Defense Priming and Jasmonates: A Role for Free Fatty Acids in Insect Elicitor-Induced Long Distance Signaling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Priming by Green Leaf Volatiles Affect Local and Distal Responses to Insect Elicitors
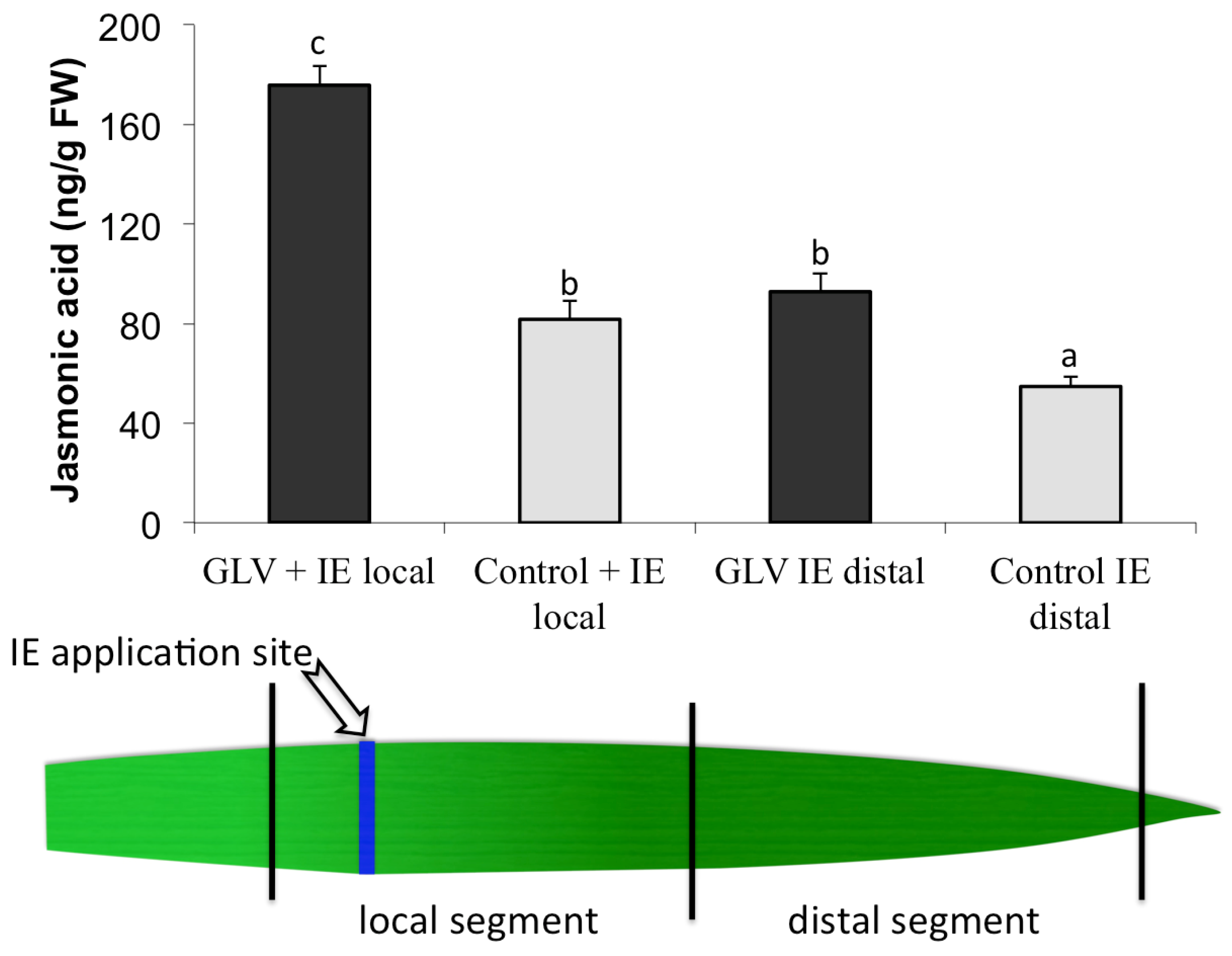
2.2. Insect Elicitor-Induced Signaling Rapidly Travels to Distant Tissues
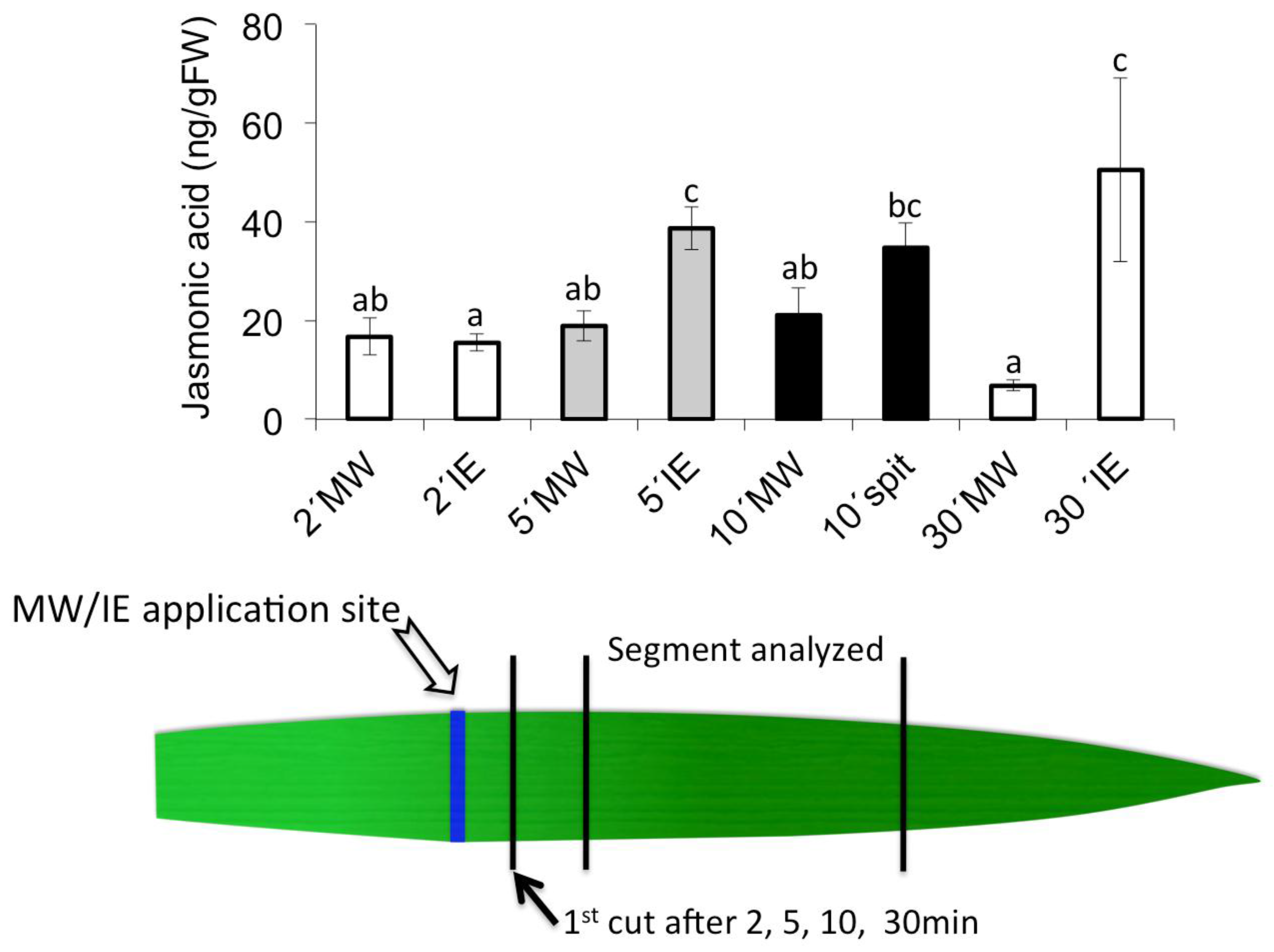
2.3. Jasmonic Acid Is Not a Mobile Signal
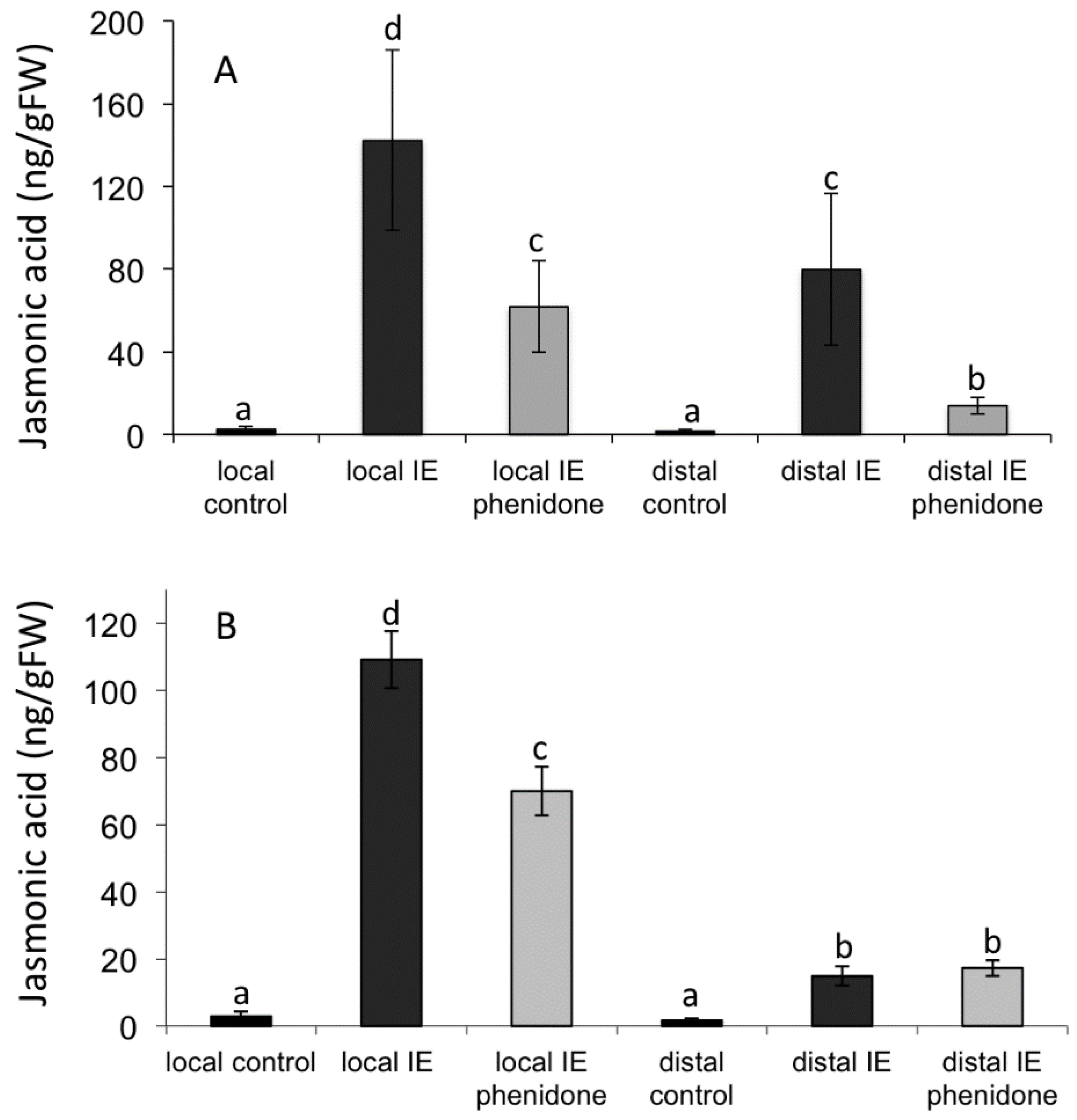
2.4. Alamethicin-Induced Signaling Leads to Distal Jasmonic Acid Accumulation

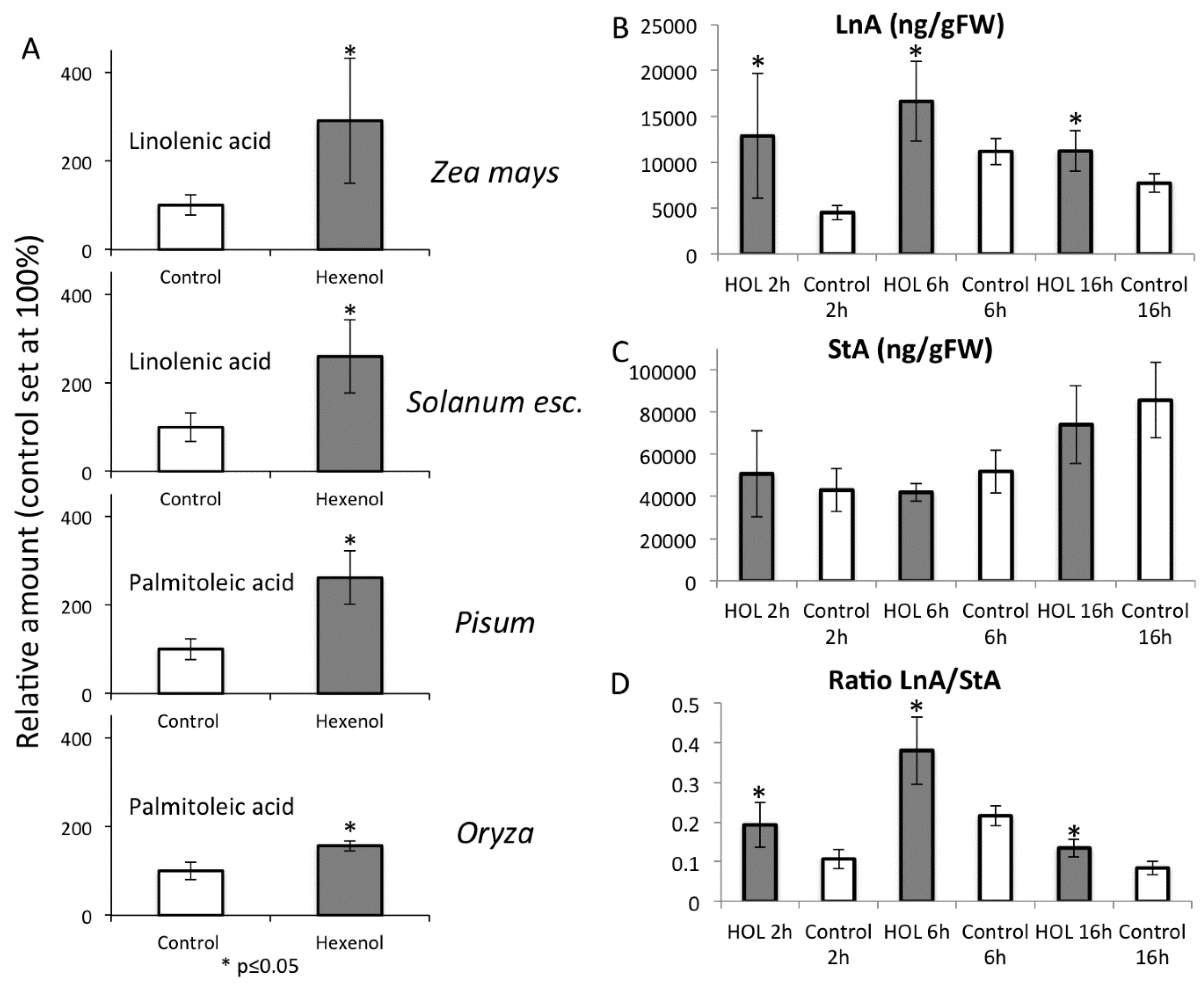
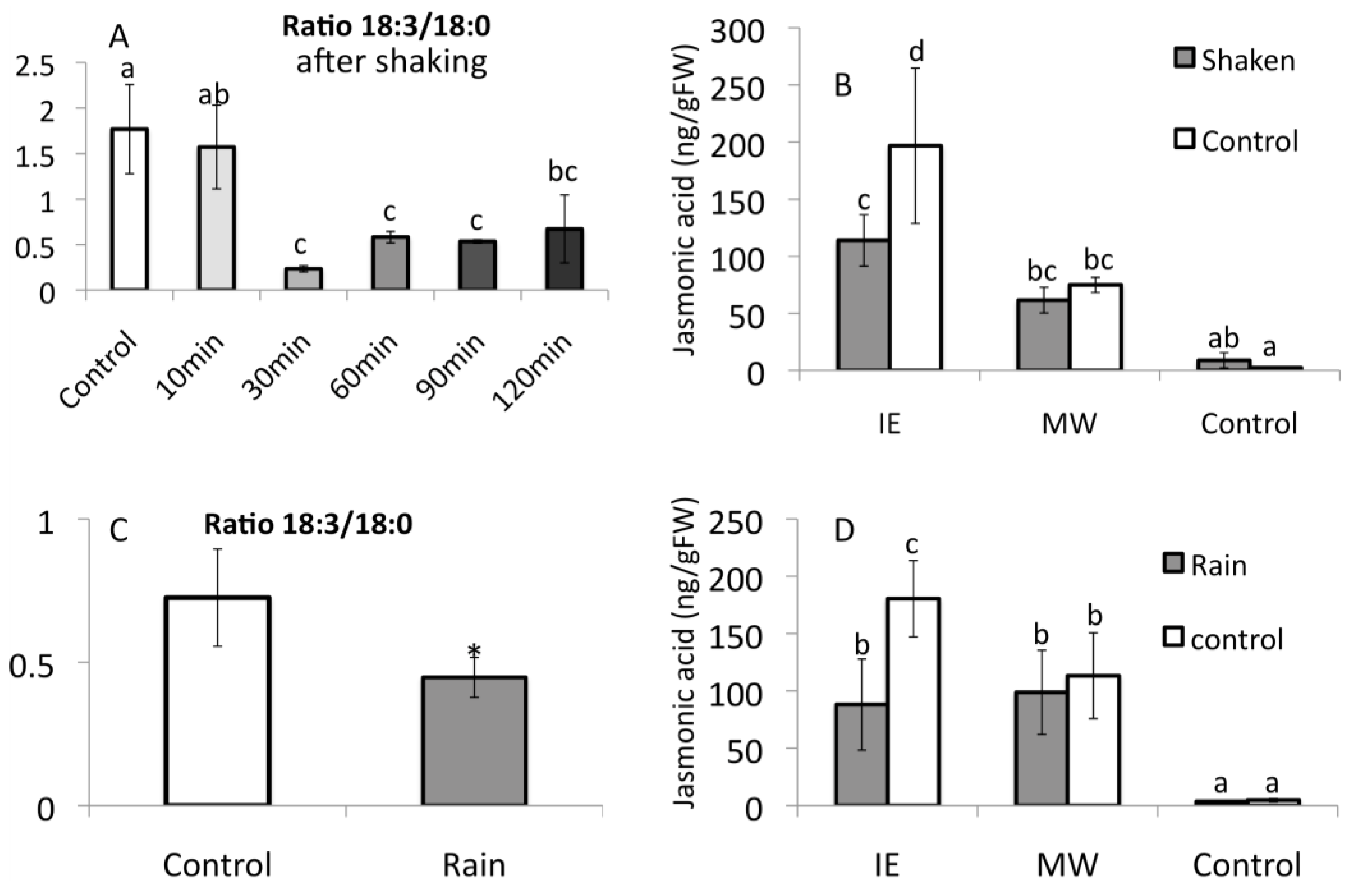
2.5. Priming by Green Leaf Volatiles Increases Distinct Free Fatty Acid in Different Plant Species
2.6. Increased Free Linolenic Acid Levels Stimulate Insect Elicitor Induced Jasmonic Acid Accumulation
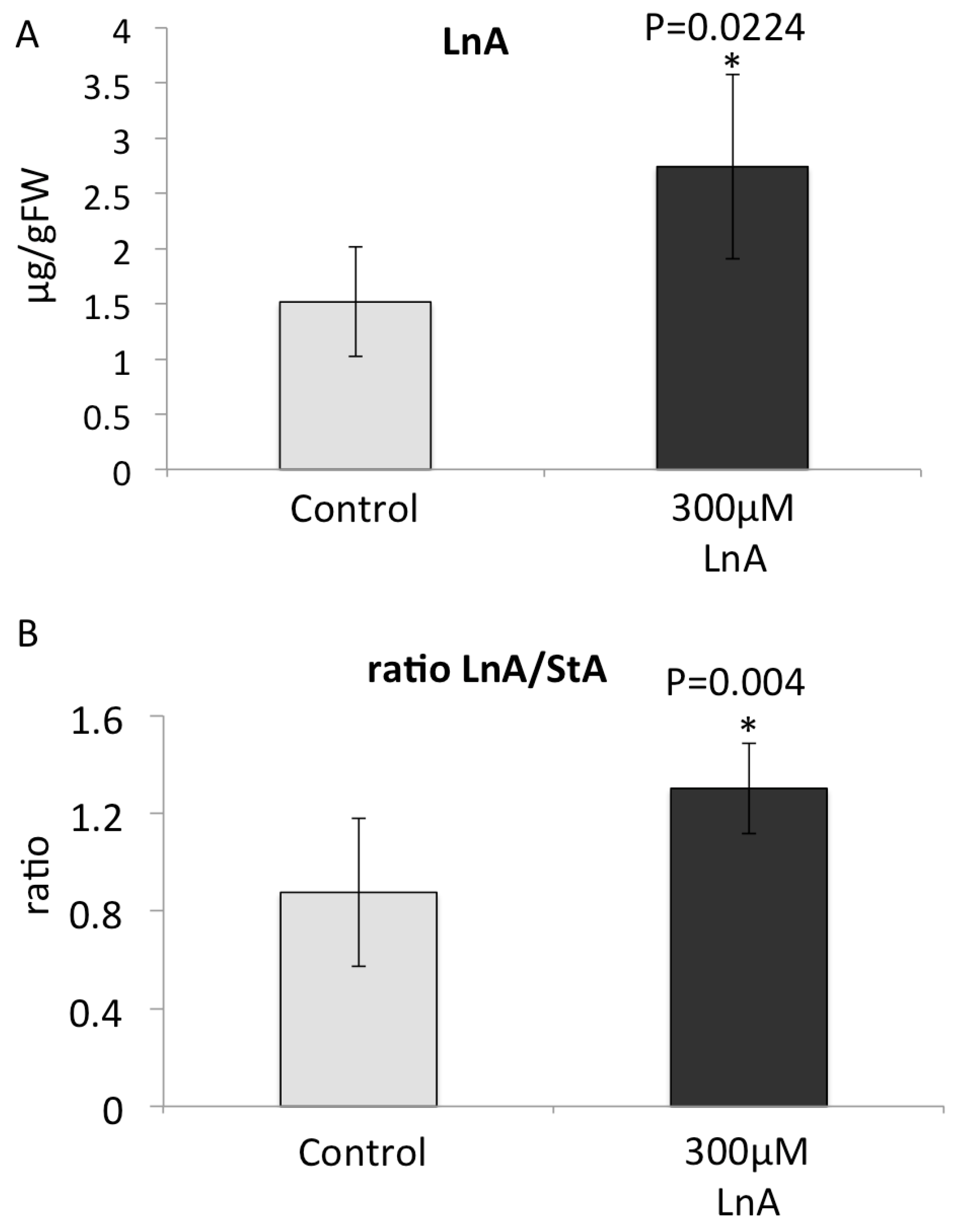
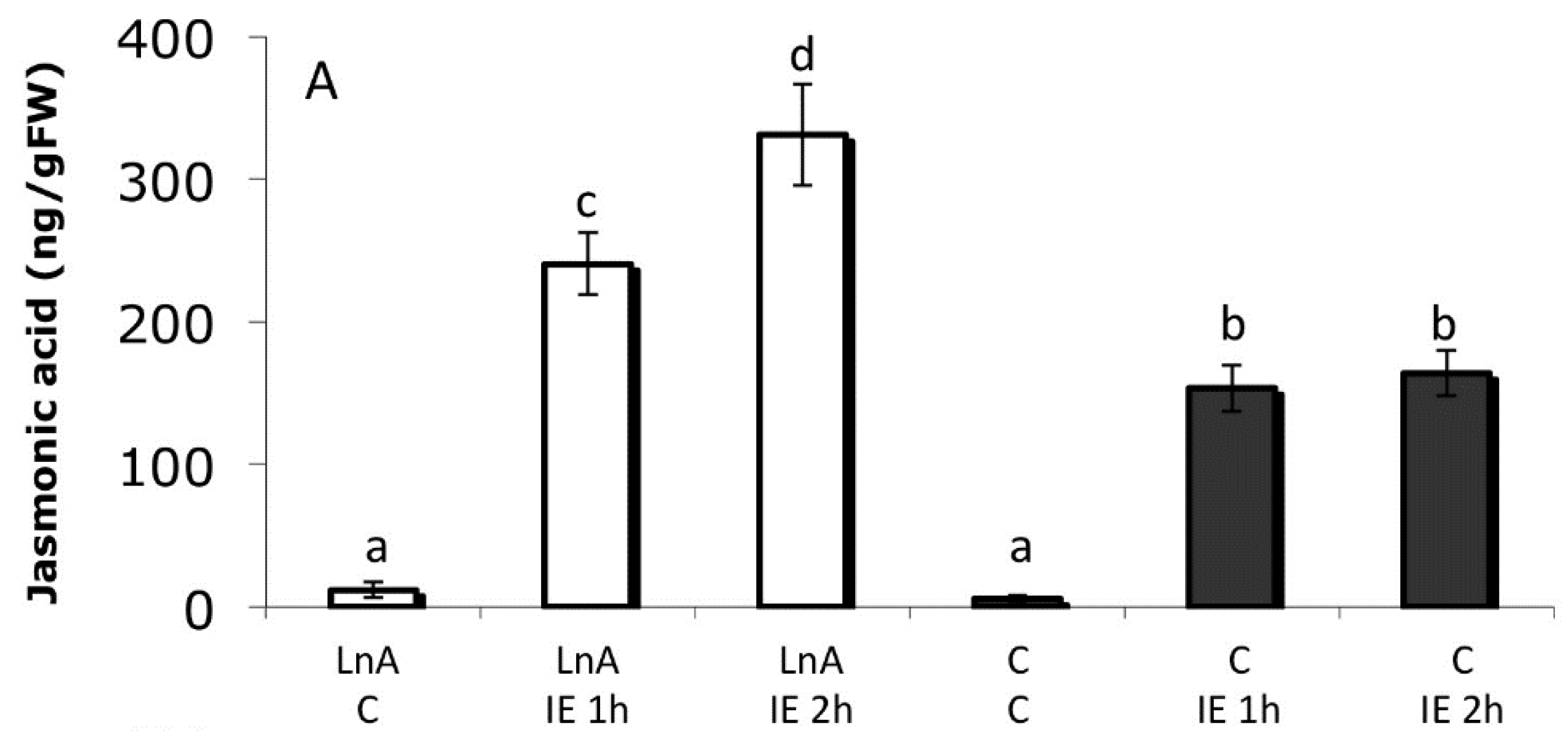
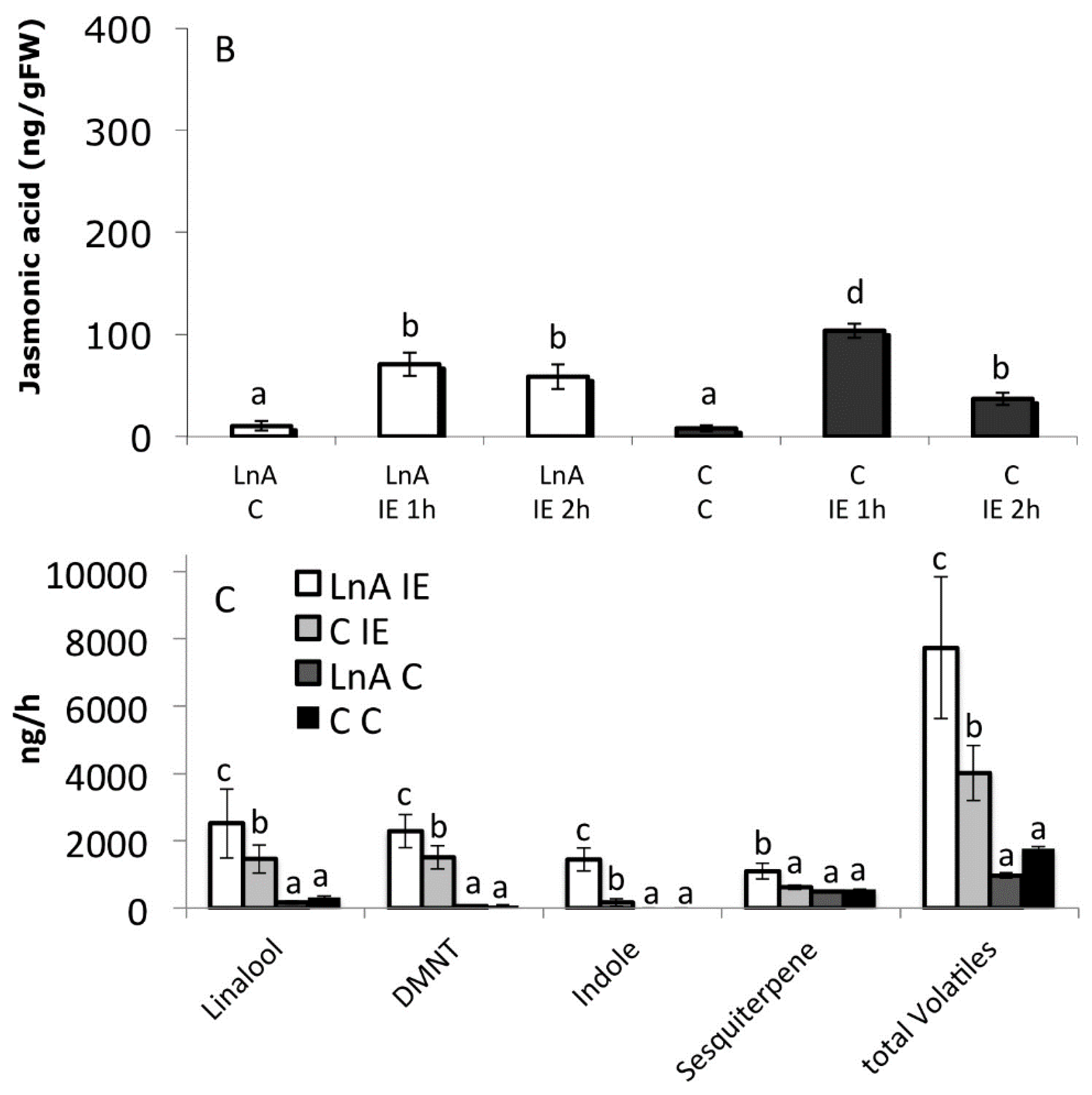
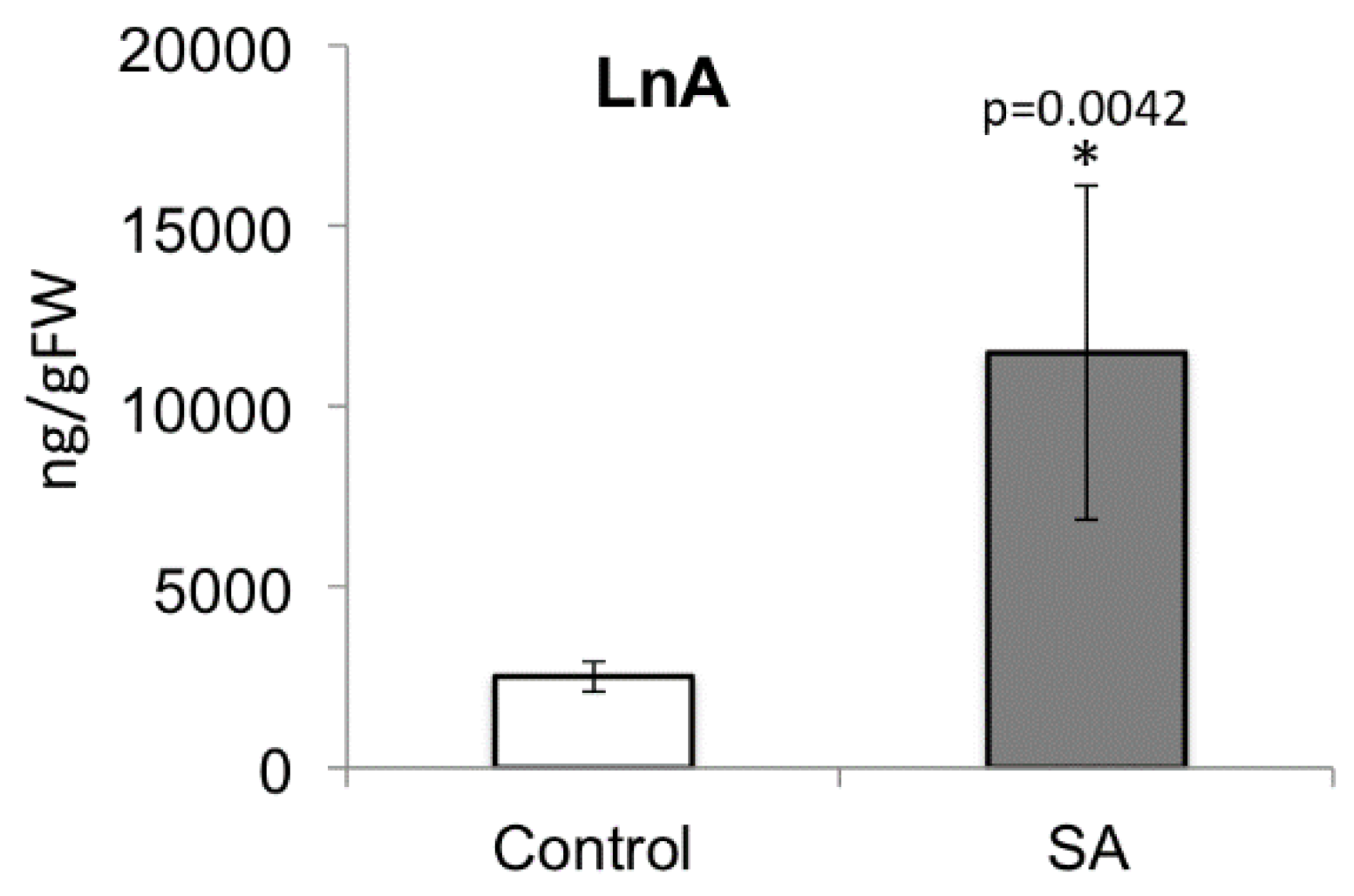
2.7. Decreased Free Linolenic Acid Levels Reduce Insect Elicitor-Induced Jasmonic Acid Accumulation
3. Experimental Section
3.1. Chemicals
3.2. Plant Material
3.3. Plant Treatments
3.3.1. Treatment of Corn Seedlings with Free α-Linolenic Acid
3.3.2. Pharmacological Effects of Phenidone on JA Accumulation
3.3.3. Effects of Movement on Free Fatty Acids and JA Accumulation
3.4. Quantification of Jasmonic Acid and Free Fatty Acids
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Campos, M.L.; Kang, J.H.; Howe, G.A. Jasmonate-triggered plant immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Howe, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochem. Biophys. Acta 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Stratmann, J.W. Long distance runs in the wound response—Jasmonic acid is pulling ahead. TIPS 2003, 8, 247–250. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Howe, G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 2005, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G. Systemin, hydroxyproline-rich systemin, and the induction of protease inhibitors. Curr. Protein Pept. Sci. 2011, 12, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Gao, X.; Jones, A.D.; Howe, G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009, 59, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.R.; Maischak, H.; Mithöfer, A.; Boland, W.; Felle, H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 2009, 149, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. Glutamate Receptor-Like genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Frasson, D. Induction of wound response gene expression in tomato leaves by ionophores. Planta 2001, 212, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Seidl-Adams, I.; Schultz, J.C.; Tumlinson, J.H. Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12-oxo phytodienoic acid reductases in Zea mays. MPMI 2007, 20, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Contreras, C.F.; Viswanathan, S. Transcriptional Analysis of Distant Signaling induced by Insect Elicitors and Mechanical Wounding in Zea mays. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [PubMed]
- Alborn, H.T.; Turlings, T.C.; Jones, T.H.; Stenhagen, G.S.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles identified from beet armyworm oral secretions. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl. Acad. Sci. USA 1998, 95, 13971–13975. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’Donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.W.; Tumlinson, J.H. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [PubMed]
- McCall, P.J.; Turlings, T.C.J.; Loughrin, J.H.; Proveaux, A.T.; Tumlinson, J.H. Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J. Chem. Ecol. 1994, 20, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Dicke, J.; van Beek, T.A.; Posthumus, M.A.; Ben Dom, N.; Van Bokhoven, H.; de Groot, A.E. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plant in its production. J. Chem. Ecol. 1990, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.J.; Turlings, T.C.J.; Lewis, W.J.; Tumlinson, J.H. Role of plant volatiles in host location by the specialist parasitoid Microplitis croceipes Cresson (Braconidae: Hymenoptera). J. Insect Behav. 1993, 6, 625–639. [Google Scholar] [CrossRef]
- Pare, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Curtius, T.; Franzen, H. Aldehyde aus gruenen Pflanzenteilen. Chem. Zentr 1911, 2, 1142–1143. [Google Scholar]
- English, J.; Bonner, J. The wound hormones of plants. I. Traumatin, the active principle of the bean test. J. Biol. Chem. 1937, 121, 791–799. [Google Scholar]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochem 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Sobhy, I.; Bosak, L.; Erb, M.; Roberts, C.; Vaughn, K.A.; Göbel, C.; Tumlinson, J.; et al. The multi-tasking lipoxygenase, ZmLOX10, regulates GLV, JA, and HIPV production for defense against insect attack. Plant J. 2012, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; de Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Shiojiri, K.; Huntzinger, M.; McCall, A.C. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology 2006, 87, 922–930. [Google Scholar] [CrossRef]
- Hirao, T.; Okazawa, A.; Harada, K.; Kobayashi, A.; Muranaka, T.; Hirata, K. Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. J. Biosci. Bioeng. 2012, 112, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defense genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [PubMed]
- Bruce, T.J.; Matthes, M.C.; Chamberlain, K.; Woodcock, C.M.; Mohib, A.; Webster, B.; Smart, L.E.; Birkett, M.A.; Pickett, J.A.; Napier, J.A. Cis-jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA 2008, 25, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Matthes, M.C.; Bruce, T.J.; Ton, J.; Verrier, P.J.; Pickett, J.A.; Napier, J.A. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 2010, 232, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.A.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Mani, B. Priming in plant-pathogen interactions. TIPS 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Monitoring plant hormones during stress responses. JoVE 2009, 28. [Google Scholar] [CrossRef] [PubMed]
- Truitt, C.L.; Wei, H.X.; Paré, P.W. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 2004, 16, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Atwell, B.J.; Kriedemann, P.E.; Turnbull, C.G.N. Plants in Action: Adaptation in Nature, Performance in Cultivation, 1st ed.; Macmillan Publishers: South Yarra, Australia, 1999. [Google Scholar]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signaling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res. Notes 2011, 4, 377. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Bossi, S.; Spiteller, D.; Mithöfer, A.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 2004, 134, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Koch, T.; Schüler, G.; Bachmann, N.; Rechtenbach, J.; Boland, W. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 2001, 125, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Koch, T.; Kühnemann, F.; Boland, W. Channel-Forming Peptaibols Are Potent Elicitors of Plant Secondary Metabolism and Tendril Coiling. Angew. Chem. Int. Ed. 2000, 39, 1860–1862. [Google Scholar] [CrossRef]
- Maischak, H.; Zimmermann, M.R.; Felle, H.H.; Boland, W.; Mithöfer, A. Alamethicin-induced electrical long distance signaling in plants. Plant Signal. Behav. 2010, 5, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early Transcriptome Analyses of Z-3-Hexenol-Treated Zea mays Revealed Distinct Transcriptional Networks and Anti-Herbivore Defense Potential of Green Leaf Volatiles. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Engelberth, M.; Viswanathan, S. Low concentrations of salicylic acid stimulate insect elicitor responses in corn (Zea mays) seedlings. J. Chem. Ecol. 2011, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Unpublished Work. Department of Biology, University of Texas at San Antonio: San Antonio, TX, USA, 2015.
- Chehab, E.W.; Eich, E.; Braam, J. Thigmomorphogenesis: A complex plant response to mechanostimulation. J. Exp. Bot. 2009, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Graber, R.; Sumida, C.; Nunez, E.A. Fatty acids and cell signal transduction. J. Lipid Mediat. Cell Signal. 1994, 9, 91–116. [Google Scholar] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods-a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochem. Biophys. Acta 2014. [Google Scholar] [CrossRef] [PubMed]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Georgiadi, A.; Kersten, S. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 2012, 3, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohammed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionary conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Conconi, A.; Miquel, M.; Browse, J.A.; Ryan, C.A. Intracellular levels of free linolenic acids increase in tomato leaves in response to wounding. Plant Physiol. 1996, 111, 797–803. [Google Scholar] [PubMed]
- Runyon, J.B.; Mescher, M.C.; Felton, G.W.; de Moraes, C.M. Parasitism by Cuscuta pentagona sequentially induces JA and SA defence pathways in tomato. Plant Cell Environ. 2010, 33, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Krumm, T.; Jung, V.; Engelberth, J.; Boland, W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid signaling pathway. Plant Physiol. 1999, 121, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, E.; Meskiene, I.; Hirt, H. Short communication: Unsaturated fatty acids inhibit MP2C, a protein phosphatase 2C involved in the wound-induced MAP kinase pathway regulation. Plant J. 1999, 20, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Blümke, A.; Falter, C.; Herrfurth, C.; Sode, B.; Bode, R.; Schäfer, W.; Feussner, I.; Voigt, C.A. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol. 2014, 165, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Sode, B.; Falter, C.; Voigt, C. Resistance of callose synthase activity to free fatty acid inhibition as an indicator of Fusarium head blight resistance in wheat. Plant Signal. Behav. 2014, 9, e28982. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Chandra-Shekara, A.C.; Jeong, R.D.; Yu, K.; Zhu, S.; Chanda, B.; Navarre, D.; Kachroo, A.; Kachroo, P. Oleci acid-dependent modulation of Nitric Oxide Associated 1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell 2012, 24, 1654–1674. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Fu, D.Q.; Havens, W.; Navarre, D.; Kachroo, P.; Ghabrial, S.A. An oleic acid-mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. MPMI 2008, 21, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.A.; Arevalo-Soliz, L.M.; Jia, L.; Navarre, D.A.; Chen, Z.; Howe, G.A.; Meng, Q.W.; Smith, J.E.; Goggin, F.L. Loss of function of FATTY ACID DESATURASE 7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012, 158, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Kim, Y.J.; Kim, K.D.; Hwang, B.K.; Ok, S.H.; Shin, J.S. Overexpression of lipid transfer protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep. 2009, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, H.J.; Crain, R.C.; Lee, A.; Korn, S.J. Polyunsaturated fatty acids modulate stomatal aperture and two distinct K+ channel currents in guard cells. Cell. Signal. 1994, 6, 181–186. [Google Scholar] [PubMed]
- Koh, E.J.; Zhou, L.; Williams, D.S.; Park, J.; Ding, N.; Duan, Y.P.; Kang, B.H. Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with Candidatus Liberibacter asiaticus. Protoplasma 2012, 249, 687–697. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Cofer, T.; Engelberth, M.; Engelberth, J. Defense Priming and Jasmonates: A Role for Free Fatty Acids in Insect Elicitor-Induced Long Distance Signaling. Plants 2016, 5, 5. https://doi.org/10.3390/plants5010005
Li T, Cofer T, Engelberth M, Engelberth J. Defense Priming and Jasmonates: A Role for Free Fatty Acids in Insect Elicitor-Induced Long Distance Signaling. Plants. 2016; 5(1):5. https://doi.org/10.3390/plants5010005
Chicago/Turabian StyleLi, Ting, Tristan Cofer, Marie Engelberth, and Jurgen Engelberth. 2016. "Defense Priming and Jasmonates: A Role for Free Fatty Acids in Insect Elicitor-Induced Long Distance Signaling" Plants 5, no. 1: 5. https://doi.org/10.3390/plants5010005
APA StyleLi, T., Cofer, T., Engelberth, M., & Engelberth, J. (2016). Defense Priming and Jasmonates: A Role for Free Fatty Acids in Insect Elicitor-Induced Long Distance Signaling. Plants, 5(1), 5. https://doi.org/10.3390/plants5010005





