Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members
Abstract
:1. Introduction
2. Results
2.1. Trimer Formation of Recombinant AOC2
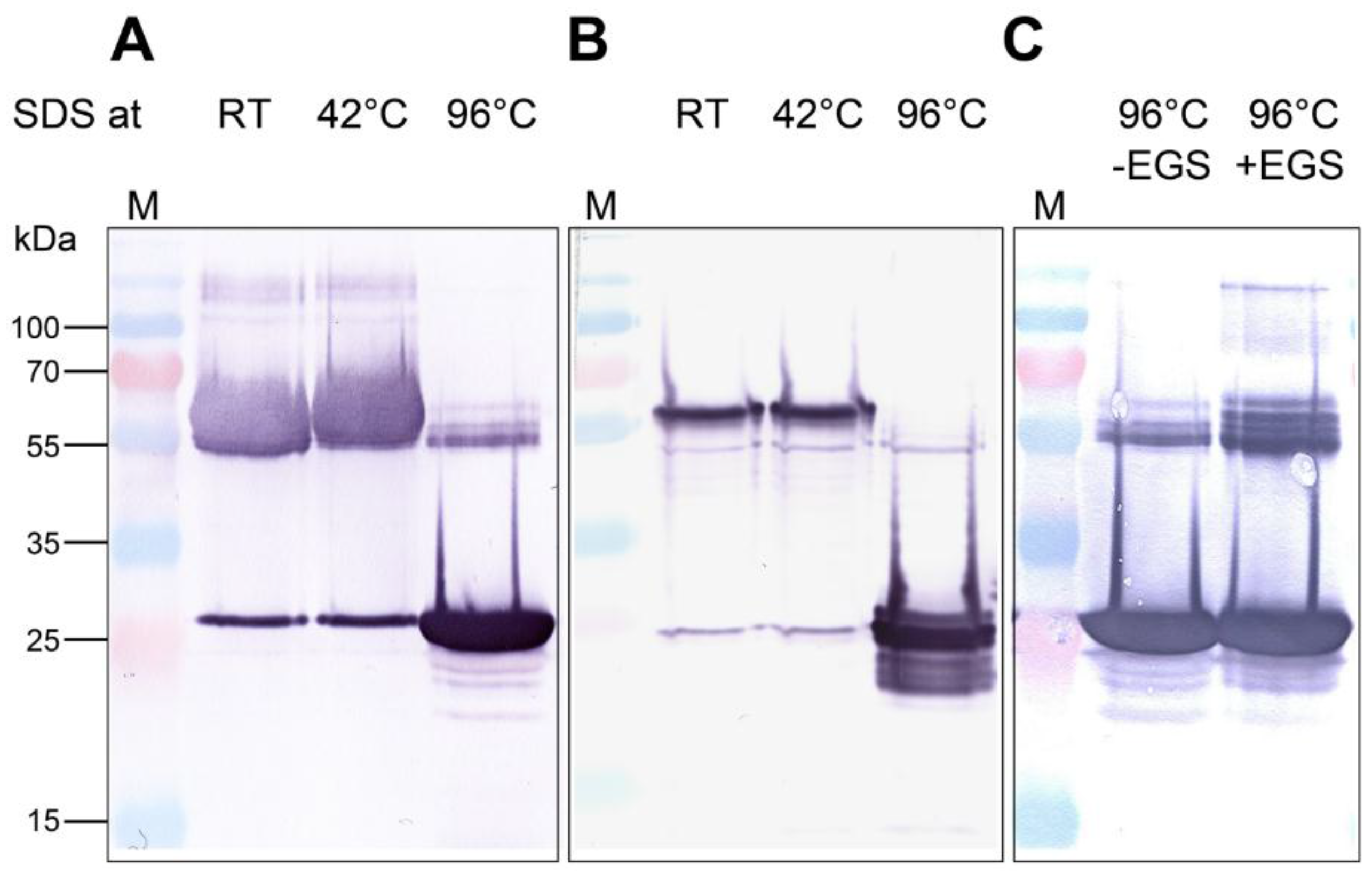
2.2. Analysis of the Quaternary Structure of AOC2
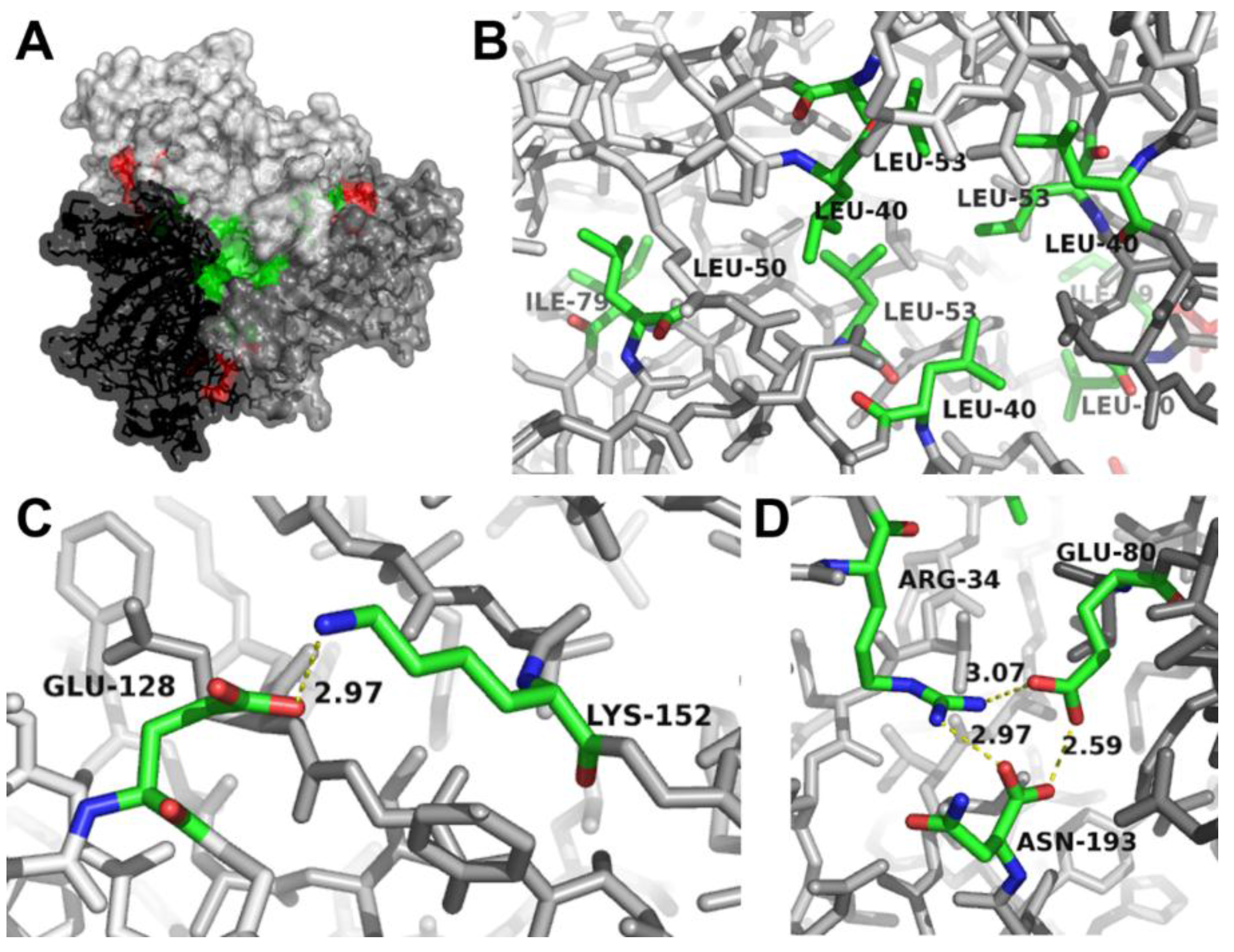
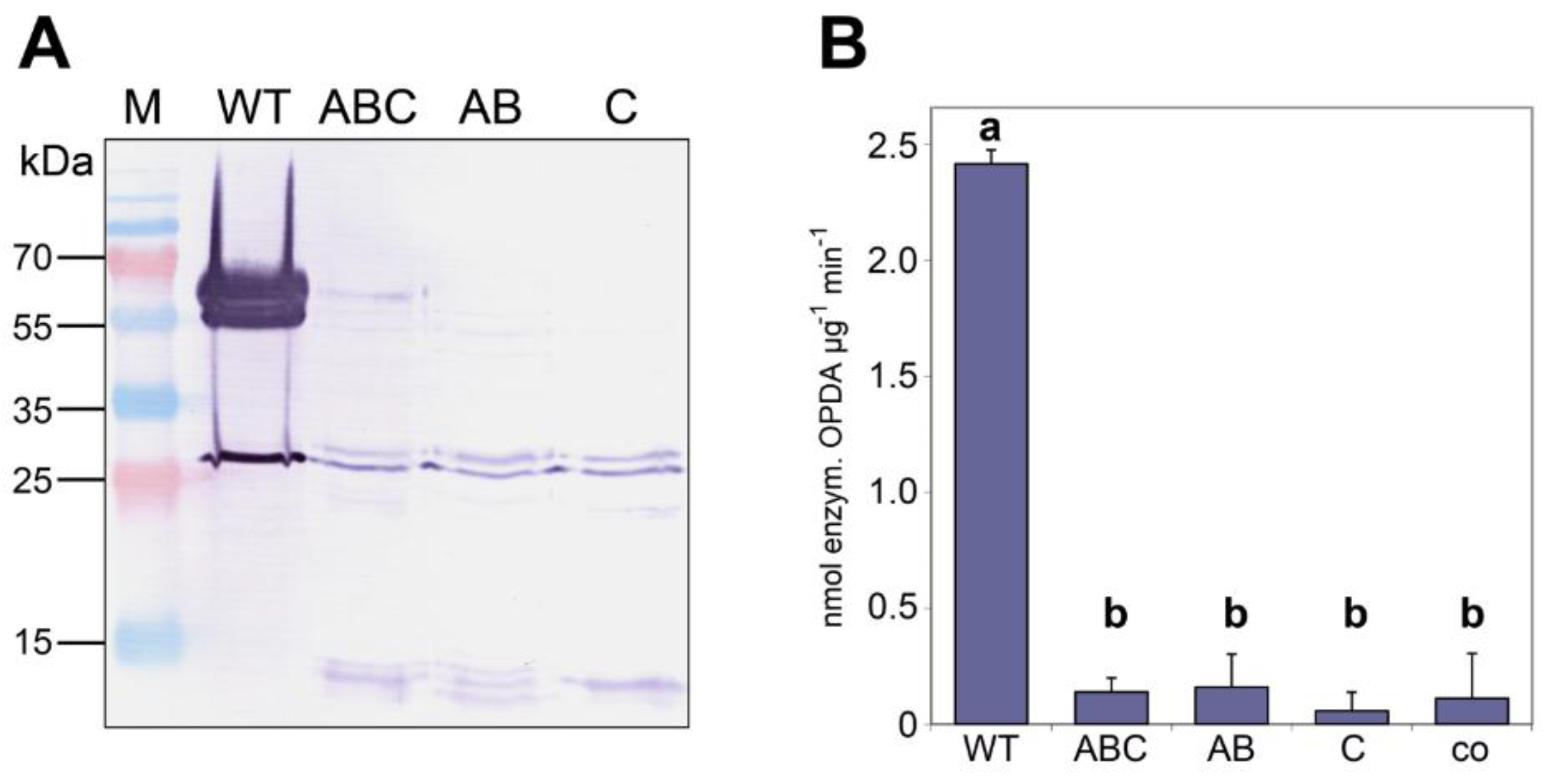
| Mutant | Exchange of Amino Acids | Expected Effects |
|---|---|---|
| AB | K152A, E80A | Disruption of salt bridges |
| C | L53S | Disruption of hydrophobic core |
| ABC | K152A, E80A; L53S | Disruption of salt bridges and hydrophobic core |
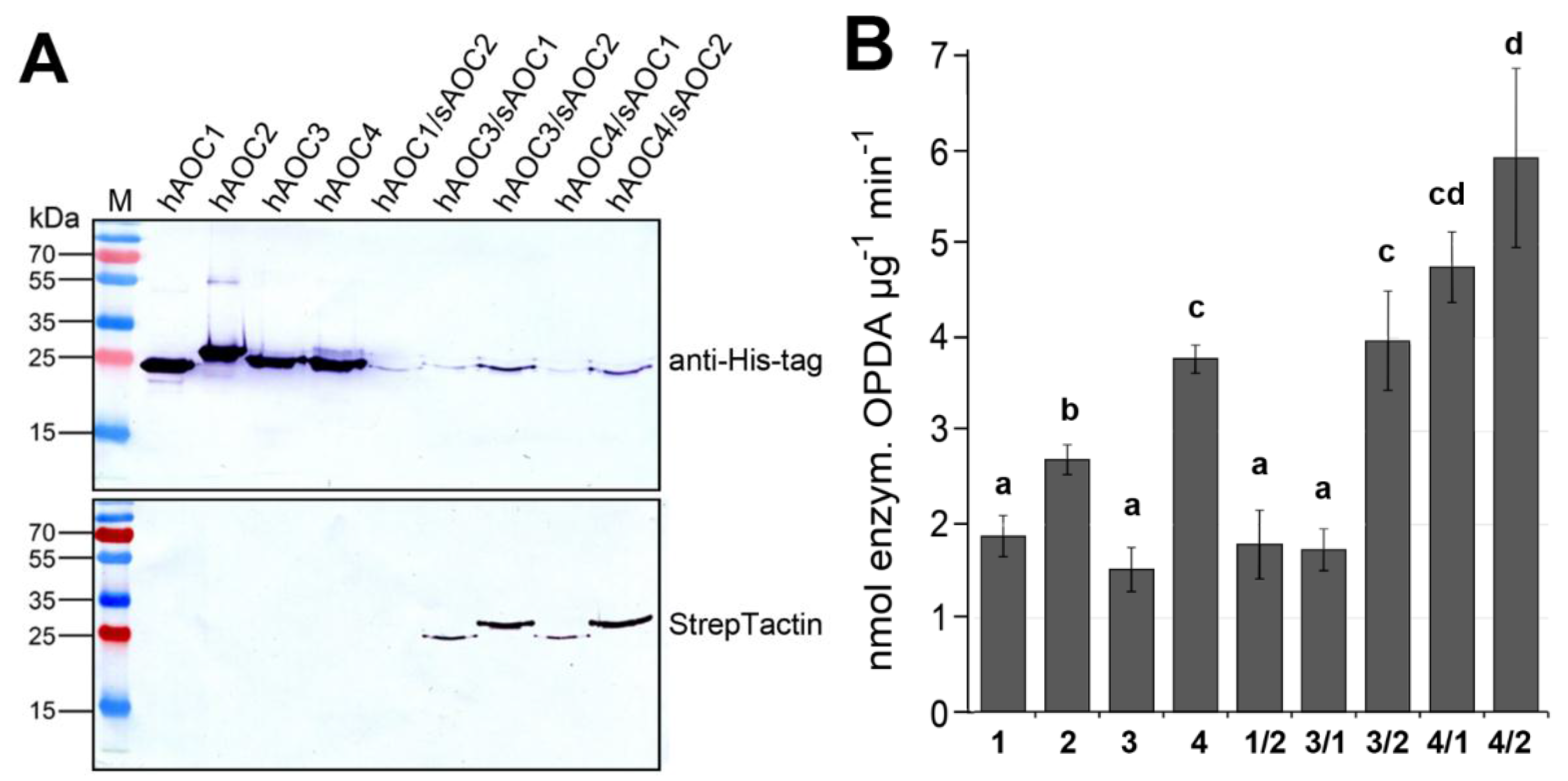
2.3. Heteromerization of AOC Gene Family Members and Its Effect on AOC Activity
3. Discussion
3.1. Recombinant AOC2 of Arabidopsis Is Active as a Trimer
3.2. Heteromers between Different AOC Isoforms Exhibit Altered Activity
4. Experimental Section
4.1. Cloning, Mutagenesis, Recombinant Expression and Purification of AOCs
4.2. Structure Analysis and Modelling of AOC Trimers
4.3. Cross-Linking, Size Exclusion Chromatography, SDS-PAGE and Immunoblot Analysis
4.4. Determination of AOC Activity
5. Conclusions
Supplemental Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Koo, A.J.K.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Laudert, D.; Schaller, F.; Weiler, E. Transgenic Nicotiana tabacum and Arabidopsis thaliana plants overexpressing allene oxide synthase. Planta 2000, 211, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato - amplification in wound signaling. Plant J. 2003, 33, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.; Stenzel, I.; Miersch, O.; Maucher, H.; Kramell, R.; Ziegler, J.; Wasternack, C. Tissue-specific oxylipin signature of tomato flowers: Allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant J. 2000, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Caldelari, D.; Wolfender, J.-L.; Farmer, E.E. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013, 197, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, D.; Chételat, A.; Acosta, I.F.; Goossens, J.; Pauwels, L.; Goossens, A.; Dreos, R.; Alfonso, E.; Farmer, E.E. Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genetics 2015, 11, e1005300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breithaupt, C.; Kurzbauer, R.; Lilie, H.; Schaller, A.; Strassner, J.; Huber, R.; Macheroux, P.; Clausen, T. Crystal structure of 12-oxophytodienoate reductase 3 from tomato: Self-inhibition by dimerization. Proc. Natl. Acad. Sci. USA 2006, 103, 14337–14342. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Otto, M.; Delker, C.; Kirmse, N.; Schmidt, D.; Miersch, O.; Hause, B.; Wasternack, C. ALLENE OXIDE CYCLASE (AOC) gene family members of Arabidopsis thaliana: tissue- and organ-specific promoter activities and in vivo heteromerization. J. Exper. Bot. 2012, 63, 6125–6138. [Google Scholar] [CrossRef] [PubMed]
- Miersch, O.; Wasternack, C. Octadecanoid and jasmonate signaling in tomato (Lycopersicon esculentum Mill.) leaves: Endogenous jasmonates do not induce jasmonate biosynthesis. Biol. Chem. 2000, 381, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Boland, W.; Mithöfer, A. Additional evidence against jasmonate-induced jasmonate induction hypothesis. Plant Sci. 2015, 239, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Zerbe, P.; Schaller, F. The crystal structure of Arabidopsis thaliana allene oxide cyclase: Insights into the oxylipin cyclization reaction. Plant Cell 2006, 18, 3201–3217. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Hause, B.; Miersch, O.; Kurz, T.; Maucher, H.; Weichert, H.; Ziegler, J.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol. Biol. 2003, 51, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Sinz, A. Chemical cross-linking and FTICR mass spectrometry for protein structure characterization. Anal. Bional. Chem. 2005, 381, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Stenzel, I.; Hause, B.; Maucher, H.; Hamberg, M.; Grimm, R.; Ganal, M.; Wasternack, C. Molecular cloning of allene oxide cyclase: The enzyme establishing the stereochemistry of octadecanoids and jasmonates. J. Biol. Chem. 2000, 275, 19132–19138. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.J.; Kondrashov, D.A.; Wesenberg, G.E.; Phillips, G.N. Ensemble refinement of protein crystal structures: validation and application. Structure 2007, 15, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Pollmann, S. Molecular mechanism of enzymatic allene oxide cyclization in plants. Plant Physiol. Biochem. 2008, 46, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Brodhun, F.; Sauer, K.; Herrfurth, C.; Hamberg, M.; Brinkmann, J.; Scholz, J.; Dickmanns, A.; Feussner, I.; Ficner, R. Crystal structures of Physcomitrella patens AOC1 and AOC2: Insights into the enzyme mechanism and differences in substrate specificity. Plant Physiol. 2012, 160, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Schaller, F.; Zerbe, P.; Reinbothe, S.; Reinbothe, C.; Hofmann, E.; Pollmann, S. The allene oxide cyclase family of Arabidopsis thaliana—localization and cyclization. FEBS J. 2008, 275, 2428–2441. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, D.; Chauvin, A.; Acosta, I.F.; Kurenda, A.; Stolz, S.; Chételat, A.; Wolfender, J.-L.; Farmer, E.E. Axial and radial oxylipin transport. Plant Physiol. 2015, 169, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 2009, 70, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Maucher, H.; Hause, B.; Feussner, I.; Ziegler, J.; Wasternack, C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): Tissue specific regulation in seedling development. Plant J. 2000, 21, 199–213. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, J.A.D.; Banavali, N.; Foloppe, N. Development and current status of the CHARMM force field for nucleic acids. Biopolymers 2001, 56, 257–265. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Lesyng, B. Generalized born model: Analysis, refinement, and applications to proteins. J. Phys. Chem. B 2004, 108, 18368–18376. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Close-range electrostatic interactions in proteins. ChemBioChem 2002, 3, 604–617. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Hamberg, M.; Miersch, O.; Parthier, B. Purification and characterization of allene oxide cyclase from dry corn seeds. Plant Physiol. 1997, 114, 565–573. [Google Scholar] [PubMed]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Wasternack, C.; Hamberg, M. On the specificity of allene oxide cyclase. Lipids 1999, 34, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Theologis, A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Theologis, A. Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otto, M.; Naumann, C.; Brandt, W.; Wasternack, C.; Hause, B. Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members. Plants 2016, 5, 3. https://doi.org/10.3390/plants5010003
Otto M, Naumann C, Brandt W, Wasternack C, Hause B. Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members. Plants. 2016; 5(1):3. https://doi.org/10.3390/plants5010003
Chicago/Turabian StyleOtto, Markus, Christin Naumann, Wolfgang Brandt, Claus Wasternack, and Bettina Hause. 2016. "Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members" Plants 5, no. 1: 3. https://doi.org/10.3390/plants5010003
APA StyleOtto, M., Naumann, C., Brandt, W., Wasternack, C., & Hause, B. (2016). Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members. Plants, 5(1), 3. https://doi.org/10.3390/plants5010003





