
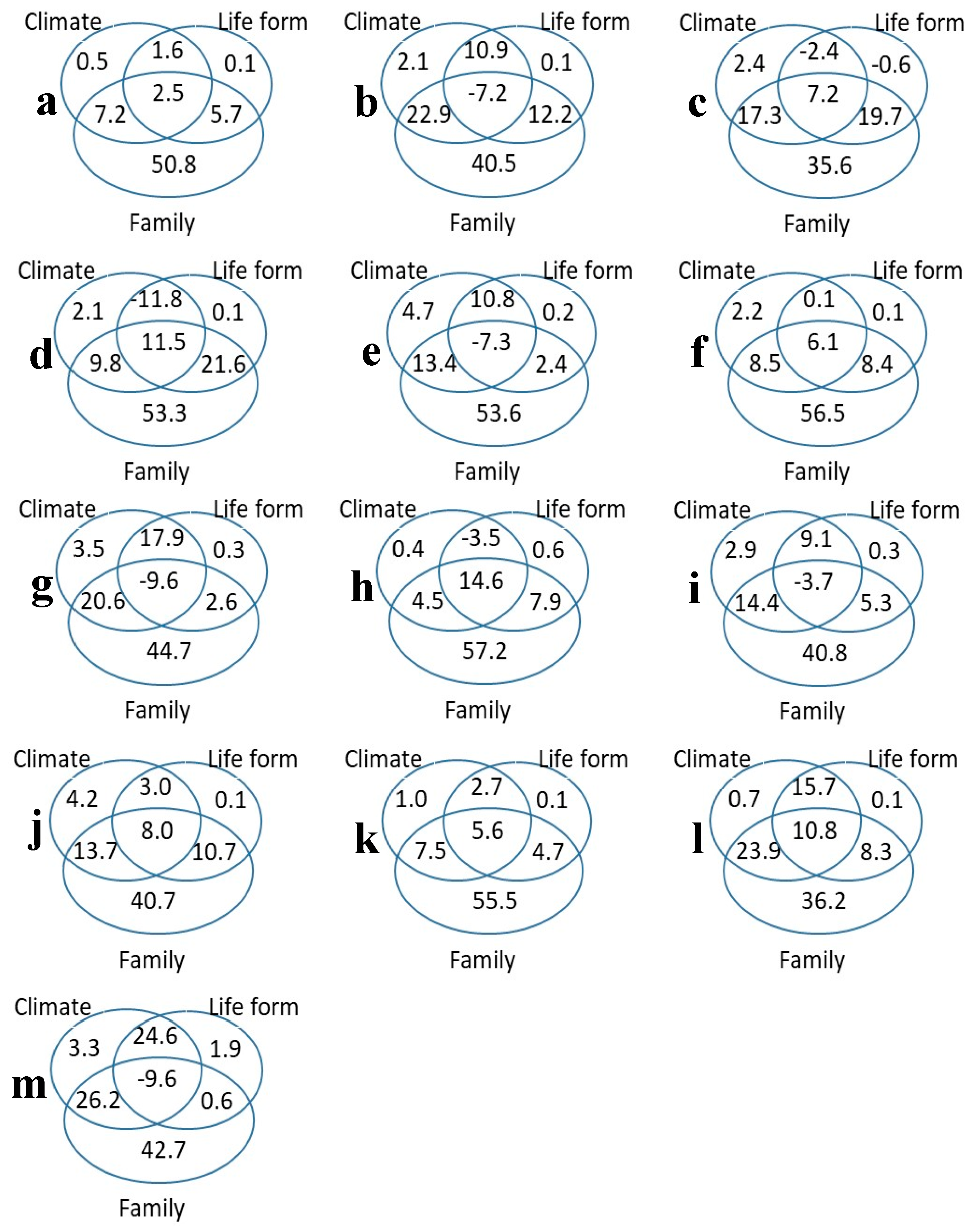
2.3. Multi-Factorial Control of Leaf Trait Variation
3. Discussion
3.1. Variation in Leaf Traits and Its Control
3.2. Covariation of Different Leaf Traits
3.3. Leaf Economics Spectrum in China
4. Materials and Methods
4.1. Plant Trait Database
4.2. Climatic, Vegetation, Family, and Life Form Variables
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruc, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Violl, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Liu, X.J.; Ma, K.P. Plant functional traits—concepts, applications and future directions. Sci. Sin. Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land−plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Ackerly, D.D.; Bongers, F.; Harms, K.E.; Ibarra-Manriquez, G.; Martinez-Ramos, M.; Mazer, S.J.; Muller−Landau, H.C.; Paz, H.; Pitman, N.C.A.; et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann. Bot. 2007, 99, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Shipley, B. Structured interspecific determinants of specific leaf area in 34 species of herbaceous angiosperms. Funct. Ecol. 1995, 9, 312–319. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Overton, J.M.; Collins, B.; Westoby, M. Shifts in trait−combinations along rainfall and phosphorus gradients. J. Ecol. 2000, 88, 964–977. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Harrison, S.P.; Prentice, I.C.; Yang, Y.; Togashi, H.F.; Wang, M.; Zhou, S.; Bai, F.; Ni, J. The China plant trait database: towards a comprehensive regional compilation of functional traits for land plants. Ecology 2018, 99, 500. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–173. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J. Trait−based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Reich, P.B. The world−wide ‘fast−slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Donovan, L.A.; Mason, C.M.; Bowsher, A.W.; Goolsby, E.W.; Ishibashi, C.D. Ecological and evolutionary lability of plant traits affecting carbon and nutrient cycling. Aust. J. Bot. 2014, 102, 302–314. [Google Scholar] [CrossRef]
- Craine, J.M.; Lee, W.G. Covariation in leaf and root traits for native and non−native grasses along an altitudinal gradient in New Zealand. Oecologia 2003, 134, 471–478. [Google Scholar] [CrossRef]
- Meng, T.T.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Ni, J.; Wang, G. Responses of leaf traits to climatic gradients: Adaptive variation versus compositional shifts. Biogeosciences 2015, 12, 5339–5352. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seed size and plant strategy across the whole life cycle. Oikos 2006, 113, 91–105. [Google Scholar] [CrossRef]
- Díaz, S.; Purvis, A.; Cornelissen, J.H.; Mace, G.M.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Z.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Wright, I.J.; Peng, C.H.; Lin, G.H. Quantifying leaf−trait covariation and its controls across climates and biomes. New Phytol. 2019, 221, 155–168. [Google Scholar] [CrossRef]
- Ackerly, D. Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proc. Natl. Acad. Sci. USA 2009, 106, 19699–19706. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2014, 205, 973–993. [Google Scholar] [CrossRef]
- Tan, Q.J.; Wang, W.L.; Chen, H.S.; Qin, Z.S.; Zheng, S.F.; Zhang, H.; Du, H.; Song, T.Q. Coupling relationships between plant community and soil characteristics in canyon karst region in south-west China. Bangladesh J. Bot. 2017, 46, 1117–1127. [Google Scholar]
- Zhang, H.; Wang, K.L.; Zeng, Z.X.; Du, H.; Zou, Z.G.; Zeng, F.P. Large−scale patterns of forest’s growth rate are mainly driven by climate variables and stand characteristics. For. Ecol. Manag. 2019, 435, 120–127. [Google Scholar] [CrossRef]
- Zou, Z.G.; Zeng, F.P.; Wang, K.L.; Zeng, Z.X.; Zhao, L.L.; Du, H.; Zhang, F.; Zhang, H. Emergy and economic evaluation of seven typical agroforestry planting patterns in the karst region of Southwest China. Forests 2019, 10, 138. [Google Scholar] [CrossRef]
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific functional variability: Extent, structure and sources of variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.B.; Ma, J.M.; Liang, S.C.; Huang, J.; Liu, R.H.; Pan, Y.F. Interspecific and intraspecific variation in functional traits of subtropical evergreen and deciduous broadleaved mixed forests in karst topography, Guilin, Southwest China. Trop. Conserv. Sci. 2016, 9. [Google Scholar] [CrossRef]
- Auger, S.; Shipley, B. Inter−specific and intra−specific trait variation along short environmental gradients in an old−growth temperate forest. J. Veg. Sci. 2013, 24, 419–428. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Huang, Y.T.; Ding, Y.; Zang, R.G. Interspecific and intraspecific variation in functional traits of subtropical evergreen and deciduous broad−leaved mixed forests. Biodivers. Sci. 2016, 24, 262–270. [Google Scholar] [CrossRef]
- He, N.P.; Tian, M.; Liu, C.C.; Li, M.L.; Yang, H.; Yu, G.R.; Guo, D.L.; Smith, M.; Yu, Q.; Hou, J.H. Variation in leaf anatomical traits from tropical to cold−temperate forests and linkage to ecosystem functions. Funct. Ecol. 2018, 32, 10–19. [Google Scholar] [CrossRef]
- Yao, T.T.; Meng, T.T.; Ni, J.; Yan, S.; Feng, X.H.; Wang, G.H. Leaf functional trait variation and its relationship with plant phylogenic background and the climate in Xinjiang Junggar Basin, NW China. Biodivers. Sci. 2010, 18, 201–211. [Google Scholar]
- Ackerly, D.D.; Reich, P.B. Convergence and correlations among leaf size and function in seed plants: A comparative test using independent contrasts. Am. J. Bot. 1999, 86, 1272–1281. [Google Scholar] [CrossRef]
- Garnier, E.; Laurent, G.; Bellmann, A.; Debain, S.; Berthelier, P.; Ducout, B.; Roumet, C.; Navas, M.L. Consistency of species ranking based on functional leaf traits. New Phytol. 2001, 152, 69–83. [Google Scholar] [CrossRef]
- Zhan, S.X.; Zhen, S.X.; Wang, Y.; Bai, Y.F. Response and correlation of above− and below−ground functional traits of Leymus chinensis to nitrogen and phosphorus additions. Chin. J. Plant Ecol. 2016, 40, 36–47. [Google Scholar]
- Figueroa, J.A.; Armesto, J.J. Community−wide germination strategies in a temperate rainforest of Southern Chile: Ecological and evolutionary correlates. Aust. J. Bot. 2001, 49, 411–425. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.G.; Song, N.P.; Yang, X.M.; Xiao, X.P.; Wang, X. A study on variations in leaf trait of 35 plants in the arid region of middle Ningxia, China. Acta Prataculturae Sin. 2014, 23, 41–49. [Google Scholar]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006, 168, 103–122. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Grubb, P. A reassessment of the strategies of plants which cope with shortages of resources. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 3–31. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Kikuta, S.B.; Lo Gullo, M.A.; Nardini, A.; Richter, H.; Salleo, S. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant Cell Environ. 1997, 20, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Oren, R.; Sperry, J.S.; Katul, G.G.; Pataki, D.E.; Ewers, B.E.; Phillips, N.; Schafer, K.V.R. Survey and synthesis of intra− and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef]
- Wu, W.H. Plant Physiology; Science Press: Beijing, China, 2005; pp. 117–175. [Google Scholar]
- He, J.S.; Wang, Z.H.; Wang, X.P. A test of the generality of leaf trait relationship on the Tibetan Plateau. New Phytol. 2006, 170, 835–848. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Lu, Q.; Wu, B.; Zhu, Y.J.; Liu, D.J.; Zhang, J.X.; Jin, Z.H. A review of leaf morphology plasticity linked to plant response and adaptation characteristics in arid ecosystems. Chin. J. Plant Ecol. 2012, 36, 88–98. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Shimizu, H.; Ito, S.; Yagasaki, Y.; Zou, C.J.; Zhou, G.S.; Zheng, Y.R. Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 2014, 239, 421–435. [Google Scholar] [CrossRef]
- Zhao, M.S.; Running, S.W. Drought−induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef]
- Maestre, F.T.; Salguero-Gómez, R.; Quero, J.L. It is getting hotter in here: determining and projecting the impacts of global environmental change on drylands. Philos. Trans. R. Soc. B 2012, 367, 3062–3075. [Google Scholar] [CrossRef] [Green Version]
- Volder, A.; Briske, D.D.; Tjoelker, M.G. Climate warming and precipitation redistribution modify tree−grass interactions and tree species establishment in a warm−temperate savanna. Glob. Chang. Biol. 2013, 19, 843–857. [Google Scholar] [CrossRef]
- Lavorel, S. Plant functional effects on ecosystem services. J. Ecol. 2013, 101, 4–8. [Google Scholar] [CrossRef]
- Wang, H.; Prentice, I.C.; Ni, J. Data−based modelling and environmental sensitivity of vegetation in China. Biogeosciences 2013, 10, 5817–5830. [Google Scholar] [CrossRef]
- Davis, T.W.; Prentice, I.C.; Stocker, B.D.; Thomas, R.T.; Whitley, R.J.; Wang, H.; Evans, B.J.; Gallego−Sala, A.V.; Sykes, M.T.; Cramer, W. Simple process−led algorithms for simulating habitats (SPLASH v. 1.0): Robust indices of radiation, evapotranspiration and plant−available moisture. Geosci. Model Dev. 2017, 10, 689–708. [Google Scholar] [CrossRef]
- Hutchinson, M. ANUSPLIN Version 4.37 User Guide; The Australian National University: Canberra, Australia, 2007. [Google Scholar]
- James, F.C.; McCulloch, C.E. Multivariate analysis in ecology and systematics: Panacea or Pandora’s Box? Annu. Rev. Ecol. Syst. 1990, 21, 129–166. [Google Scholar] [CrossRef]
- Zhang, H.; Song, T.Q.; Wang, K.L.; Wang, G.X.; Liao, J.X.; Xu, G.H.; Zeng, F.P. Biogeographical patterns of biomass allocation vary with climate, soil and forest characteristics in China. Environ. Res. Lett. 2015, 10, 044014. [Google Scholar] [CrossRef]

| Functional Traits | Mean | Minimum | Maximum | Standard Deviation | Variable Coefficient |
|---|---|---|---|---|---|
| LA | 0.0036 | 1.67−07 | 0.24012 | 0.0098 | 2.7222 |
| SLA | 20.3092 | 1.6420 | 205.6915 | 12.9413 | 0.6372 |
| LMA | 0.0712 | 0.0049 | 0.6090 | 0.0546 | 0.7669 |
| LDMC | 336.9785 | 62.0915 | 950.5650 | 116.2379 | 0.3449 |
| C mass | 436.7534 | 168.9521 | 979.3636 | 85.6143 | 0.1960 |
| N mass | 19.5915 | 0.0870 | 122.9118 | 8.9276 | 0.4557 |
| P mass | 2.4990 | 0.0600 | 7.8705 | 1.2507 | 0.5005 |
| K mass | 14.7673 | 0.1210 | 102.5344 | 10.3826 | 0.7031 |
| N area | 1.3227 | 0.0054 | 16.0167 | 0.9466 | 0.7157 |
| P area | 0.1487 | 0.0025 | 1.2868 | 0.1086 | 0.7303 |
| K area | 0.8250 | 0.0030 | 10.9283 | 0.7188 | 0.8713 |
| d13C:12C | −28.5584 | −39.0705 | −11.8300 | 4.4495 | −0.1558 |
| d15N:14N | −0.2624 | −7.4005 | 12.1350 | 2.8134 | −10.7218 |
| LA | SLA | LMA | LDMC | C Mass | N Mass | P Mass | K Mass | N Area | P Area | K Area | d13C:12C | d15N:14N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | 0.2195 | 0.1841 | 0.4822 | 0.4698 | 0.5431 | 0.0685 | 0.4745 | 0.1463 | 0.1263 | 0.8353 | <0.0001 | 0.1828 | |
| SLA | 0.054 | <0.0001 | <0.0001 | 0.0026 | <0.0001 | 0.2830 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0007 | <0.0001 | |
| LMA | −0.059 | −0.617 | <0.0001 | 0.2980 | <0.0001 | 0.0105 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0070 | 0.0100 | |
| LDMC | −0.031 | −0.482 | 0.281 | 0.0021 | <0.0001 | 0.0592 | <0.0001 | 0.0007 | <0.0001 | 0.8982 | <0.0001 | 0.2827 | |
| C mass | −0.032 | −0.133 | -0.046 | 0.135 | <0.0001 | 0.0118 | 0.5186 | <0.0001 | 0.0287 | 0.4322 | 0.0171 | <0.0001 | |
| N mass | −0.027 | 0.203 | −0.247 | −0.233 | 0.181 | 0.7016 | <0.0001 | <0.0001 | <0.0001 | 0.0006 | 0.0004 | 0.0002 | |
| P mass | 0.080 | 0.047 | −0.113 | −0.083 | 0.111 | 0.017 | 0.8934 | 0.0362 | <0.0001 | 0.0487 | <0.0001 | 0.8104 | |
| K mass | 0.032 | 0.257 | −0.222 | −0.335 | −0.029 | 0.335 | 0.006 | 0.1288 | <0.0001 | <0.0001 | <0.0001 | 0.5534 | |
| N area | −0.064 | −0.492 | 0.694 | 0.149 | 0.184 | 0.365 | −0.092 | 0.067 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| P area | 0.067 | −0.485 | 0.556 | 0.297 | 0.096 | −0.187 | 0.630 | −0.183 | 0.367 | <0.0001 | 0.0050 | <0.0001 | |
| K area | −0.009 | −0.276 | 0.363 | 0.006 | −0.035 | 0.151 | −0.087 | 0.711 | 0.495 | 0.179 | <0.0001 | <0.0001 | |
| d13C:12C | −0.225 | −0.148 | 0.119 | 0.175 | 0.105 | 0.154 | −0.258 | 0.197 | 0.214 | −0.124 | 0.326 | 0.0002 | |
| d15N:14N | 0.059 | −0.237 | 0.113 | 0.047 | −0.247 | 0.163 | −0.011 | 0.026 | 0.283 | 0.213 | 0.275 | 0.161 |
| Leaf Traits | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| LA | 0.95 | −0.01 | 0.05 | −0.19 |
| SLA | 0.84 | 0.17 | 0.00 | 0.43 |
| LMA | −0.19 | 0.93 | −0.02 | 0.18 |
| LDMC | 0.38 | 0.88 | −0.01 | 0.02 |
| C mass | −0.13 | −0.01 | 0.96 | 0.04 |
| N mass | 0.51 | −0.02 | 0.78 | −0.14 |
| P mass | −0.05 | 0.15 | −0.03 | 0.95 |
| K mass | −0.64 | 0.00 | −0.04 | 0.23 |
| N area | 0.16 | −0.13 | 0.02 | −0.09 |
| P area | 0.12 | 0.07 | 0.05 | 0.11 |
| K area | 0.07 | −0.03 | 0.07 | 0.12 |
| d13C:12C | −0.04 | 0.02 | 0.04 | −0.02 |
| d15N:14N | 0.08 | 0.17 | −0.15 | 0.06 |
| Eigenvalue | 2.53 | 1.73 | 1.57 | 1.26 |
| Explained (%) | 19.43 | 13.31 | 12.09 | 9.70 |
| Cumulative (%) | 19.43 | 32.74 | 44.83 | 54.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zeng, Z.; Zou, Z.; Zeng, F. Climate, Life Form and Family Jointly Control Variation of Leaf Traits. Plants 2019, 8, 286. https://doi.org/10.3390/plants8080286
Zhang H, Zeng Z, Zou Z, Zeng F. Climate, Life Form and Family Jointly Control Variation of Leaf Traits. Plants. 2019; 8(8):286. https://doi.org/10.3390/plants8080286
Chicago/Turabian StyleZhang, Hao, Zhaoxia Zeng, Zhigang Zou, and Fuping Zeng. 2019. "Climate, Life Form and Family Jointly Control Variation of Leaf Traits" Plants 8, no. 8: 286. https://doi.org/10.3390/plants8080286




