Abstract
The availability of water is the critical factor driving plant growth, physiological responses, population and community succession in arid and semiarid regions, thus a precipitation addition-reduction platform with five experimental treatments, was established to explore the growth and physiology of two psammophytes (also known as psammophiles) to precipitation manipulation in Horqin Sandy Land. Changes in coverage and density were measured, and antioxidant enzymes and osmoregulatory substances in both of the studied species were determined. Investigation results showed that the average vegetation coverage increased with an increasing precipitation, and reached a maximum in July. Under the −60% precipitation treatment, Tribulus terrestris accounted for a large proportion of the area, but Bassia dasyphylla was the dominant species in the +60% treatment. T. terrestris was found to have higher a drought stress resistance than B. dasyphylla. From days 4 to 7 after rainfall, B. dasyphylla under precipitation reduction showed obvious water stress. The malondialdehyde (MDA) content of B. dasyphylla was higher than that of T. terrestris, but that of B. dasyphylla had the lower relative water content (RWC). The MDA content in the precipitation reduction treatments of the two studied species was higher than that in the precipitation addition treatments from days 4 to 10. Peroxidase (POD) and superoxide dismutase (SOD) activity and the soluble proteins and free proline content of T. terrestris were higher than those of B. dasyphylla. The free proline content of T. terrestris and B. dasyphylla increased with increasing drought stress. Our data illustrated that T. terrestris had a higher drought stress resistance than B. dasyphylla, which was correlated with the augmentation of some antioxidant enzymes and osmoregulatory substance. The adaptive mechanism provides solid physiological support for an understanding of psammophyte adaptation to drought stress, and of community succession or species manipulation for desertified land restoration.
1. Introduction
Water is a key driving factor in arid and semi-arid sand ecosystems. Plant growth and physiological processes are closely correlated with water availability. Precipitation is the most important source of water in arid and semi-arid sand areas, and is also a main constraint on the formation and development of a psammophyte community [1]. With the intensification of global climate change, rainfall events characterized by long intervals and high single rainfall amounts will increase. It is widely accepted that climate variation has caused changes in vegetation distribution patterns and productivity [2]. Furthermore, plant physiological features, such as photosynthesis and transpiration, antioxidant enzymes and osmoregulatory substances, are affected by climate variation [3,4]. As such, the responses of the above physiological features influence plant community dynamics, such as degradation, restoration and succession.
Horqin Sandy Land is located in the transitional zone of semi-arid and semi-humid regions in eastern Inner Mongolia of China [5]. The annual precipitation is approximately 351.7 mm, but this has shown a decreasing trend in recent years [6]. The effect of precipitation upon vegetation in this area is mainly reflected in the change of annual herbaceous plant species [7]. The disappearance and invasion of annual herbaceous plants occurs frequently with changes in precipitation in fixed dunes [8].
For grasslands in semi-arid regions, the rainfall timing pattern and the number of single rainfall events during the growing season have a greater impact on the net primary productivity than the total rainfall [9]. The Memorial model, as an estimate index of grassland potential productivity, points out that precipitation is one of the rigid limiting factors for the improvement of grassland in eastern Inner Mongolia [10]. In different months of the growing season, precipitation had different effects on grassland vegetation growth, especially from June to August (summer) [11]. In addition, the species diversity index varied with precipitation change, and the impact of this growing season precipitation on species diversity was greater than the impact of the total annual precipitation [10]. Studies revealed that vegetation coverage in most parts of inland China increased significantly. Precipitation in Inner Mongolia showed a bipolar change [12,13], with the western part showing a trend of warming and humidification, and the central and eastern parts characterized by warming and drying. Therefore, precipitation change inevitably affected not only plant community composition, diversity and productivity on a small scale, but also the structure and functioning of the entire ecosystem on a regional or even a global scale [14].
A study of the physiological responses of psammophytes to adverse stresses, which were mainly concentrated on sand burial, cold, drought and other stresses [15], indicated that antioxidant enzymes and osmoregulatory substances played an important role in resisting or repairing any damage caused by stress. However, when stress exceeded the tolerance limits of the plants, it resulted in plant growth stagnation or even death [16]. Under conditions of soil drought, rehydration, frequent dehydration and rehydration cycles, the responses of psammophytes are demonstrated, and many studies are manually simulated with a short-term regulation of soil moisture. It is difficult to systematically reflect the true responses of plants in their natural state. Studies on the physiological responses of Digitaria sanguinalis and Setaria viridis under simulated drought have been carried out [17,18]. The results show that osmoregulatory substances increase under drought stress, and moderate drought induces an increase in antioxidant enzyme activity, but severe drought damages the antioxidant enzyme system.
Tribulus terrestris is distributed in sandy land, uncultivated land, hillsides and residential areas. There are also many distributions in agricultural and forestry production land in the protected areas and the interlaced zone between the oasis edge and the agricultural area. Its stems lie flat and up to about 1m in length [19]. B. dasyphylla is a pioneer plant in semi-fixed or fixed dunes, flat sandy land and moderately saline-alkali land. Its stem is erect and its height is about 30–50 cm. It is often scattered or clustered in the grasslands, semi-deserts and desert areas of northern China [20,21]. Both plants are annual herbs [19,20,21], but their coverage and density varies greatly under different precipitation, thus it is necessary to reveal the differences in their physiological levels.
Studies on the physiological resistance of psammophytes to stress have mostly focused on short-term drought stress [22], which makes it difficult to precisely trace the physiological adaptation processes of the different plants of different families and genera in a short processing time and under a high intensity of treatments. Thus, the objectives of this study were as follows: (1) To reveal the growth and physiological responses of two psammophytes to precipitation gradients, including extreme drought under natural conditions, and (2) to examine the adaptive mechanisms of the two species to a sand habitat.
2. Materials and Methods
2.1. Site Description
This study was carried out in the Naiman Desertification Research Station (42°58′N, 120°43′E), Chinese Academy of Sciences, which is located in the south-eastern part of Horqin Sandy Land, eastern Inner Mongolia, China. This area is characteristic of a continental semi-arid monsoon climate, with a mean annual precipitation of 351.7 mm, 70–80% of which falls from June to August (summer). The annual mean potential evaporation is 1935 mm and the annual frost-free period is approximately 150 days. The landscape of this region is characterized by sand areas and lowland areas. Most of the lowlands have been reclaimed into cropland, while the sand areas are used as pasture. The sandy soil mainly consists of coarse sand and silt. The natural vegetation mainly consists of Agriophyllum squarrosum, Setaria viridis, Corispermum marocarpum, Salsola collina, Artemisia halodendron, Caragana microphylla, Chenopodium glaucum, Bassia dasyphylla and Tribulus terrestris [23,24].
2.2. Experimental Design
A precipitation addition-reduction structure was constructed in April 2011 and used in this study from 10 May to 20 August 2016. The device was 2 m (length) × 2 m (width) × 1.5 m (height). The structure had a square steel frame, with the top tilted by 15 degrees to support rain intercept grooves for rain reduction, and a rain tank for rain collection (Figure 1). The rain intercept groove was made of highly transparent 5 mm-thick Perspex with a light transmission of 95%. The rain tank was made of a stainless-steel plate with a thickness of 3 mm and sealed ends. White aluminum-plastic pipes were connected to the bottom of the rain tank for rain addition, and nine isometric holes were made evenly along each of the pipes for the redistribution of the collected rain [25].

Figure 1.
Precipitation addition-reduction apparatus.
According to the annual precipitation data for the last 49 years in this area, the maximum precipitation reached 567.1 mm in 1986, which was 61.2% over the average, and the minimum precipitation was 213.1 mm in 2000, 39.4% less than the average precipitation. Five treatments were set up: (a) Control, without precipitation addition or reduction; (b) precipitation added by 30% (+30%); (c) precipitation added by 60% (+60%); (d) precipitation decreased by 30% (−30%); and (e) precipitation decreased by 60% (−60%). Each treatment consisted of four replicate plots. To avoid mutual interference, there were buffer strips of 2 m between the plots.
2.3. Analytical Methods and Statistical Analysis
The species number, coverage and density of these plants in the different sample plots were examined monthly from April 20, 2016 to August 16, 2016. Five sampling plots of 50 cm × 50 cm were set in each plot (one sampling plot was placed at the center, and the four others were placed near the corners). The rainfall was 34.4 mm on July 28, after which there was no precipitation for 15 days. Leaves of B. dasyphylla and T. terrestris were randomly cut on days 1, 4, 7 and 10 after the rainfall event. Several of them were taken immediately to the laboratory to measure the relative water content (RWC) of the leaf; the rest were placed in a liquid nitrogen tank for an observation of enzyme activities and osmoregulatory substances.
Leaf samples were extracted with a chilled buffer (50 mM phosphate, 1% (w/v) polyvinylpolypyrrolidone) and were centrifuged at 15,000 g for 20 min. The supernatants were stored at 4 °C for physiological indices. The malondialdehyde (MDA) content was measured using the thiobarbituric acid method [26]. Peroxidase (POD) activity was determined spectrophotometrically at 470 nm due to the oxidation of guaiacol. Superoxide dismutase (SOD) activity was measured spectrophotometrically using the nitroblue tetrazolium photoreduction method. Catalase (CAT) activity was determined with the iodine–perhydrol method [24,27,28]. The soluble proteins content was measured spectrophotometrically using the Coomassie blue dye combination method. The soluble sugars content was determined with the method described by An et al. [29]. The free proline concentration was measured spectrophotometrically with the ninhydrin method [30,31]. The activities of POD and SOD, as well as the amount of MDA and osmoregulatory substances were determined using a spectrophotometer (Shimadzu Corporation, Japan).
All variables (density, RWC, MDA, antioxidant enzymes and osmotic regulatory substances) were analyzed and assessed using SPSS version 20.0 and Analysis of Variance (ANOVA). A least significant difference (LSD) test was performed to determine any differences in drought resistance between the two species. Significant differences were defined at P < 0.05.
3. Results
3.1. Characteristics of Rainfall and Plant Growth
Rainfall patterns have an important influence on plant coverage, species composition and plant population size in the study area. The annual rainfall measured at the Naiman Desertification Research Station in 2016 was 392.8 mm, 69.3% of that rainfall falling from June to August (summer), with 84.2 mm, 128.0 mm and 60.2 mm falling in June, July and August, respectively (Figure 2A). The average coverage of plants increased from the −60% treatment to the +60% treatment (Figure 2B). The coverage was found to be only 5% in treatment −60% in June, and it reached the most (58%) under treatment +60% in July. The mean plant coverage in July for all treatments was the highest compared to that in June and August, with significant differences among the control, −60% and +60% treatments. In June, the coverage increased by 60% in treatment +30% and decreased by 40% in treatment −30% compared with that in the control. In August, the coverage was 53% in treatment +30% and 51% in treatment +60%, with no significant difference between the two precipitation treatments, but they were found to be significantly higher than those in the other treatments.

Figure 2.
Rainfall change during the study period (A) and the influence of different precipitation treatments on plant coverage (B).
The rainfall in July was much higher than that in June and August, but the plant density in June was significantly higher than in July and August (Table 1). All four species, T. terrestris, B. dasyphylla, S. collina and C. glaucum were found in each of the plots. However, the dominant species under these precipitation addition treatments was B. dasyphylla, accounting for 44.9% of the plants under treatment +60% in August. T. terrestris became dominant under treatment −60% and ranged up to 80.0% in July and 79.4% in August.

Table 1.
Influence of different precipitation treatments on plant density.
3.2. Changes in Leaf RWC and MDA
The relative water content (RWC) and MDA are widely used to assess cellular damage. RWC is a relatively sensitive index of leaf water status, and the rate of decline under drought stress is related to drought resistance. When plants suffer from extremely high temperature, drought, salt and high radiation stress, the balance of the active oxygen metabolism system is disrupted, leading to the accumulation of reactive oxygen species (ROS). ROS accumulation causes metabolic disorder and cell membrane lipid peroxidation. MDA is the first product in cell membrane lipid peroxidation, and is widely used to determine the extent of this cell damage [32,33,34]. RWC in the leaves of both species decreases with time. The RWC of T. terrestris is higher than that of B. dasyphylla and decreases by 18.3–30.3% in the drier treatments (Figure 3A,B), while the RWC in B. dasyphylla decreases by 26.2–47.6%. The RWC of both species shows three phases: A slow decline (days 1 to 4), followed by a rapid decrease (days 4 to 7), and finally a moderate drop (days 7 to 10). RWC in all treatments on day 1 does not differ significantly. On day 10, B. dasyphylla has the lowest RWC (43.95%) under the −60% treatment, whereas RWC in T. terrestris is seen at 62.20%.

Figure 3.
Influence of different precipitation treatments on relative water content (RWC) in T. terrestris (A), B. dasyphylla (B) and malondialdehyde (MDA) of T. terrestris (C), B. dasyphylla (D). Values are assigned as the mean ± SD.
There is no significant difference in MDA content between the studied species on day 1, while MDA content in the precipitation reduction treatment is higher than that in the precipitation addition treatment from days 4 to 10 (Figure 3C,D). The MDA content of T. terrestris increases continuously over the whole stressed period except for the precipitation addition treatment, but is lower than that of B. dasyphylla. The MDA content in the control, precipitation reduction and precipitation addition treatments differs significantly from days 4 to 7. Under the −60% treatment, the MDA content was 2.927 (day 7) and 3.307 mmol g−1 FW (day 10). The MDA content of B. dasyphylla increases from days 1 to 7 and then decreases. On day 7, the content in treatment −60% becomes the highest (4.565 mmol g−1 FW), being 2.74 times that of the control and 1.90 times that of the +60% treatment.
3.3. Changes in Antioxidant Enzymes
POD activity in T. terrestris and B. dasyphylla was weak, with values below 1 U g−1 FW (Figure 4A,B), but POD activity in T. terrestris was relatively higher than that in B. dasyphylla. The activity of T. terrestris first increased (days 1 to 4), and then decreased (days 4 to 7), and finally increased again (days 7 to 10), except for in treatment +60%. On day 10, POD activity in treatment −60% was found to be the highest (0.709 U g−1 FW) and was significantly different from the control and precipitation addition treatments. The POD activity of B. dasyphylla first decreased (days 1 to 4), then increased (days 4 to 7), and finally decreased again (days 7 to 10) except for in treatment +60%. No significant difference in POD activity was detected between the control and +60% treatment.

Figure 4.
Influence of different precipitation treatments on antioxidant enzymes. (A): Peroxidase (POD) of T. terrestris; (B): POD of B. dasyphylla; (C): Superoxide dismutase (SOD) of T. terrestris; (D): SOD of B. dasyphylla; (E): Catalase (CAT) of T. terrestris; (F): CAT of B. dasyphylla. Values are assigned as the mean ± SD.
The SOD activity of T. terrestris was significantly higher than that of B. dasyphylla (Figure 4C,D). The SOD activity of T. terrestris first increased (days 1 to 4), then decreased (days 4 to 7), and finally increased (days 7 to 10). From days 4 to 10, SOD activity in treatment −60% was higher than SOD activity in the other treatments. SOD activity in the precipitation addition treatment was significantly lower than that under precipitation reduction from days 1 to 7. The SOD activity of B. dasyphylla first decreased (days 1 to 4) and then increased (days 4 to 10). The SOD activity in treatment −60% was the highest (155.041 U g−1 FW) on day 10 and was higher than in other treatments. The SOD activity in the precipitation reduction treatment was significantly higher than that in the precipitation addition treatment from days 7 to 10.
From days 0 to 4, CAT activity of T. terrestris was significantly higher than that of B. dasyphylla (Figure 4E,F). The activity of T. terrestris first increased and then decreased, except for in treatment +60%. On day 10, CAT activity in treatment −60% was the lowest (37.418 U g−1 FW) and was significantly lower than that in the other treatments; CAT activity under precipitation addition was significantly higher than that in precipitation reduction. The CAT activity of B. dasyphylla in precipitation reduction first decreased (days 0 to 4) and then increased (days 4 to 10), but the activity in treatment +60% increased during the whole study period. On day 10, CAT activity under precipitation addition and precipitation reduction was significantly higher than in the control, while the activity in treatment +60% was found to be the highest (108.782 U g−1 FW), with significant differences from all other treatments.
3.4. Changes in Osmoregulatory Substances
The soluble proteins content of T. terrestris was significantly higher than that of B. dasyphylla (Figure 5A,B). From days 0 to 10, the content of T. terrestris was 23.212–40.733 mg g−1 FW versus 9.541–23.171 mg g−1 FW in B. dasyphylla. The soluble proteins content of T. terrestris first increased (days 0 to 7) and then decreased (days 7 to 10). On day 7, its content in treatment −60% (40.733 mg g−1 FW) was significantly higher than that in the other treatments. The soluble proteins content in the precipitation reduction treatment of B. dasyphylla increased, and was significantly higher than that in the precipitation addition from days 4 to 10. On day 10, the soluble proteins content in treatment −60% (23.171 mg g−1 FW) was the highest. From days 0 to 10, the soluble proteins content in treatment +60% continuously increased from 11.406 to 12.262 mg g−1 FW, but the differences were not significant.
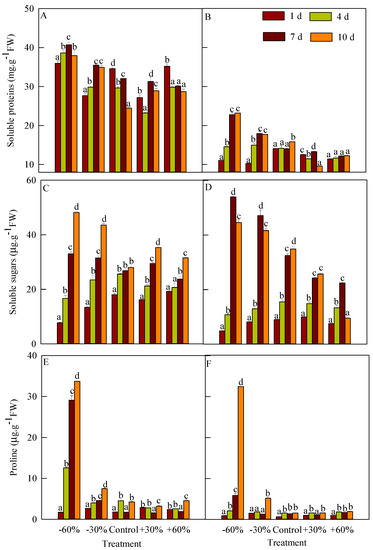
Figure 5.
Influence of different precipitation treatments on osmoregulatory substances. (A): Soluble proteins of T. terrestris; (B): Soluble proteins of B. dasyphylla; (C): Soluble sugars of T. terrestris; (D): Soluble sugars of B. dasyphylla; (E): Free proline of T. terrestris; (F): Free proline of B. dasyphylla. Values are assigned as the mean ± SD.
The soluble sugars content on day 4 of T. terrestris and B. dasyphylla in the control was higher than those in the precipitation addition and reduction treatments (Figure 5C,D). The soluble sugars content of T. terrestris increased continuously. From days 4 to 10, the soluble sugars content in the control ranged from 25.556 to 27.986 µg g−1 FW and the differences were not significant, but were significantly higher than on day 1. On day 10, the soluble sugars content under precipitation addition was significantly lower than that in the precipitation reduction treatment and higher than that in the control; its content (48.145 µg g−1 FW) in treatment −60% was the highest, and was significantly different from all other treatments. The soluble sugars content of B. dasyphylla continued to increase in the control and +30% treatment. Its soluble sugars content first increased (days 0 to 7) and then decreased (days 7 to 10) in the precipitation reduction and +60% treatments. The soluble sugars content of B. dasyphylla in the control was lower than that in the precipitation reduction treatment, but higher than that in precipitation addition treatments. On day 7, the soluble sugars content in treatment −60% was 53.945 µg g−1 FW and was significantly different from the other treatments.
The free proline content of T. terrestris was significantly higher than that of B. dasyphylla (Figure 5E,F). Under −60% treatment, the free proline content of both species increased, but the content of B. dasyphylla was significantly lower than that of T. terrestris. From days 4 to 7, the free proline content of T. terrestris in the precipitation reduction treatment continued to increase and showed significant differences from the other treatments. The proline content in treatment −60% was 6.35–18.76 times and 4.50–10.63 times higher than in other treatments on days 7 and 10, respectively. From days 4 to 10, the free proline content of B. dasyphylla in treatment +60% did not differ significantly from the control, but was significantly higher than its free proline content on day 1. Under treatment −60%, the free proline content on day 10 was 5.54 times higher than on day 7 and was 6.27–20.99 times higher than those in the other treatments.
3.5. Correlation Analysis
The results of the correlation analysis (Table 2) show that RWC was significantly positively correlated CAT activity of T. terrestris, but negatively correlated with the other parameters in both studied psammophytes. For T. terrestris, MDA was significantly positively correlated with the three osmotic regulators, but negatively correlated with CAT. POD was significantly positively correlated with soluble proteins and SOD, but CAT was significantly negatively correlated with soluble sugars and prolines. Proline was significantly positively correlated with SOD, soluble sugars and proteins. For B. dasyphylla, there were significant positives correlations among the three osmotic regulators and they were significantly positively correlated with POD, SOD and MDA. CAT was significantly positively correlated with soluble sugars and SOD, and MDA was significantly positively correlated with the three antioxidase activities.

Table 2.
Pearson correlation matrix of physiological characteristics of T. terrestris and B. dasyphylla under precipitation manipulation.
4. Discussion
Precipitation is one of the most important ecological factors that shapes plant community succession [35]. Precipitation patterns and quantities are decisive factors in plant colonization and growth. Changes in precipitation have different effects on sand area plant community structure, biodiversity and productivity, and therefore determine the characteristics of the whole ecosystem [36]. It is shown that in spare vegetation coverage areas where precipitation is less than 100 mm per year, plant coverage increases with the increase in precipitation [34]. In our study area, the south-eastern part of Horqin Sandy Land, eastern Inner Mongolia, precipitation also significantly affected the rate of vegetation cover and the vegetation density. In this area, the beginning of vegetation growth is in May, while most precipitation falls in June, July and August (summer). The precipitation in July accounts for one-third of the annual rainfall. After plants germinate in May, later precipitation significantly affects the growth of the vegetation, which is presented in vegetation coverage and density.
It is shown in this study that in all precipitation manipulation treatments, the average vegetation coverage reaches its maximum in July. In addition, an increase along the gradient from precipitation reduction throughout the control to precipitation addition treatment is found. This result is consistent with Meserve’s study, which explored the relationship between the coverage of desert ephemeral plants and precipitation in northern Chile for 13 years inclusive (1989–2001) [37]. Our study shows that coverage and other indices of the plants in August decreases in the +60% treatment and are higher than those in the control, but are lower than those in the +30% treatment. As most plants in our study area were psammophytes, suitable precipitation increases vegetation coverage and density, but high-intensity precipitation inhibits plant growth. A high precipitation rate causes these psammophytes to reduce their coverage and density, this phenomenon was also observed for other vegetation characteristics. T. terrestris, B. dasyphylla, S. collina and C. glaucum were present in all the plots, with significant differences in their density. The reason for this difference might be their different responses to precipitation during seed germination and during the growing season [38]. T. terrestris is in the Zygophyllaceae family, and the other three plants belong to Chenopodiaceae [39,40,41], but are of different genera.
In the early stages of drought, RWC and MDA were not significantly different among the different treatments. With increasing drought stress, RWC decreased; RWC with precipitation addition was found to be higher than that under precipitation reduction. Precipitation addition led to an increase in RWC, but this response lagged behind the precipitation by 1–4 days. On day 10, RWC in B. dasyphylla in treatment −60% was significantly lower than that found in T. terrestris. Fresh leaves had the ability to store and retain water, which was one of the reasons for the high survival rate of T. terrestris under drought stress. Under extreme drought (−60%), The MDA of T. terrestris reached its highest amount, indicating that T. terrestris suffered the most severely. The MDA of T. terrestris continued to increase over the whole stress period except for precipitation addition, but MDA in B. dasyphylla was higher than that in T. terrestris. This finding leads to the idea that T. terrestris has a better water retention capacity and a lower membrane lipid peroxidation than does B. dasyphylla, which makes T. terrestris more adaptable under drought stress. This coincides with the result that the dominant species found in treatment −60% was T. terrestris. It is shown that precipitation addition protects the plants from cell injury. With prolonged drought stress, the MDA of B. dasyphylla decreased from days 7 to 10, likely because of the extreme drought that seriously damaged its cells beyond the range of drought stress that B. dasyphylla could withstand.
The balance of active oxygen metabolism in plants is destroyed when plants are subjected to environmental stress [42]. However, the accumulation of oxygen free radicals can induce the antioxidant enzyme system. The synthesis of antioxidant enzymes in cells can eliminate or alleviate the toxic effects of reactive oxygen species and thus relieve the damage to the organism [43].
At the same time, the plants also reduce cytoplasmic infiltration by increasing the solute content of the cytoplasm to prevent cytoplasmic leakage, thus maintaining cell swelling and growth [44]. The three antioxidant enzyme activities of T. terrestris were stimulated and triggered at the early stage of drought. CAT activity was decreased except for treatment +60% on day 10, implying that the resistance of T. terrestris to drought stress via CAT activity was restricted. CAT activity cannot provide good protection under extreme drought stress. The antioxidant enzyme activities in B. dasyphylla remained at a low level in the early period of drought. This was probably because antioxidant enzymes were not stimulated under mild drought, whereas the activities of B. dasyphylla in SOD and CAT increased with prolonged drought stress. In addition, it was found that the activities of POD and SOD in T. terrestris were the highest under severe drought. The accumulation of oxygen free radicals activated the antioxidant enzyme protection system, and the activity of antioxidant enzymes increased, which maintained the balance of oxygen free radical metabolism. The antioxidant enzyme system is commonly assessed to reveal the ability of plants to resist stress [45]. The activities of POD and SOD in T. terrestris were higher than those in B. dasyphylla, thus, the drought resistance of T. terrestris was stronger than that of B. dasyphylla. In addition to CAT in T. terrestris, the antioxidant enzyme activities and osmoregulation substances of the studied species were positively correlated. To prevent excessive water loss under environmental stress, plants usually maintain cell expansion via decreasing cytoplasmic osmotic potential or increasing osmoregulatory substance content [24]. In our study, we found that osmoregulation substances were negatively correlated with RWC. The osmoregulation substance contents of the studied species increased at the early stage of drought. With prolonged drought stress, the free proline content of the two psammophytes and the soluble sugars content of T. terrestris increased by several dozen times, but the soluble sugars content of B. dasyphylla was reduced under severe drought. Drought inhibited photosynthesis and the stored soluble sugars in leaves was consumed as an energy source. The main osmoregulatory substances of T. terrestris were soluble proteins and free proline while soluble sugars and free proline were the main osmoregulatory substances in B. dasyphylla. The differences in the physiological adaptation strategies of the two psammophyte species are controlled by heredity, which is the basis for the emergence of psammophytes as target genes for stress-resistance breeding.
5. Conclusions
According to the above analysis, it was concluded that precipitation reduction significantly reduced vegetation coverage and density. The average vegetation coverage increased with the increase of precipitation, and reached a maximum in July. The +60% precipitation treatment was conducive to early plant growth, but not to vegetation coverage or density in the later period. Our results indicate that T. terrestris is better adapted to drought stress than B. dasyphylla in Horqin Sandy Land, eastern Inner Mongolia. The MDA of B. dasyphylla was higher than that of T. terrestris. POD, SOD, soluble proteins and free proline of T. terrestris were higher than those of B. dasyphylla. The free proline content of T. terrestris and B. dasyphylla increased several folds with prolonged drought stress. The changes in RWC content in T. terrestris leaves were small, and the antioxidant enzymes and osmoregulatory substances increased greatly, which effectively alleviated the damage to the cell membrane. Future studies on the adversity stress of both studied plants will evolve from the eco-physiological level to the molecular level.
Author Contributions
Conceptualization, X.Z. and X.L.; Investigation, J.C. and Y.L. (Yayong Luo); Writing-original draft preparation, J.C.; Software, Y.L. (Yongqing Luo) and Y.Z.; Writing-review and editing, R.Z. and Z.H.; Data curation, J.C. and R.Z.; All authors have read the final manuscript and approved the submission.
Funding
This study was financially supported by the National Basic Resources Investigation Program of China (2017FY100200), Inner Mongolia autonomous region science and technology major project (Y749BJ1001) and the National Natural Science Foundation of China (41371053).
Acknowledgments
We thank all members of Naiman Desertification Research Station, Chinese Academy of Sciences, for their help in field and laboratory work. We also wish to thank the anonymous reviewers for their valuable comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wilhelmi, O.V.; Wilhite, D.A. Assessing vulnerability to agricultural drought: A Nebraska case study. Nat. Hazards 2002, 25, 37–58. [Google Scholar] [CrossRef]
- Yue, X.F.; Zhang, T.H.; Zhao, X.Y.; Liu, X.P.; Ma, Y.H. Effects of rainfall patterns on annual plants in Horqin Sandy Land, Inner Mongolia of China. J. Arid Land 2016, 8, 389–398. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Zunzunegui, M.; Barradas, M.C.D.; Tirado, R.; Clavijo, A.; Novo, F.G. Comparison of proline accumulation in two mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Thomey, M.L.; Collins, S.L.; Friggens, M.T.; Brown, R.F.; Pockman, W.T. Effects of monsoon precipitation variability on the physiological response of two dominant C4 grasses across a semiarid ecotone. Oecologia 2014, 176, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.A.; Zhang, J.; Zhou, X.; Zhao, X.Y.; Wang, S.K.; Lian, J.; Lv, P.; Knops, J. Changes in carbon and nitrogen storage along a restoration gradient in a semiarid sandy grassland. Acta Oecol. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Liu, X.P.; He, Y.H.; Zhang, T.H.; Zhao, X.Y.; Li, Y.Q.; Zhang, L.M.; Wei, S.L.; Yun, J.Y.; Yue, X.F. The response of infiltration depth, evaporation, and soil water replenishment to rainfall in mobile dunes in the Horqin Sandy Land, Northern China. Environ. Earth Sci. 2015, 73, 8699–8708. [Google Scholar] [CrossRef]
- Xu, C.L.; Li, Z.Z. Population regulation and dynamical simulation of annual plant Eragrostis poaeoides in desert region. Acta Bot. Boreali-Occident. Sin. 2002, 22, 1415–1420. [Google Scholar]
- Chang, X.L.; Zhao, A.F.; Li, S.G. Responses of species discovery to precipitation change on fixed-dunes of the Naiman banner region. Acta Physiologia Sinica 2000, 24, 147–151. (In Chinese) [Google Scholar]
- Swemmer, A.M.; Knapp, A.K.; Snyman, H.A. Intra-seasonal precipitation patterns and above-ground productivity in three perennial grasslands. J. Ecol. 2007, 95, 780–788. [Google Scholar] [CrossRef]
- Yang, Z.L.; Du, W.X.; Hou, Q.; Li, X.; Wang, B. Regional analysis of climate change in the east of Inner Mongolia and its potential productivity of grassland. Chin. J. Grassl. 2008, 30, 62–66. (In Chinese) [Google Scholar]
- Cheng, X.L.; An, S.Q.; Li, B.; Chen, J.Q.; Lin, G.H.; Liu, Y.H.; Luo, Y.Q.; Liu, S.R. Summer rain pulse size and rainwater uptake by three dominant desert plants in a desertified grassland ecosystem in northwestern China. Plant Ecol. 2006, 184, 1–12. [Google Scholar] [CrossRef]
- Shi, Z.J.; Gao, J.X.; Xu, L.H.; Feng, C.; Lv, S.; Shang, J. Effect of vegetation on changes of temperature and precipitation in Inner Mongolia, China. Ecol. Environ. Sci. 2011, 20, 1594–1601. (In Chinese) [Google Scholar]
- Wang, Z.Y.; Ding, Y.H.; He, J.H. An updating analysis of the climate change in China in recent 50 years. Acta Meteorol. Sin. 2004, 62, 228–236. (In Chinese) [Google Scholar]
- Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gross, K.L.; Gherardi, L.A.; Hallett, L.M.; Hobbs, R.J.; Hsu, J.S.; Turnbull, L. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 2013, 94, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.L.; Yang, S.Q.; Huang, Q.R.; Zuo, J.C.; Chen, J.L.; Li, Z. The relationship between growth of Caraganas stenophylla and the activities and isoforms of protective enzymes under different depths of sand burial. Acta Ecol. Sin. 2015, 35, 3014–3022. (In Chinese) [Google Scholar]
- Poulson, T. Autogenic, Allogenic, and Individualistic Mechanisms of Dune Succession at Miller, Indiana. Nat. Areas J. 1999, 19, 172–176. [Google Scholar]
- Luo, Y.Y.; Zhao, X.Y.; Zhou, R.l.; Zuo, X.A.; Zhang, J.H.; Li, Y.Q. Physiological acclimation of two psammophytes to repeated soil drought and rewatering. Acta Physiol. Plant. 2011, 33, 79–91. [Google Scholar] [CrossRef]
- Zhou, S.S.; Li, M.J.; Guan, Q.M.; Liu, F.; Zhang, S.; Chen, W.; Yin, L.; Qin, Y.; Ma, F. Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in Malus. Plant Sci. 2015, 236, 44–60. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, D.M.; Hu, S.X.; Gao, W.L.; Zhao, Q.Y.; Xie, R.D.; Pan, C.H. Medicinal plant resources of Polygonaceae and Convolvulaceae in Minqin Lian Ancient City National Nature Reserve, Gansu Province. Gansu Technol. 2017, 33, 138–140. (In Chinese) [Google Scholar]
- Li, X.; Zhao, W.Z. Effects of salt-alkaline mixed stresses on seed germination and seedling growth of Bassia dasyphylla in desert region. J. Desert Res. 2018, 38, 300–306. [Google Scholar]
- Su, Y.G.; Li, X.R.; Zheng, J.G.; Huang, G. The effect of biological soil crusts of different successional stages and conditions on the germination of seeds of three desert plants. J. Arid Environ. 2009, 73, 931–936. [Google Scholar] [CrossRef]
- Zhou, R.L.; Hou, Y.P.; Wang, Y.; Zuo, J.C. The physiological adaptation mechanisms of four common desert species in response to desert environments. Acta Ecol. Sin. 2015, 35, 14–24. [Google Scholar]
- Zhao, H.L.; Zhou, R.L.; Zhang, T.H.; Zhao, X.Y. Effects of desertification on soil and crop growth properties in Horqin sandy cropland of Inner Mongolia, north China. Soil Tillage Res. 2006, 87, 175–185. [Google Scholar] [CrossRef]
- Li, J.; Qu, H.; Zhao, H.L.; Zhou, R.L.; Yun, J.Y.; Pan, C.C. Growth and physiological responses of Agriophyllum squarrosum to sand burial stress. J. Arid Land 2015, 7, 94–100. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, X.P.; Zhao, X.Y.; Zhang, T.H.; Yue, X.F.; Yun, J.Y. Response of sandy vegetation characteristics to precipitation change in Horqin Sandy Land. Acta Ecol. Sin. 2014, 34, 2737–2745. (In Chinese) [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Drazkiewicz, M.; Skórzyńska-Polit, E.; Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 2004, 17, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sundar, D.; Perianayaguy, B.; Reddy, A.R. Localization of antioxidant enzymes in the cellular compartments of sorghum leaves. Plant Growth Regul. 2004, 44, 157–163. [Google Scholar] [CrossRef]
- An, Y.Y.; Liang, Z.S.; Zhao, R.K.; Zhang, J.; Wang, X.J. Organ-dependent responses of Periploca sepium to repeated dehydration and rehydration. South Afr. J. Bot. 2011, 77, 446–454. [Google Scholar] [CrossRef][Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Shen, S. Effect of salt on malondialdehyde and antioxidant enzymes in seedling roots of Jerusalem artichoke (Helianthus tuberosus L.). Acta Physiol. Plant. 2011, 33, 273–278. [Google Scholar] [CrossRef]
- Han, C.; Liu, Q.; Yang, Y. Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regul. 2009, 58, 153–162. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, W.X.; Cheng, Z.G.; Liu, Y. Spatial-temporal changes of climate and vegetation cover in the semi-arid and arid regions of China during 1982–2006. Ecol. Environ. Sci. 2012, 21, 268–272. [Google Scholar]
- Dube, O.P.; Pickup, G. Effects of rainfall variability and communal and semi-commercial grazing on land cover in Southern African rangelands. Clim. Res. 2001, 17, 195–208. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Tian, C.Y.; Li, X.L. Response of grass growth and productivity to enhanced water input in ephemeral desert grassland in Gurbantunggut desert. Acta Ecol. Sin. 2009, 29, 1859–1868. (In Chinese) [Google Scholar]
- Meserve, P.L.; Kelt, D.A.; Milstead, W.B.; Gutiérrez, J.R. Thirteen years of shifting top-down and bottom-up control. Bioscience 2003, 53, 633–646. [Google Scholar] [CrossRef]
- Barker, M.G.; Booth, W.E. Vertical profiles in a Brunei rain forest: II. Leaf characteristics of dryobalanops lanceolata. J. Trop. For. Sci. 1996, 9, 52–66. [Google Scholar]
- Huang, Y.X.; Zhao, X.Y.; Zhou, D.W.; Zhao, H.L.; Zhang, H.X.; Zuo, X.A.; Mao, W. Allometry of Salsola collina in response to soil nutrients, water supply and population density. Nord. J. Bot. 2010, 27, 539–547. [Google Scholar] [CrossRef]
- Tobe, K.; Zhang, L.; Omasa, K. Seed germination and seedling emergence of three annuals growing on desert sand dunes in China. Ann. Bot. 2005, 95, 649–659. [Google Scholar] [CrossRef]
- Liu, Y.X.; Lan, X.X.; Cao, J.; Zhang, J.H.; Lan, H.Y. Screening of qRT-PCR reference genes for Chenopodium album and C. glaucum of Chenopodiaceae. Guihaia 2016, 36, 1511–1518. (In Chinese) [Google Scholar]
- Cakmak, I.; Marschner, H. Effect of zinc nutritional status on activities of superoxide radical and hydrogen peroxide scavenging enzymes in bean leaves. Plant Soil 1993, 155–156, 127–130. [Google Scholar] [CrossRef]
- Jouili, H.; Ferjani, E. Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess. C. R. Biol. 2003, 326, 639–644. [Google Scholar] [CrossRef]
- Cutler, J.M.; Shahan, K.W.; Steponkus, P.L. Influence of water deficits and osmotic adjustment on leaf elongation in rice. Crop Sci. 1980, 20, 314–318. [Google Scholar] [CrossRef]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2010, 190, 6–12. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).