Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review
Abstract
:1. Introduction
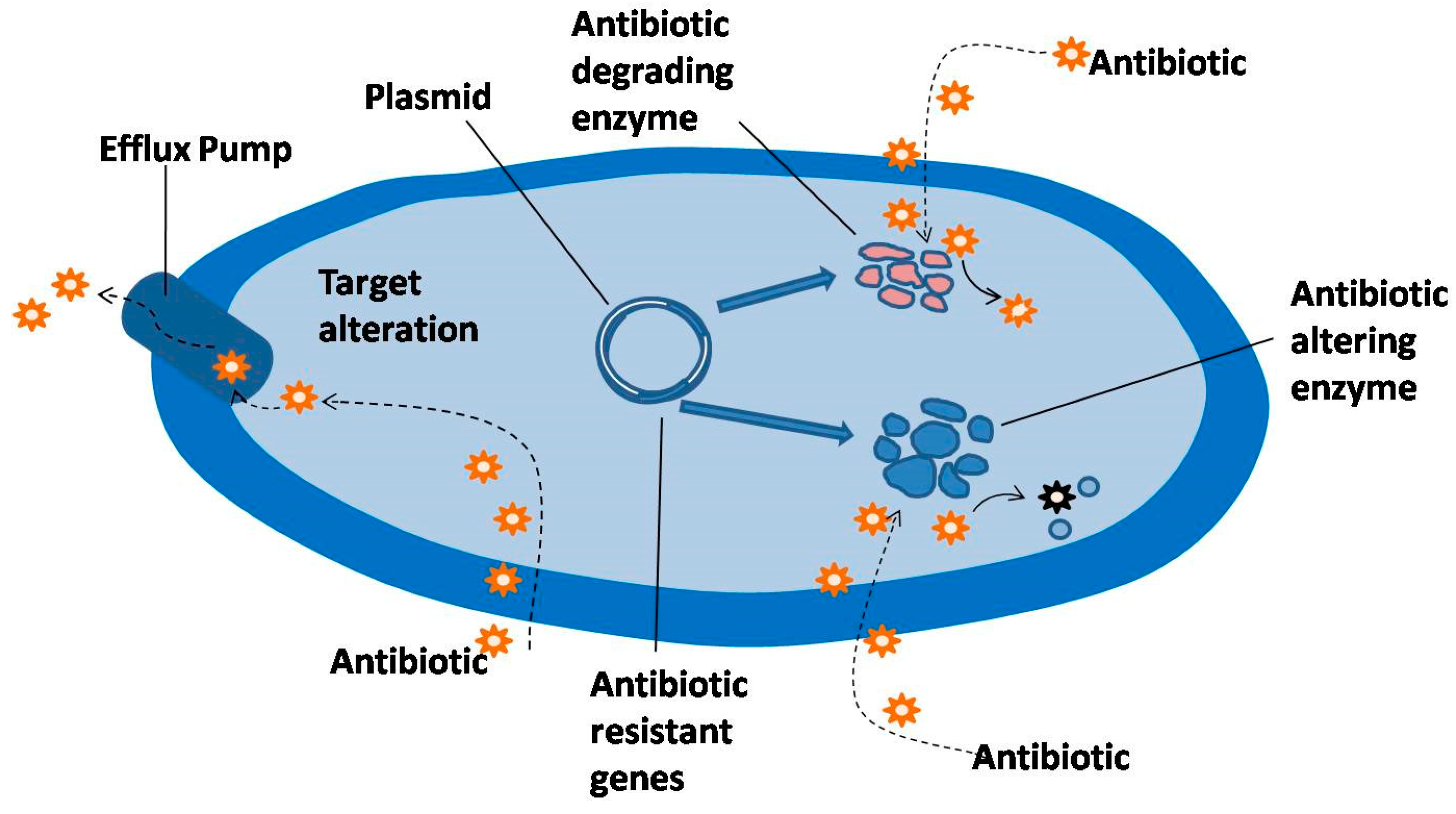
2. Mechanism of Resistance to Antibacterial Agents
2.1. Antibiotic Inactivation
2.2. Target Modification
2.3. Efflux Mechanism of Resistance
2.4. Plasmidic Efflux
3. Active Compounds of Plants with Antimicrobial Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| S. No. | Name of Plants | Antimicrobials | Microbes treated | References |
|---|---|---|---|---|
| 1. | Acacia nilotica | Terpenoids, flavanoid, Saponins, Tannins | S. viridians, S. aureus, E. coli, B. subtilis, Shigella sonnei, MDR E. coli, C. albican, K. pneumoniae | [35,48] |
| 2. | Allium cepa | Flavanoid, Polyphenol | MDR Pseudomonas aeruginosa, S. Typhi, E. coli | [49] |
| 3. | Allium sativum | Organosulphur compounds (Phenolic compounds), Allicin | Campylobacter jejuni, MDR E. coli, C. albican, Entamoeba histolytica, Giardia lamblia | [50,51] |
| 4. | Angelica lucida L. | Coumarins | S. viridians, S. mutans | [33] |
| 5. | Chelidonian majus | Glycoprotein | B. cereus, Staphylococcus spp. | [52] |
| 6. | Cinnamomum spp. | Cinnamaldehyde (essential oil) | Legionella pneumophila, MDR E. coli, C. albican, K. pneumoniae | [53] |
| 7. | Cirsium hypoleucum | Flavones | MDR K. pneumoniae | [31] |
| 8. | Curcuma longa | Curcuminoid (A phenolic compound), turmerone, curlone, Essential oil, curcumins, turmeric oil | S. typhi, E. coli, S. aureus, B. cereus, B. subtilis, Ps. aeruginosa, B. coagulans, A. niger, P. digitatum, Antifungal and antiviral activity | [54] |
| 9. | Cymbopogan citratus | Essential oil | C. albicals, Aspergillus flavus, A. parasiticus | [36] |
| 10. | Galium fissurense | Flavones | MDR K. pneumoniae | [31] |
| 11. | Hypericum perforatum | Hypericin (anthraquinone) | Methicillin Resistant Staphylococcus aureus and Methicillin sensitive Staphylococcus | [25] |
| 12. | Lawsonia inermis | Quinones | MDR Pseudomonas aeruginosa | [24] |
| 13. | Medicago sativa | Saponins, Canavanine | Enterococcus faecium, S. aureus, Antifungal | [55] |
| 14. | Mentha longifoilia | Essential oil | MDR Staphylococcus aureus | [56] |
| 15. | Ocimum basilicum | Essential oil | MDR Staphylococcus aureus, S. Typhi, Aeromonas hydrophila, Pseudomonas spp. | [57] |
| 16. | Onobrychis sativa | AMPs (antimicrobial peptides) | E. faecium, S. aureus | [55] |
| 17. | Origanum vulgare | Essential oil | B. subtilis, B. cereus, MDR Staphylococcus aureus | [58] |
| 18. | Piper longum | Piperine, Saponin, alkaloid | MDR B. subtilis, Shigella sonnei | [59] |
| 19. | Raphanus sativum | RsAFP2 (Antifungal peptide) | C. albicans | [60] |
| 20. | Rhazy astricta | Alkaloids and Non alkaloids | MDR E. coli, K. pneumoniae (ESBL), E. faecium(VRE) | [61] |
| 21. | Rosmarinus officinalis | Essential oil | Streptococcus mutans | [58] |
| 22. | Sanguisorba officianalis | Alkaloids, antimicrobial peptides | Ps. aeruginosa, E. coli | [52] |
| 23. | Sorghum spp. | Tannins | S.aureus, S. typhimurium, A. niger, A. flavus, S. cerevisae | [39] |
| 24. | Stephania glabra | Alkaloids | S. aureus, S. mutans, Microsporum gypseum, M. canis and Trichophyton rubrum | [27] |
| 25. | Syzygium aromaticum | Essential oil, Eugenol | Streptococcus mutans, Staphylococcus aureus, Lactobacillus acidophilus, Candida albicans and Saccharomyces cerevisiae, MDR E. coli, K. pneumoniae | [59] |
| 26. | Vetiveria ziznioides | Vetivone (vetiver oil) | Enterobacter spp. | [62] |
| 27. | Viscum album | Flavones | MDR K. pneumoniae | [31] |
| 28. | Zingiber officinale | Gingerol | E. coli, Enterobacter spp., P. aeruginosa, Proteus spp., Klebsiella spp., S. aureus and Bacillus spp. | [49,63] |
References
- Iwu, M.W.; Duncan, A.R.; Okunji, C.O. New antimicrobials of plant origin. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 457–462. [Google Scholar]
- Bush, K. Antibacterial drug discovery in the 21st century. Clin. Microbiol. Inf. 2004, 10, 10–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antimicrobial Resistance; Fact Sheet No. 194; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Styers, D.; Sheehan, D.J.; Hogan, P.; Sahm, D.F. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microb. Antimicrob. 2006, 5. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Moll, A.; Sturm, A.W.; Pawinski, R.; Govender, T.; Lalloo, U.; Zeller, K.; Andrews, J.; Friedland, G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006, 368, 1575–1580. [Google Scholar] [CrossRef]
- Venkateswaran, P.S.; Millman, I.; Blumberg, B.S. Effect of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: In vitro and in vivo studies. Proc. Natl. Acad. Sci. USA 1987, 84, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Thakur, B.; Singh, P.; Singh, H.B.; Sharma, V.D.; Katoch, V.M.; Chauhan, S.V.S. Anti-tuberculosis activity of selected medicinal plants against multi drug resistant Mycobacterium tuberculosis isolates. Ind. J. Med. Res. 2010, 131, 809–813. [Google Scholar]
- Anderson, D.I. The ways in which bacteria resist antibiotics. Int. J. Risk Saf. Med. 2005, 17, 111–116. [Google Scholar]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C.J. β-Lactam antibiotic resistance: A current structural perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamase from Gram-negative bacteria. Ann. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Kataja, J.; Seppala, H.; Skurnik, M.; Sarkkinen, H.; Huovinen, P. Different erythromycin resistance mechanisms in group C and group G Streptococci. Antimicrob. Agents Chemother. 1998, 42, 1493–1494. [Google Scholar] [PubMed]
- Okuma, K.; Iwakawa, K.; Turnidge, J.D.; Grubb, W.B.; Bell, J.M.; O’Brien, F.G.; Coombs, G.W.; Pearman, J.W.; Tenover, F.C.; Kapi, M.; et al. Dissemination of New Methicillin-Resistant Staphylococcus aureus Clones in the Community. J. Clin. Microbiol. 2002, 40, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K. Vancomycin-resistant Staphylococcus aureus: A new model of antibiotic resistance. Lancet Infect. Dis. 2001, 1, 147–155. [Google Scholar] [CrossRef]
- Happi, C.T.; Gbotosho, G.O.; Folarin, O.A.; Akinboye, D.O.; Yusuf, B.O.; Ebong, O.O.; Sowunmi, A.; Kyle, D.E.; Milhous, W.; Wirth, D.T.; et al. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfaxine-pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop. 2005, 95, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Taylor, D.E. Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 1998, 42, 1952–1958. [Google Scholar] [PubMed]
- Suzuki, Y.; Katsukawa, C.; Tamaru, A.; Abe, C.; Makino, M.; Mizuguch, Y.; Taniguchi, I.H. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J. Clin. Microbiol. 1998, 36, 1220–1225. [Google Scholar] [PubMed]
- Lomovskaya, O.; Bostian, K.A. Practical applications and feasibility of efflux pump inhibitors in the clinic—A vision for applied use. Biochem. Pharmacol. 2006, 7, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Michel, L.O.; Zhang, Q. Cme ABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002, 46, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Wardal, E.; Sadowy, E.; Hryniewicz, W. Complex nature of Enterococcal pheromone-responsive plasmids. Pol. J. Microbiol. 2010, 59, 79–87. [Google Scholar] [PubMed]
- Martinez-Martinez, L.; Pascual, A.; Jacoby, G.A. Quinolone resistance from a transferable plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Habbal, O.; Hasson, S.S.; El-Hag, A.H.; Al-Mahrooqui, Z.; Al-Hashmi, N.; Al-Balushi, M.S.; Al-Jabri, A.A. Antibacterial activity of Lawsonia inermis Linn (Henna) against Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2011, 1, 173–176. [Google Scholar] [CrossRef]
- Dadgar, T.; Asmar, M.; Saifi, S.; Mazandarani, M.; Bayat, H.; Moradi, A.; Bazueri, M.; Ghaemi, F. Antibacterial activity of certain Iranian medicinal plants against methicillin resistant and methicillin sensitive Staphylococcus aureus. Asian J. Plant Sci. 2006, 5, 861–866. [Google Scholar]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217–5223. [Google Scholar] [PubMed]
- Semwal, D.K.; Rawat, U. Antimicrobial hasubanalactam alkaloid from Stephania glabra. Planta Med. 2009, 75, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Gabor, M. Anti-inflammatory and anti-allergic properties of flavonoids. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological and Structure-Activity Relationships; Cody, V., Middleton, V.E., Harbourne, J.B., Eds.; Alan R. Liss: New York, NY, USA, 1986; pp. 471–480. [Google Scholar]
- Beschia, M.; Leonte, A.; Oancea, I. Phenolic compounds with biological activity. Bull. Univ. Galati Faso 1984, 6, 23–27. [Google Scholar]
- Thara, S.; Ingham, J.; Nakahara, S.; Mizutani, J.; Harborne, J.B. Fungitoxic dihydrofuranoisoflavones and related compounds in white lupin, Lupinus albus. Phytochemistry 1984, 23, 1889–1900. [Google Scholar] [CrossRef]
- Ozcelik, B.; Deliorman, O.D.; Ozgen, S.; Ergun, F. Antimicrobial Activity of Flavonoids against Extended-Spectrum Beta Lactamase (ESBL)-Producing Klebsiella pneumoniae. Trop J. Pharm. Res. 2008, 7, 1151–1157. [Google Scholar] [CrossRef]
- O'Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Widelski, J.; Popova, M.; Graikou, K.; Glowniak, K.; Chinou, I. Coumarins from Angelica lucida L.—Antibacterial Activities. Molecules 2009, 14, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of Coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Banso, A. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J. Med. Plants Res. 2009, 3, 82–85. [Google Scholar]
- Ragaso, C.Y. Antimicrobial and Cytotoxic terpenoid from Cymbopogon citratus Stapf. Philipp. Sci. 2008, 45, 111–122. [Google Scholar] [CrossRef]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. Red wine, tea and anti-oxidants. Lancet 1994, 344, 626. [Google Scholar] [CrossRef]
- Jones, S.B., Jr.; Luchsinger, A.E. Plant Systematics, 2nd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1986. [Google Scholar]
- Moneim, A.; Suleman, E.; Issa, F.M.; Elkhalifa, E.A. Quantitative determination of tannin content in some sorghum cultivars and evaluation of its antimicrobial activity. Res. J. Microbiol. 2007, 2, 284–288. [Google Scholar]
- Garcia-Olmedo, F.; Molina, A.; Sefura, A.; Moreno, M. The defensive role of nonspecific lipid transfer proteins in plants. Trends Microbiol. 1995, 3, 72–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Lewis, K. Fabatins: New antimicrobial plant peptides. FEMS Microbiol. Lett. 1997, 149, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Segura, A.; Garcia-Olmedo, F. Pseudothionin-St1, a potato peptide active against potato pathogens. Eur. J. Biochem. 1994, 223, 135–139. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Chu, H.B.; Zhang, Y.M.; Han, H.J.; Yan, H.; Zeng, G.Z.; Fu, Z.H.; Olubanke, O.; Tan, N.H. Antimicrobial, cytotoxic lignans and terpenoids from the twigs of Pseudolarix kaempferi. Planta Med. 2011, 77, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Hakala, E.; Hanski, L.; Uvell, H.; Yrjonen, T.; Vuorela, H.; Elofsson, M.; Vuorela, P.M. Dibenzocyclooctadiene lignans from Schisandra spp. selectively inhibit the growth of the intracellular bacteria Chlamydia pneumoniae and Chlamydia trachomatis. J. Antibiot. 2015, 68, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Mazumdaer, A.; Dwivedi, A.; du Plessis, J. Sinigrin and its therapeutic bebefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Al-Gendy, A.A.; Nematallah, K.A.; Zaghloul, S.S.; Ayoub, N.A. Glucosinolates profile, volatile constituents, antimicrobial and cytotoxic activities of Lobularia libyca. Pharm. Biol. 2016, 54, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Losee, L.L.; Tanja, S.; Aaron, J.P.; Amy, L.S.; Ina, E.; Brian, P.C.; Anna, M.; Till, F.S.; Dallas, E.H.; Slava-Michael, J.; et al. A new antibiotic kills pathogen without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar]
- Riaz, S.; Faisal, M.; Hasnain, S.; Khan, N.A. Antibacterial and cytotoxic activities of Acacia nilotica L (Mimosaceae) methanol extract against extended spectrum beta lactamase producing E. coli and Klebsiella species. Trop. J. Pharm. Res. 2011, 10, 785–791. [Google Scholar] [CrossRef]
- Adesino, G.O.; Jibo, S.; Aggu, V.E.; Ehinmidu, J.O. Antibacterial activity of fresh juices of Allium cepa and Zingiber ofiicinale against multidrug resistant bacteria. Int. J. Pharm. Biol. Sci. 2011, 2, 289–295. [Google Scholar]
- Lu, X.; Rasco, B.A.; Jabal, J.M.; Aston, D.E.; Lin, M.; Konkel, M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 2011, 77, 5257–5269. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microb. Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Janovska, D.; Kubikova, K.; Kokoska, L. Screening for antimicrobial activity of some medicinal plants species of traditional Chinese medicine. Czech J. Food Sci. 2003, 21, 107–110. [Google Scholar]
- Chang, C.; Chang, W.; Chang, S.; Cheng, S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Gul, P.; Bakht, J. Antimicrobial activity of turmeric extract and its potential use in food industry. J. Food Sci. Technol. 2015, 52, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Aliahmadi, A.; Roghanian, R.; Emtiazi, G.; Mirzajani, F.; Ghassempour, A. Identification and primary characterization of a plant antimicrobial peptide with remarkable inhibitory effects against antibiotic resistant bacteria. Afr. J. Biotechnol. 2012, 11, 9672–9676. [Google Scholar]
- Al-Ali, K.; Abdelrazik, M.; Hemeg, H.; Ozbak, H. Antibacterial activity of four herbal extract against methicillin resistant bacterial strains isolated from patient in Almadinah hospitals, Saudi Arabia. Int. J. Acad.Sci. Res. 2015, 3, 34–40. [Google Scholar]
- Wan, J.; Wilcock, A.; Coventry, M.J. The effect of essential oil of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. J. Appl. Microbiol. 1998, 84, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Falco, E.D.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [PubMed]
- Kumar, V.; Shriram, V.; Mulla, J. Antibiotic resistance reversal of multiple drug resistant bacteria using Piper longum fruit extract. J. Appl. Pharm. Sci. 2013, 3, 112–116. [Google Scholar]
- Aerts, A.M.; Carmona-Gutierrez, D.; Lefevre, S.; Govaert, G.; Francois, I.E.; Madeo, F.; Santos, R.; Cammue, B.P.; Thevissen, K. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009, 583, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Baeshen, M.N.; Saini, K.S.; Bora, R.S.; Al-Hejin, A.M.; Baeshen, N.A. Antibacterial activities of Rhazyastricta leaf extracts against multidrug resistant human pathogens. Biotechnol. Biotechnol. Equip. 2016, 30, 1016–1025. [Google Scholar] [CrossRef]
- Srivastava, J.; Chandra, H.; Singh, N. Allelopathic response of Vetiveria zizanioides(L.) Nash on members of Enterobacteriaceae and Pseudomonas spp. Environmentalist 2007, 27, 253–260. [Google Scholar] [CrossRef]
- Karuppiah, P.; Rajaram, S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple drug resistant chemical pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 597–601. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. https://doi.org/10.3390/plants6020016
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants. 2017; 6(2):16. https://doi.org/10.3390/plants6020016
Chicago/Turabian StyleChandra, Harish, Parul Bishnoi, Archana Yadav, Babita Patni, Abhay Prakash Mishra, and Anant Ram Nautiyal. 2017. "Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review" Plants 6, no. 2: 16. https://doi.org/10.3390/plants6020016
APA StyleChandra, H., Bishnoi, P., Yadav, A., Patni, B., Mishra, A. P., & Nautiyal, A. R. (2017). Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants, 6(2), 16. https://doi.org/10.3390/plants6020016








