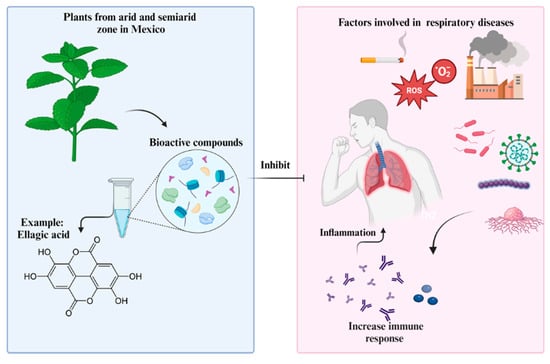
Plants from Arid and Semi-Arid Zones of Mexico Used to Treat Respiratory Diseases: A Review
Abstract
:1. Introduction
2. Respiratory Diseases
3. Scientific Evidence of Semi-Desert Plants Used to Treat Respiratory Diseases
3.1. Jatropha dioica
3.2. Turnera diffusa
3.3. Larrea tridentata
3.4. Opuntia spp.
3.5. Flourensia cernua
3.6. Fouquieria splendes
3.7. Prosopis glandulosa
4. Mechanisms of Action of Bioactive Compounds and/or Crude Extracts against the Disease
5. Ethnopharmacology of Plants from Arid and Semi-Arid Zones of Mexico
6. Commercially Available Important Plants in Mexico
7. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- INAH 2023 Códice de La Cruz-Badiano. Available online: https://www.mediateca.inah.gob.mx/repositorio/islandora/object/codice:851#page/1/mode/2up (accessed on 30 May 2023).
- Comisión Nacional Para El Conocimiento y Uso de La Biodiversidad|Gobierno|Gob.Mx. Available online: https://www.gob.mx/conabio (accessed on 9 May 2023).
- Bigurra-Alzati, C.A.; Ortiz-Gómez, R.; Vázquez-Rodríguez, G.A.; López-León, L.D.; Lizárraga-Mendiola, L. Water Conservation and Green Infrastructure Adaptations to Reduce Water Scarcity for Residential Areas with Semi-Arid Climate: Mineral de La Reforma, Mexico. Water 2021, 13, 45. [Google Scholar] [CrossRef]
- Hernández-Magaña, R.; Hernández-Oria, J.G.; Chávez, R. Floristic Conservation Data Based on the Geographical Extent of the Species in the Semidesert Queretano, Mexico. Acta Bot. Mex. 2012, 99, 105–140. [Google Scholar] [CrossRef]
- Carlos-Hernández, S.; Carrillo-Parra, A.; Díaz-Jiménez, L.; Salas-Cruz, L.R.; Rosales-Serna, R.; Ngangyo-Heya, M. Transformation Processes for Energy Production Alternatives from Different Biomass Sources in the Highlands and Semi-Desert Areas of Mexico. Resources 2023, 12, 103. [Google Scholar] [CrossRef]
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Encina-Domínguez, J.A.; Jurado-Ybarra, E.; Cuéllar-Rodríguez, L.G.; Garza-Zambrano, P.; Arévalo-Sierra, J.R.; Cantú-Ayala, C.M.; Himmelsbach, W.; Salinas-Rodríguez, M.M.; et al. Ethnobotanical Biocultural Diversity by Rural Communities in the Cuatrociénegas Valley, Coahuila; Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Rodríguez-Herrera, R.; Aguilar-González, C. Análisis de Ácido Elágico En Algunas Plantas Del Semidesierto Mexicano. Rev. Mex. Cienc. Farm. 2013, 44, 36–40. [Google Scholar]
- Armijo-Nájera, G.M.; Moreno-Reséndez, A.; Blanco-Contreras, E.; Borroel-García, J.V.; Reyes-Carrillo, L.J. Mesquite Pod (Prosopis Spp.) Food for Goats in the Semi-Desert. Rev. Mex. Cienc. Agric. 2019, 10, 113–122. [Google Scholar]
- Torres-León, C.; Rebolledo Ramírez, F.; Aguirre-Joya, J.A.; Ramírez-Moreno, A.; Chávez-González, M.L.; Aguillón-Gutierrez, D.R.; Camacho-Guerra, L.; Ramírez-Guzmán, N.; Hernández Vélez, S.; Aguilar, C.N. Medicinal Plants Used by Rural Communities in the Arid Zone of Viesca and Parras Coahuila in Northeast Mexico. Saudi Pharm. J. 2023, 31, 21–28. [Google Scholar] [CrossRef] [PubMed]
- González Elizondo, M.; López Enriquez, I.L.; González Elizondo, M.S.; Tena Flores, J.A. Plantas Medicinales Del Estado de Durango y Zonas Aledañas; CIIDIR Durango, Ed.; Instituto Politécnico Nacional: Durango, México, 2004. [Google Scholar]
- Dimayuga, R.E.; Virgen, M.; Ochoa, N. Antimicrobial Activity of Medicinal Plants from Baja California Sur (Mexico). Pharm. Biol. 1998, 36, 33–43. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Venditti, A. Plants Adapted to Arid Areas: Specialized Metabolites. Nat. Prod. Res. 2021, 35, 3314–3331. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; Torres-Moreno, H.; Ireta-Paredes, A.d.R.; Charles-Rodríguez, A.V.; Flores-López, M.L. Chemical and Bioactive Compounds from Mexican Desertic Medicinal Plants. In Aromatic and Medicinal Plants of Drylands and Deserts: Ecology, Ethnobiology, and Potential Uses; CRC Press: Boca Raton, FL, USA, 2023; pp. 189–218. [Google Scholar]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.F.; Figueroa, G. Fitoquímica; UNAM; FES Zaragoza: Ciudad de México, Mexico, 2022; Volume 1, ISBN 978-607-30-6019-6. [Google Scholar]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and Nanoencapsulation of Bioactive Compounds for Agri-Food Applications: A Review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Torres-Moreno, H.; López-Romero, J.C.; Vidal-Gutiérrez, M.; Villarreal-Quintanilla, J.A.; Carrillo-Lomelí, D.A.; Robles-Zepeda, R.E.; Vilegas, W. Antioxidant, Anti-Inflammatory, and Antiproliferative Activities of Flourensia spp. Biocatal. Agric. Biotechnol. 2023, 47, 102552. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Balciunaite, R.; Simaitiene, Z.; Bernatoniene, J. Herbal Medicine Uses for Respiratory System Disorders and Possible Trends in New Herbal Medicinal Recipes during COVID-19 in Pasvalys District, Lithuania. Int. J. Environ. Res. Public Health 2022, 19, 8905. [Google Scholar] [CrossRef]
- Lammi, C.; Arnoldi, A. Food-Derived Antioxidants and COVID-19. J. Food Biochem. 2021, 45, e13557. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Pagliaro, B.; Santolamazza, C.; Simonelli, F.; Rubattu, S. Phytochemical Compounds and Protection from Cardiovascular Diseases: A State of the Art. BioMed Res. Int. 2015, 2015, 918069. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A Review of Dietary Phytochemicals and Their Relation to Oxidative Stress and Human Diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Aminian, A.R.; Mohebbati, R.; Boskabady, M.H. The Effect of Ocimum basilicum L. and Its Main Ingredients on Respiratory Disorders: An Experimental, Preclinical, and Clinical Review. Front. Pharmacol. 2022, 12, 805391. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia Ficus-Indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Goh, N.-Y.; Razali, R.A.; Abdullah, N.A.H.; Yazid, M.D.; Sulaiman, N. Role of Olive Bioactive Compounds in Respiratory Diseases. Antioxidants 2023, 12, 1140. [Google Scholar] [CrossRef]
- Yumura, M.; Nagano, T.; Nishimura, Y. Novel Multitarget Therapies for Lung Cancer and Respiratory Disease. Molecules 2020, 25, 3987. [Google Scholar] [CrossRef]
- Zong, X.; Liang, N.; Wang, J.; Li, H.; Wang, D.; Chen, Y.; Zhang, H.; Jiao, L.; Li, A.; Wu, G.; et al. Treatment Effect of Qingfei Paidu Decoction Combined With Conventional Treatment on COVID-19 Patients and Other Respiratory Diseases: A Multi-Center Retrospective Case Series. Front. Pharmacol. 2022, 13, 849598. [Google Scholar] [CrossRef]
- WHO. Un Nuevo Informe Insta a Actuar Con Urgencia Para Prevenir Una Crisis Causada Por La Resistencia a Los Antimicrobianos. Available online: https://www.who.int/es/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 21 January 2024).
- Derouiche, S. Oxidative Stress Associated with SARS-CoV-2 (COVID-19) Increases the Severity of the Lung Disease—A Systematic Review. J. Infect. Dis. Epidemiol. 2020, 6, 121–126. [Google Scholar] [CrossRef]
- Koya, T.; Hasegawa, T. Aggravation of Asthma by Cold, Fatigue, Stress, or Discontinuation of Medicines: What Should We Measures and Prevents Worse of Asthma Control Induced by the Aggravation of the Environmental Hygiene and/or the Stopping Medicine? In Disaster and Respiratory Diseases; Fujimoto, K., Ed.; Series: Diagnostic Tools and Disease Managements; Springer: Singapore, 2019; pp. 67–78. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Chen, Z.; Zu, B.; Zhao, Y. Effects of Variations in Meteorological Factors on Daily Hospital Visits for Asthma: A Time-Series Study. Environ. Res. 2020, 182, 109115. [Google Scholar] [CrossRef]
- Kobayashi, S. Exacerbation of COPD by Air Pollution, Cold Temperatures, or Discontinuation of Medicine: What Should Be Measured to Help Prevent It? In Disaster and Respiratory Diseases; Fujimoto, K., Ed.; Respiratory Disease Series: Diagnostic Tools and Disease Managements; Springer: Singapore, 2019; pp. 79–90. [Google Scholar] [CrossRef]
- Ostridge, K.; Gove, K.; Paas, K.H.W.; Burke, H.; Freeman, A.; Harden, S.; Kirby, M.; Peterson, S.; Sieren, J.; McCrae, C.; et al. Using Novel Computed Tomography Analysis to Describe the Contribution and Distribution of Emphysema and Small Airways Disease in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2019, 16, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Sethi, S.; Murphy, T.; Nariya, S.; Boushey, H.A.; Lynch, S.V. Airway Microbiome Dynamics in Exacerbations of Chronic Obstructive Pulmonary Disease. J. Clin. Microbiol. 2014, 52, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Aredo, J.V.; Luo, S.J.; Gardner, R.M.; Sanyal, N.; Choi, E.; Hickey, T.P.; Riley, T.L.; Huang, W.Y.; Kurian, A.W.; Leung, A.N.; et al. Tobacco Smoking and Risk of Second Primary Lung Cancer. J. Thorac. Oncol. 2021, 16, 968–979. [Google Scholar] [CrossRef]
- Ho, L.J.; Yang, H.Y.; Chung, C.H.; Chang, W.C.; Yang, S.S.; Sun, C.A.; Chien, W.C.; Su, R.Y. Increased Risk of Secondary Lung Cancer in Patients with Tuberculosis: A Nationwide, Population-Based Cohort Study. PLoS ONE 2021, 16, e0250531. [Google Scholar] [CrossRef]
- Fujimoto, D.; Yomota, M.; Sekine, A.; Morita, M.; Morimoto, T.; Hosomi, Y.; Ogura, T.; Tomioka, H.; Tomii, K. Nivolumab for Advanced Non-Small Cell Lung Cancer Patients with Mild Idiopathic Interstitial Pneumonia: A Multicenter, Open-Label Single-Arm Phase II Trial. Lung Cancer 2019, 134, 274–278. [Google Scholar] [CrossRef]
- Herrera-Lara, S.; Fernández-Fabrellas, E.; Cervera-Juan, Á.; Blanquer-Olivas, R. Do Seasonal Changes and Climate Influence the Etiology of Community Acquired Pneumonia? Arch. Bronconeumol. 2013, 49, 140–145. [Google Scholar] [CrossRef]
- Kharwadkar, S.; Attanayake, V.; Duncan, J.; Navaratne, N.; Benson, J. The Impact of Climate Change on the Risk Factors for Tuberculosis: A Systematic Review. Environ. Res. 2022, 212, 113436. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, M.L.; Gonzalez-Carrasco, E.; Bracamonte, T.; Molinero, M.; Pozo, F.; Casas, I.; Calvo, C. Impact of Prematurity and Severe Viral Bronchiolitis on Asthma Development at 6–9 Years. J. Asthma Allergy 2020, 13, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Leimanis, M.L.; Adams, M.; Bachmann, A.S.; Uhl, K.L.; Bupp, C.P.; Hartog, N.L.; Kort, E.J.; Olivero, R.; Comstock, S.S.; et al. Balancing Precision versus Cohort Transcriptomic Analysis of Acute and Recovery Phase of Viral Bronchiolitis. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L1147–L1157. [Google Scholar] [CrossRef]
- Nowicki, J.; Murray, M.T. Bronchitis and Pneumonia. In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1196–1201. [Google Scholar] [CrossRef]
- Passos, S.D.; Gazeta, R.E.; Felgueiras, A.P.; Beneli, P.C.; Coelho, M.D.S.Z.S. Do Pollution and Climate Influence Respiratory Tract Infections in Children? Rev. Assoc. Med. Bras. 2014, 60, 276–283. [Google Scholar] [CrossRef]
- Bocanegra-García, V.; Del Rayo Camacho-Corona, M.; Ramírez-Cabrera, M.; Rivera, G.; Garza-Gonzlez, E. The Bioactivity of Plant Extracts against Representative Bacterial Pathogens of the Lower Respiratory Tract. BMC Res. Notes 2009, 2, 95. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, Phytochemistry, and Bioactivity of the Genus Turnera (Passifloraceae) with a Focus on Damiana—Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pawar, R.S.; Ali, Z.; Khan, I.A. Phytochemical Investigation of Turnera diffusa. J. Nat. Prod. 2007, 70, 289–292. [Google Scholar] [CrossRef]
- Serrano-Gallardo, L.-B.; Castillo-Maldonado, I.; Borjón-Ríos, C.-G.; Rivera-Guillén, M.-A.; Morán-Martínez, J.; Téllez-López, M.-A.; García-Salcedo, J.-J.; Pedroza-Escobar, D.; Vega-Menchaca, M. del C. Antimicrobial Activity and Toxicity of Plants from Northern Mexico; NISCAIR-CSIR: New Delhi, India, 2017; Volume 16. [Google Scholar]
- WHO. 2020 Respiratory Diseases. Available online: https://platform.who.int/mortality/themes/theme-details/topics/topic-details/MDB/respiratory-diseases (accessed on 27 May 2023).
- Datos Abiertos Dirección General de Epidemiología|Secretaría de Salud|Gobierno|Gob.Mx. Available online: https://www.gob.mx/salud/documentos/datos-abiertos-152127 (accessed on 27 May 2023).
- Ramírez-Moreno, A.; Delgadillo-Guzmán, D.; Bautista-Robles, V.E.; Marszalek, J.; Keita, H.; Kourouma, A.; Ramírez-García, S.A.; Rodríguez Amado, J.R.; Tavares-Carvalho, J.C. Jatropha Dioica, an Aztec Plant with Promising Pharmacological Properties: A Systematic Review. Afr. J. Pharm. Pharmacol. 2020, 14, 169–178. [Google Scholar] [CrossRef]
- UNAM. Biblioteca Digital de La Medicina Tradicional Mexicana. 2010. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=jatropha-dioica (accessed on 28 May 2023).
- Basilio Heredia, J.; Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; Soto-Landeros, F. Recent Studies on Jatropha Research; Plant Science Research and Practices; Nova Science Publishers: Hauppauge, NY, USA, 2021; ISBN 9781536194944. [Google Scholar]
- Silva-Belmares, Y.; Rivas-Morales, C.; Viveros-Valdez, E.; de la Cruz-Galicia, M.G.; Carranza-Rosales, P. Antimicrobial and Cytotoxic Activities from Jatropha dioica Roots. Pak. J. Biol. Sci. 2014, 17, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, L.M.; Albarado, I.L.; Betancourt, J.; Medina, B. Bacterias Patógenas En Infecciones Del Tracto Respiratorio: Servicio Autónomo Hospital Universitario “Antonio Patricio de Alcalá”. Cumaná, Estado Sucre. Kasmera 2005, 33, 16–26. [Google Scholar]
- Urbizu-González, A.L.; Castillo-Ruiz, O.; Martínez-Ávila, G.C.G.; Torres-Castillo, J.A. Natural Variability of Essential Oil and Antioxidants in the Medicinal Plant Turnera diffusa. Asian Pac. J. Trop. Med. 2017, 10, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Sun, Y.T.; Su, X.M.; He, M.; Dai, B.; Kang, J. Eucalyptol Protects Lungs against Bacterial Invasion through Attenuating Ciliated Cell Damage and Suppressing MUC5AC Expression. J. Cell Physiol. 2019, 234, 5842–5850. [Google Scholar] [CrossRef]
- Snowden, R.; Harrington, H.; Morrill, K.; Jeane, L.D.; Garrity, J.; Orian, M.; Lopez, E.; Rezaie, S.; Hassberger, K.; Familoni, D.; et al. A Comparison of the Anti-Staphylococcus aureus Activity of Extracts from Commonly Used Medicinal Plants. J. Altern. Complement. Med. 2014, 20, 375–382. [Google Scholar] [CrossRef]
- Pivard, M.; Moreau, K.; Vandenesch, F. Staphylococcus aureus Arsenal to Conquer the Lower Respiratory Tract. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Núñez-Mojica, G.; Vázquez-Ramírez, A.L.; García, A.; Rivas-Galindo, V.M.; Garza-González, E.; Cuevas González-Bravo, G.E.; Toscano, R.A.; Moo-Puc, R.E.; Villanueva-Toledo, J.R.; Marchand, P.; et al. New Cyclolignans of Larrea Tridentata and Their Antibacterial and Cytotoxic Activities. Phytochem. Lett. 2021, 43, 212–218. [Google Scholar] [CrossRef]
- Reyes-Melo, K.Y.; Galván-Rodrigo, A.A.; Martínez-Olivo, I.E.; Núñez-Mojica, G.; Ávalos-Alanís, F.G.; García, A.; del Rayo Camacho-Corona, M. Larrea tridentata and Its Biological Activities. Curr. Top. Med. Chem. 2021, 21, 2352–2364. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and Antimycobacterial Lignans and Flavonoids from Larrea Tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E. Prickly Pear (Opuntia spp.): A Valuable Fruit Crop for the Semi-Arid Lands of Mexico. J. Arid. Environ. 1994, 28, 1–11. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) Plant Compounds, Biological Activities and Prospects—A Comprehensive Review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and Isolation of Anti-Pneumonia Bioactive Compounds from: Opuntia ficus-indica Fruit Waste Peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Ledesma, N.E.; González-Hernández, M.D.; Rojas, R.; Paz-González, A.D.; Rivera, G.; Luna-Sosa, B.; Martínez-Ávila, G.C.G. Essential Oil and Polyphenolic Compounds of Flourensia Cernua Leaves: Chemical Profiling and Functional Properties. Agronomy 2022, 12, 2274. [Google Scholar] [CrossRef]
- Alvarez-Pérez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdés, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as Source of Antioxidants and Antifungal Bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- GLOBOCAN 2020—Oncologia.Mx. Available online: https://oncologia.mx/tag/globocan-2020/ (accessed on 5 April 2023).
- Molina-Salinas, G.M.; Ramos-Guerra, M.C.; Vargas-Villarreal, J.; Mata-Cárdenas, B.D.; Becerril-Montes, P.; Said-Fernández, S. Bactericidal Activity of Organic Extracts from Flourensia Cernua DC against Strains of Mycobacterium tuberculosis. Arch. Med. Res. 2006, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Nevárez-Prado, L.O.; Rocha-Gutiérrez, B.A.; Neder-Suárez, D.; Cordova-Lozoya, M.T.; Ayala-Soto, J.G.; Salazar-Balderrama, M.I.; de Ruiz-Anchondo, T.J.; Hernández-Ochoa, L.R. The Genus Fouquieria: Description and Review of Ethnobotanical, Phytochemical, and Biotechnological Aspects. Tecnociencia Chihuah. 2021, 15, 186–220. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Torres-Moreno, H.; Rodríguez-Martínez, K.L.; Ramírez-Audelo, V.; Vidal-Gutiérrez, M.; Hernández, J.; Robles-Zepeda, R.E.; Ayala-Zavala, J.F.; González-Ríos, H.; Valenzuela-Melendres, M. Fouquieria splendens: A Source of Phenolic Compounds with Antioxidant and Antiproliferative Potential. Eur. J. Integr. Med. 2022, 49, 102084. [Google Scholar] [CrossRef]
- Rodríguez Garza, R.G. Tamizaje Fitoquímico y Actividad Biológica de Fouquieria splendens (Engelmann), Ariocarpus retusus (Scheidweiler) y Ariocarpus kotschoubeyanus (Lemaire). Ph.D. Thesis, Universidad Autónoma de Nuevo León, Nuevo León, México, 2010. [Google Scholar]
- Monreal-García, H.M.; Almaraz-Abarca, N.; Ávila-Reyes, J.A.; Torres-Ricario, R.; González-Elizondo, M.S.; Herrera-Arrieta, Y.; Gutiérrez-Velázquez, M.V. Phytochemical Variation among Populations of Fouquieria splendens (Fouquieriaceae). Bot. Sci. 2019, 97, 398–412. [Google Scholar] [CrossRef]
- Vega Menchaca, M.D.C.; Rivas Morales, C.; Verde Star, J.; Oranday, C.A.; Rubio Morales, M.E.; Núñez González, M.A.; Serrano Gallardo, L.B. Antimicrobial Activity of Five Plants from Northern Mexico on Medically Important Bacteria. Afr. J. Microbiol. Res. 2013, 7, 5011–5017. [Google Scholar] [CrossRef]
- Nevárez-Prado, L.O.; Amaya-Olivas, N.; Sustaita-Rodriguez, A.; Rodríguez-Zapién, J.; Zúñiga-Rodríguez, E.; Cordova-Lozoya, M.; García-Triana, A.; Sandoval-Salas, F.; Hernández-Ochoa, L. Chemical Composition and Toxicity of Extracts of Fouquieria splendens. AIMS Agric. Food 2022, 7, 357–369. [Google Scholar] [CrossRef]
- Orozco Meléndez, L.R.; García Muñoz, S.A.; Leyva Chávez, A.N.; González Aldana, R.A.; Villalobos Pérez, E.; Yáñez Muñoz, R.M. Insecticidal Properties of Secondary Metabolites of Fouquieria splendens Engelm (Ocotillo). Biol. Agropecu. Tuxpan 2017, 6, 1763–1774. [Google Scholar]
- Zhou, Z.; Ma, C.; Zhang, H.; Bi, Y.; Chen, X.; Tian, H.; Xie, X.; Meng, Q.; Lewis, P.J.; Xu, J. Synthesis and Biological Evaluation of Novel Ocotillol-Type Triterpenoid Derivatives as Antibacterial Agents. Eur. J. Med. Chem. 2013, 68, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ma, C.; Zhou, Z.; Zhang, T.; Zhang, H.; Zhang, X.; Lu, J.; Meng, Q.; Lewis, P.J.; Xu, J. Synthesis and Antibacterial Evaluation of Novel Hydrophilic Ocotillol-Type Triterpenoid Derivatives from 20(S)-Protopanaxadiol. Rec. Nat. Prod. 2015, 9, 356–368. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and Other Triterpenoids from Camellia Oil and Their Inhibitory Effects on Epstein–Barr Virus Activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cepeda, A.; Macclesh del Pino-Pérez, L.A.; Marmolejo, M.; Rivera-Armenta, J.L.; Peraza-Vázquez, H. Isolation of Ocotillol/Ocotillone from Fouquieria splendens (Ocote) Using a Batch Reactor. Prep. Biochem. Biotechnol. 2022, 52, 540–548. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Valdez-Salas, B.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Grimaldo-Juarez, O.; González-Mendoza, D. Silver Nanoparticles from Prosopis glandulosa and Their Potential Application as Biocontrol of Acinetobacter Calcoaceticus and Bacillus Cereus. Chem. Speciat. Bioavailab. 2017, 29, 1–5. [Google Scholar] [CrossRef]
- SEINet Portal Network—Prosopis Glandulosa. Available online: https://swbiodiversity.org (accessed on 20 November 2023).
- Felker, P.; Takeoka, G.; Dao, L. Pod Mesocarp Flour of North and South American Species of Leguminous Tree Prosopis (Mesquite): Composition and Food Applications. Food Rev. Int. 2013, 29, 49–66. [Google Scholar] [CrossRef]
- Rahman, A.A.; Samoylenko, V.; Jacob, M.R.; Sahu, R.; Jain, S.K.; Khan, S.I.; Tekwani, B.L.; Muhammad, I. Antiparasitic and Antimicrobial Indolizidines from the Leaves of Prosopis glandulosa Var glandulosa. Planta Med. 2011, 77, 1639–1643. [Google Scholar] [CrossRef]
- Kumar Raju, S.; Shridharshini, K.; Mohanapriya, K.; Praveen, S.; Maruthamuthu, M.; Anajana, E.; Mythili, A. An Updated Review on Phytochemical Composition and Pharmacological Activities of Prosopis Glandulosa Torr.: An Invasive Exotic Plant. Indian J. Nat. Sci. 2022, 13, 46100–46110. [Google Scholar]
- Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, Antiinfective and Antiparasitic Compounds from Prosopis glandulosa Torr. Var. glandulosa. Planta Med. 2009, 75, 399–457. [Google Scholar] [CrossRef]
- Kumar, R.S.; Rajkapoor, B.; Perumal, P.; Dhanasekaran, T.; Jose, M.A.; Jothimanivannan, C. Antitumor Activity of Prosopis Glandulosa Torr. on Ehrlich Ascites Carcinoma (EAC) Tumor Bearing Mice. Iran. J. Pharm. Res. 2011, 10, 505. [Google Scholar]
- Moorthy, K.; Kumar, R.S. Phytochemical and Antimicrobial Studies of Leaf Extract of Prosopis glandulosa. J. Ecotoxicol. Environ. Monit. 2011, 21, 143. [Google Scholar]
- Ali, M.S.; Azhar, I.; Ahmad, F.; Ahmad, V.U.; Usmanghani, K. Antimicrobial Screening of Mimoaceous Plants. Pharm. Biol. 2001, 39, 43–46. [Google Scholar] [CrossRef]
- Imam, R.; Rafiq, M.; Sheng, Z.; Naqvi, S.H.A.; Talpur, F.N.; Abdelkhalek, A.; Jokhio, M.A. Evaluation of Physicochemical Properties and Antimicrobial Activity of Essential Oils from Seeds of Prosopis juliflora, P. Glandulosa and P. Cineraria. J. Essent. Oil Bear. Plants 2019, 22, 554–562. [Google Scholar] [CrossRef]
- López-Anchondo, A.N.; López-de la Cruz, D.; Gutiérrez-Reyes, E.; Castañeda-Ramírez, J.C.; De la Fuente-Salcido, N.M. Antifungal Activity In Vitro and In Vivo of Mesquite Extract (Prosopis glandulosa) against Phytopathogenic Fungi. Indian J. Microbiol. 2021, 61, 85–90. [Google Scholar] [CrossRef]
- Gupta, A.; Chaphalkar, S.R. Virucidal Potential of Prosopis spicigera and Mangifera indica on Human Peripheral Blood Mononuclear Cells. J. HerbMed Pharmacol. 2016, 5, 162–165. [Google Scholar]
- Patel, N.; Sharath Kumar, L.; Gajera, J.; Jadhav, A.; Muguli, G.; Babu, U. Isolation and Characterization of Flavonoid C-Glycosides from Prosopis glandulosa Torr. Leaves. Pharmacogn. Mag. 2018, 14, 451–454. [Google Scholar] [CrossRef]
- Odoh, U.E.; Uzor, P.F.; Eze, C.L.; Akunne, T.C.; Onyegbulam, C.M.; Osadebe, P.O. Medicinal Plants Used by the People of Nsukka Local Government Area, South-Eastern Nigeria for the Treatment of Malaria: An Ethnobotanical Survey. J. Ethnopharmacol. 2018, 218, 1–15. [Google Scholar] [CrossRef]
- Samoylenko, V.; Chuck Dunbar, D.; Jacob, M.R.; Joshi, V.C.; Ashfaq, M.K.; Muhammad, I. Two New Alkylated Piperidine Alkaloids from Western Honey Mesquite: Prosopis Glandulosa Torr. Var. Torreyana. Nat. Prod. Commun. 2008, 3, 35–40. [Google Scholar] [CrossRef]
- Ashfaq, M.K.; Abdel-Bakky, M.S.; Maqbool, M.T.; Samoylenko, V.; Rahman, A.A.; Muhammad, I. Efficacy of Prosopilosidine from Prosopis Glandulosa Var. Glandulosa against Cryptococcus Neoformans Infection in a Murine Model. Molecules 2018, 23, 1674. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Phytoconstituents—Active and Inert Constituents, Metabolic Pathways, Chemistry and Application of Phytoconstituents, Primary Metabolic Products, and Bioactive Compounds of Primary Metabolic Origin. In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2. Progress in Drug Research; Springer: Cham, Switzerland, 2018; Volume 74, pp. 25–164. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yang, W.K.; Park, Y.R.; Park, Y.C.; Park, I.J.; Lee, G.J.; Kang, H.S.; Kim, B.K.; Kim, S.H. Opuntia ficus-indica Alleviates Particulate Matter 10 Plus Diesel Exhaust Particles (PM10D)—Induced Airway Inflammation by Suppressing the Expression of Inflammatory Cytokines and Chemokines. Plants 2022, 11, 520. [Google Scholar] [CrossRef]
- Yoo, G.; Oh, Y.; Yang, H.; Kim, T.; Sung, S.; Kim, S. Efficient Preparation of Narcissin from Opuntia ficus-indica Fruits by Combination of Response Surface Methodology and High-Speed Countercurrent Chromatography. Pharmacogn. Mag. 2018, 14, 338–343. [Google Scholar] [CrossRef]
- Linares-Braham, A.; Palomo-Ligas, L.; Nery-Flores, S.D. Bioactive Compounds and Pharmacological Potential of Hojasen (Flourensia cernua): A Mini Review. Plant Sci. Today 2023, 10, 304–312. [Google Scholar] [CrossRef]
- Mansouri, Z.; Dianat, M.; Radan, M.; Badavi, M. Ellagic Acid Ameliorates Lung Inflammation and Heart Oxidative Stress in Elastase-Induced Emphysema Model in Rat. Inflammation 2020, 43, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Madariaga-Navarrete, A.; Higuera-Piedrahita, R.I.; Delgadillo-Ruiz, L.; Bañuelos-Valenzuela, R.; Zaragoza-Bastida, A. Phytochemical Compounds and Pharmacological Properties of Larrea Tridentata. Molecules 2022, 27, 5393. [Google Scholar] [CrossRef]
- Marin-Tinoco, R.I.; Ortega-Ramírez, A.T.; Esteban-Mendez, M.; Silva-Marrufo, O.; Barragan-Ledesma, L.E.; Valenzuela-Núñez, L.M.; Briceño-Contreras, E.A.; Sariñana-Navarrete, M.A.; Camacho-Luis, A.; Navarrete-Molina, C. Antioxidant and Antibacterial Activity of Mexican Oregano Essential Oil, Extracted from Plants Occurring Naturally in Semiarid Areas and Cultivated in the Field and Greenhouse in Northern Mexico. Molecules 2023, 28, 6547. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Hernández, T.; Canales, M.; Duran, A.; María García, A.; Guillermo Avila, J.; Hernández-Portilla, L.; Alvarado, M.; Romero, M.; Terán, B.; Dávila, P.; et al. Variation in the Hexanic Extract Composition of Lippia graveolens in an Arid Zone from Mexico: Environmental Influence or True Chemotypes? Open Plant Sci. J. 2009, 3, 29–34. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bae, C.S.; Choi, Y.H.; Seo, N.S.; Na, C.S.; Yoo, J.C.; Cho, S.S.; Park, D.H. Opuntia Humifusa Modulates Morphological Changes Characteristic of Asthma via Il-4 and Il-13 in an Asthma Murine Model. Food Nutr. Res. 2017, 61, 1393307. [Google Scholar] [CrossRef]
- Martins, S.; Teixeira, J.A.; Mussatto, S.I. Solid-State Fermentation as a Strategy to Improve the Bioactive Compounds Recovery from Larrea Tridentata Leaves. Appl. Biochem. Biotechnol. 2013, 171, 1227–1239. [Google Scholar] [CrossRef]
- Loukili, E.H.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Zidi, K.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M.; et al. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia dillenii Fruits Collected from Morocco. J. Food Qual. 2022, 2022, 9471239. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.; Nevárez-Moorillón, G.V.; Aguilar, C.N. Total Phenolic Content, in Vitro Antioxidant Activity and Chemical Composition of Plant Extracts from Semiarid Mexican Region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular Docking Analysis of Rutin Reveals Possible Inhibition of SARS-CoV-2 Vital Proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and Biology of Selected Mexican Medicinal Plants. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2019; Volume 108, pp. 1–142. [Google Scholar]
- García-Andrade, M.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Rosales-Castro, M.; Medina-Torres, L. Mesquite Leaves (Prosopis laevigata), a Natural Resource with Antioxidant Capacity and Cardioprotection Potential. Ind. Crops Prod. 2013, 44, 336–342. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Duran-Hernandez, D.; Gutierrez-Miceli, F. Changes in the Phenylalanine Ammonia Lyase Activity, Total Phenolic Compounds, and Flavonoids in Prosopis glandulosa Treated with Cadmium and Copper. An. Acad. Bras. Cienc. 2018, 90, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Andrade-Cetto, A.; Cárdenas, R. Larrea Tridentata (Creosote Bush), an Abundant Plant of Mexican and US-American Deserts and Its Metabolite Nordihydroguaiaretic Acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Govea-Salas, M.; Morlett-Chávez, J.; Rodriguez-Herrera, R.; Ascacio-Valdés, J. Some Mexican Plants Used in Traditional Medicine. In Aromatic and Medicinal Plants—Back to Nature; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Favela-Hernández, J.M.J.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; Camacho-Corona, M.D.R. Potential Mechanism of Action of 3′-Demethoxy-6-O-Demethylisoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Kim, K.Y. Eucalyptol and Its Role in Chronic Diseases. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 929, pp. 389–398. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Duarte, G.; Navarrete, A.; Bye, R.; Linares, E.; Mata, R. Chemical Composition and Antimicrobial and Spasmolytic Properties of Poliomintha longiflora and Lippia graveolens Essential Oils. J. Food Sci. 2011, 76, C309–C317. [Google Scholar] [CrossRef] [PubMed]
- Kheiry, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V. P-Coumaric Acid Attenuates Lipopolysaccharide-Induced Lung Inflammation in Rats by Scavenging ROS Production: An In Vivo and In Vitro Study. Inflammation 2019, 42, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Hernandez, J.I.; Roncal, E. Comparison of Isolation Procedures for Mexican Oregano Oil. Food/Nahr. 1990, 34, 825–830. [Google Scholar] [CrossRef]
- Liu, M.; Niu, W.; Ou, L. β-Caryophyllene Ameliorates the Mycoplasmal Pneumonia through the Inhibition of NF-ΚB Signal Transduction in Mice. Saudi J. Biol. Sci. 2021, 28, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Estell, R.E.; Havstad, K.M.; Fredrickson, E.L.; Gardea-Torresdey, J.L. Secondary Chemistry of the Leaf Surface of Flourensia cernua. Biochem. Syst. Ecol. 1994, 22, 73–77. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M.; Mota Flores, J.M. Petiveria Alliacea Suppresses Airway Inflammation and Allergen-Specific Th2 Responses in Ovalbumin-Sensitized Murine Model of Asthma. Chin. J. Integr. Med. 2018, 24, 912–919. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Pérez-López, A.; Becerril-Montes, P.; Salazar-Aranda, R.; Said-Fernández, S.; Torres, N.W. de Evaluation of the Flora of Northern Mexico for in Vitro Antimicrobial and Antituberculosis Activity. J. Ethnopharmacol. 2007, 109, 435–441. [Google Scholar] [CrossRef]
- Wächter, G.A.; Hoffmann, J.J.; Furbacher, T.; Blake, M.E.; Timmermann, B.N. Antibacterial and Antifungal Flavanones from Eysenhardtia texana. Phytochemistry 1999, 52, 1469–1471. [Google Scholar] [CrossRef]
- Riaz, B.; Sohn, S. Neutrophils in Inflammatory Diseases: Unraveling the Impact of Their Derived Molecules and Heterogeneity. Cells 2023, 12, 2621. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Zapata-Morales, J.R.; Ruiz-Padilla, A.J.; Solorio-Alvarado, C.R.; Rangel-Velázquez, J.E.; Cruz-Jiménez, G.; Orozco-Castellanos, L.M.; Domínguez, F.; Maldonado-Miranda, J.J.; Carranza-Álvarez, C.; et al. Use of Medicinal Plants by Health Professionals in Mexico. J. Ethnopharmacol. 2017, 198, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea Genus: A Review on Phytochemistry, Pharmacology, and Toxicology. Evid.-Based Complement. Altern. Med. 2018, 2018, 9070927. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Lu, P.; Wu, H.; Liu, Z.; Sharifi-Rad, J.; Setzer, W.N.; Suleria, H.A.R. Current Insights into Phytochemistry, Nutritional, and Pharmacological Properties of Prosopis Plants. Evid.-Based Complement. Altern. Med. 2022, 2022, 2218029. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Franco, C.; Maldonado Aguirre, L.J. Overview of Past, Current and Potential Uses of Mesquite in Mexico. In Prosopis; Semiarid Fuelwood and Forage Tree Building Consensus for the Disenfranchised; U.S. National Academy of Sciences Building: Washington, DC, USA, 1996; Volume 13. [Google Scholar]
- Kaushik, V.; Niketan, S.; Sachdeva, S.; Saini, V. A Review on Phytochemical and Pharmacological Potential of Prosopis cineraria. Int. J. Ethnobiol. Ethnomed. 2020, 1, 1–4. [Google Scholar]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Ben Amira, A.; Attia, H. Phenolic Profiles, Phytchemicals and Mineral Content of Decoction and Infusion of Opuntia ficus-indica Flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Fackler, C. Three Ethnobotanically Important Plants of Texas: Southern Prickly Ash, Ocotillo, and Jimson Weed. American Botanical Council. HerbalEGram 2016, 13. [Google Scholar]
- Villagomez-Ibarra, J.R.; Sánchez, M.; Espejo, O.; Zuniga-Estrada, A.; Torres-Valencia, J.M.; Joseph-Nathan, P. Antimicrobial Activity of Three Mexican Gnaphalium Species. Fitoterapia 2001, 72, 692–694. [Google Scholar] [CrossRef]
- Listados de Registros Sanitarios de Medicamentos|Comisión Federal Para La Protección Contra Riesgos Sanitarios|Gobierno|Gob.Mx. Available online: https://www.gob.mx/cofepris/documentos/registros-sanitarios-medicamentos (accessed on 17 January 2024).
- Mpiana, P.T.; Ngbolua, K.-T.; Tshibangu, D.S.; Kilembe, J.T.; Gbolo, B.Z.; Mwanangombo, D.T.; Inkoto, C.L.; Lengbiye, E.M.; Mbadiko, C.M.; Matondo, A.; et al. Identification of Potential Inhibitors of SARS-CoV-2 Main Protease from Aloe Vera Compounds: A Molecular Docking Study. Chem. Phys. Lett. 2020, 754, 137751. [Google Scholar] [CrossRef]
- Timalsina, D.; Pokhrel, K.P.; Bhusal, D. Pharmacologic Activities of Plant-Derived Natural Products on Respiratory Diseases and Inflammations. BioMed Res. Int. 2021, 2021, 1636816. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Pishkar, L.; Eftekhari, Z.; Barzin, G.; Babaeekhou, L. The Human Host Defense Peptide LL-37 Overexpressed in Lung Cell Lines by Methanolic Extract of Valeriana officinalis. Braz. J. Pharm. Sci. 2023, 59, e21025. [Google Scholar] [CrossRef]
- Šutovská, M.; Capek, P.; Kočmalová, M.; Pawlaczyk, I.; Zaczyńska, E.; Czarny, A.; Uhliariková, I.; Gancarz, R.; Fraňová, S. Characterization and Pharmacodynamic Properties of Arnica Montana Complex. Int. J. Biol. Macromol. 2014, 69, 214–221. [Google Scholar] [CrossRef] [PubMed]



| Respiratory Diseases | Infection Factors | Climatic/Pollution and Other Factors | References |
|---|---|---|---|
| Bronchial asthma | Rhinoviruses, influenza virus, and respiratory syncytial virus | Cold air can exacerbate asthma symptoms by triggering the release of inflammatory mediators from mast cells, leading to airway contraction. Additionally, factors such as fatigue, infection, stress, and allergen exposure can further exacerbate asthma symptoms. | [31,32] |
| Chronic obstructive pulmonary disease COPD | A chronic lung disease characterized by airflow limitation. Some factors are respiratory pathogens (Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis). | Environmental pollutions, cigarette smoke, anxiety, depression, and other factors contribute to an increase in airway inflammation. | [33,34,35] |
| Lung cancer | Pneumonia and tuberculosis. Idiopathic interstitial pneumonia has been linked to lung carcinogenesis, with causative agents including Mycobacterium tuberculosis or S. pneumoniae. | Tobacco smoking is the primary risk factor responsible for 80 to 90% of lung cancer diagnoses. | [36,37,38] |
| Pneumonia | Pneumococcal disease, commonly caused by S. pneumoniae, affects lungs (pneumonia), bloodstream (bacteremia), and the tissues and fluids surrounding the brain and spinal cord (meningitis). | Microorganisms are the main factor of respiratory diseases, with their prevalence influenced by seasonal climates. S. pneumoniae is more common in winter, while Legionella pneumophila predominates in summer. | [36,39,40] |
| Tuberculosis | Infectious disease transmitted through cough aerosols and is caused by M. tuberculosis. While it primarily affects the lungs, it can also elevate the risk of developing lung cancer. | There is a positive correlation between climate change and increased susceptibility to tuberculosis, particularly in developing countries. | [38,41] |
| Bronchitis | Viruses are responsible for about 90% of acute bronchitis cases, with multiple viruses implicated including RSV, human rhinovirus, parainfluenza virus, human metapneumovirus, coronavirus, adenovirus, influenza, and enterovirus. | Exposure to air pollutants can lead to decreased absorption rates in the airways, potentially compromising the individual’s immune system and increasing susceptibility to acute infections. | [42,43,44,45] |
| Compound | Examples of Medicinal Plants | Effectiveness in Respiratory Disease | Description Compound | Chemical Structure | References |
|---|---|---|---|---|---|
| Quercetin | Opuntia humifusa; O. dillenii; Larrea tridentata; Flourensia cernua; Turnera diffusa; Salvia officinalis. | Potential against influenza A virus (IFV-A), rhinovirus, Respiratory Syncytial Virus (RSV). Inhibitory effect against pneumonia pathogens like Moraxella catarrhalis, K. pneumoniae, S. pneumoniae, and P. aeruginosa. Also exhibits anti-asthmatic efficacy. | It is one of the most abundant flavonoids. It exhibits antibacterial, antiviral, antioxidant, protein kinase inhibition, antineoplastic properties, and acts as a free radical scavenger. |  | [21,66,102,108,109,110,111,112] |
| Rutin | Schinus molle; Prosopis laevigata; P. glandulosa; O. dillenii | Effective against avian influenza virus, herpes simplex virus 1 and 2 (HSV-1, HSV-2), and parainfluenza-3 virus. It inhibits essential proteins of SARS-CoV-2. | It is a flavonoid with potent antioxidant properties and is widely utilized in medicine. Besides, it shows antiprotozoal, antibacterial, and antiviral properties. |  | [21,85,110,113,114,115,116] |
| Ellagic Acid | Fouquiera splendens; F. cernua; L. tridentata | It exhibits anti-proliferative, anti-inflammatory, and antioxidant effects, stimulating the activity of SOD and CAT enzymes. It mitigates pulmonary emphysema by inhibiting ROS, reducing lipid peroxidation, and enhancing antioxidant defenses. | It is a trihydroxybenzoic acid, primarily known for its antioxidant and anti-proliferative effects in therapeutic action. |  | [14,102,103,104] |
| Juliprosopin | P. glandulosa; P. flexuosa | Effective against respiratory disease-causing microorganisms such as S. aureus, M. intracellulare and C. neoformans. | Alkaloid with antimicrobial potential |  | [85] |
| Nordihydroguaiaretic acid (NDGA) | L. tridentata and L. divaricata | Antibacterial effect against S. aureus, S. pneumoniae, and antiviral against H. influenzae. Lung cancer | It has a role as an antioxidant, anti-inflammatory, antitumoral, ferroptosis inhibitor, lipoxygenase inhibitor, and geroprotectant. It is found in various molecules including catechols and lignans. |  | [46,104,117,118] |
| Thymol | L. graveolens; Lavandula angustifolia; L. tridentata | It has been utilized for its antiseptic, antibacterial, and antifungal actions in respiratory system diseases. In P. aeruginosa and S. aureus, it disrupts the cell membrane, increases membrane permeability, and decreases cytoplasmic pH in these bacteria. | It is a phenolic compound, a monoterpene derived from cymene. It acts as a volatile component present in the oils of various vegetal plants. |  | [104,105,106,107] |
| Carvacrol | L. graveolens; L. angustifolia; L. tridentata | In P. aeruginosa and S. aureus, it disrupts the cell membrane, enhances membrane permeability, and reduces cytoplasmic pH in these bacteria. | It is a type of phenol with antimicrobial and anti-inflammatory properties, capable of inhibiting the production of microbial toxins. |  | [104,105,106,107] |
| 3′-Demethoxy-6-O-demethylisoguaiacin | L. tridentata | Effective against methicillin-resistant S. aureus (MRSA) and exhibits moderate activity against a drug-resistant strain of M. tuberculosis. | Antibacterial, antiprotozoal, anthelmintic, antifungal, antiviral, anticancer, and antioxidant activities. It disrupts bacterial ABC transporters, affecting substrate transport across the membrane. |  | [119,120] |
| β-sitosterol | J. dioica | Effective against Cryptococcus neoformans; the symptoms produced by this microorganism are pneumonia and meningitis. | A high antibacterial and antifungal activity. |  | [55] |
| Eucalyptol or 1,8-Cineole | T. diffusa, Poliomintha longiflora; L. graveolens; S. officinalis. | This compound has antitussive effects, regulates mucus hypersecretion and asthma by inhibiting anti-inflammatory cytokinins, and reduces inflammation and pain when applied topically. | It is a monoterpene with antibacterial and antioxidant properties. Eucalyptol has the ability to penetrate the blood–brain barrier. |  | [57,112,121,122] |
| p-cumaric acid/Coumaric acid | Fouquieria spp.; F. cernua; S. officinalis. | Attenuates lipopolysaccharide-induced pulmonary inflammation in rats (from Gram-negative bacteria). It also exhibits antibacterial effects against M. tuberculosis. | It is a flavonoid and one of the three isomers of hydroxycinnamic acid. It has biochemical activities such antioxidant, an antimutagenic, and anti-ulcer properties. |  | [111,123] |
| Caffeic acid | O. dillenii; F. cernua; S. officinalis. | Regulates the proliferation, migration, and apoptosis of lung cancer cells. | It is a hydroxycinnamic acid derivative, namely cinnamic acid, with potential antioxidant, anti-inflammatory, and antineoplastic activities. |  | [102,110,124] |
| Linalool | L. angustifolia | Bactericidal activity against K. pneumoniae | It is a monoterpenoid, a volatile oil component, and an antimicrobial agent. |  | [106] |
| β-Caryophyllene | F. cernua; P. longiflora; L. graveolens; S. officinalis. | Effective against Mycoplasma pneumoniae. | It is a sesquiterpene with anti-inflammatory properties. |  | [102,112,122,125,126,127] |
| Plant | Part Used | Extraction Solvent | Dose | Active Compound Isolated | Mechanism of Action | Activity | References |
|---|---|---|---|---|---|---|---|
| Larrea tridentata | Leaves | Chloroformic (CLO) and methanol (MET) extracts | MIC 80 = 31.25 μg/mL against S. pneumoniae | Antioxidant NDGA | Unspecified | The MET and CLO extracts of L. tridentata were also effective against S. aureus, S. pneumoniae, and the MET was also active against H. influenzae. | [46,117] |
| Petiveria alliacea | Leaves | Metanol extract | Oral gavage to mice at 100–400 mg/kg body weight once daily from days 18 to 23. | No active compound was reported, but the extract exhibited high antioxidant activity against DPPH radical scavenging. | Inhibited the production of chemokines, eotaxin, TNF-α, IgE, TGF-α, IgE, and TGF-β1. In addition, it reduced cytokine levels of IL-4, IL-5, IL-13, and ICAM-1 in bronchoalveolar lavage fluid. | Administration could inhibit airway inflammation, regulate cytokines and chemokines, and improve pulmonary conditions in an allergic murine model of asthma. | [128] |
| Fouquieria splendens | Leaves | Methanol extract | MIC 25.0 μg/mL in S. aureus | ND | Unspecified | Antimicrobial activity against S. aureus and K. pneumoniae | [75] |
| Leucophyllum frutescens | Leaves | Methanol extract | MIC 25.4 μg/mL in S. aureus. | ND | Unspecified | Antimicrobial activity against M. tuberculosis, S. aureus, and H. influenzae b type | [75] |
| L. frutescens | Roots and leaves | Methanol extract | MIC 62.5 (roots), 125 (leaves) g/mL | ND | Unspecified | Antimicrobial activity against the drug-resistant strains of M. tuberculosis | [129] |
| Chrysanctinia mexicana | Roots | Ethyl ether extract | MIC 62.5 g/mL | ND | Unspecified | Antimicrobial activity against the drug-resistant strains of M. tuberculosis | [129] |
| Cordia boissieri | leaves | Methanol extract | MIC250 g/mL | ND | Unspecified | Antimicrobial activity against the drug-resistant strains of S. aureus | [129] |
| Schinus molle | fruits | Hexane-based extract | MIC 62.5 g/mL | ND | Unspecified | Antimicrobial activity against the drug-resistant strain of Streptococcus pneumoniae | [129] |
| Opuntia ficus indica (OFI) | Stems | OFI extracts (water, 30% ethanol, or 50% ethanol extracts) | 100 and 200 mg/kg OFI extracts | Narcissin in 50% ethanol extracts | All extracts suppressed neutrophil infiltration and the number of immune cells (CD3+/CD4+, CD3+/CD8+, and Gr-1+/CD11b) in bronchoalveolar lavage fluid (BALF) and lungs. OFI extracts also decreased the expression of cytokines and chemokines, including chemokine, including interleukins, macrophage inflammatory protein-2, and tumor necrosis factor (TNF)-α. | OFI extracts may be used to prevent and treat airway inflammation and respiratory diseases. | [100] |
| Eysenhardtia texana | Leaves | Methanol-dichloromethane extract | 0.1 mg/mL | 4′,5,7-trihydroxy-8-methyl-6-(3-methyl-[2-butenyl])-(2S)-flavanone, 4′,5,7-trihydroxy-6-methyl-8-(3-methyl-[2-butenyl])-(2S)-flavanone | Flavonoids often inhibit fungal growth through diverse mechanisms, such as disrupting the plasma membrane, inducing mitochondrial dysfunction, and inhibiting processes like cell wall formation, cell division, RNA and protein synthesis, and the efflux-mediated pumping system. | Activity against S. aureus and inhibited the growth of Candida albicans | [99,130] |
| Carya illinoensis, Jatropha dioica, Selaginella lepidophylla, Euphorbia antisyphilitica | Leaves | Methanol extracts | MIC 500 µg/mL and LD50 of 1000 µg/mL. | Phytochemical tests were positive for flavonoids, lactones, quinones, triterpenes, and sterols. | Unspecified | Activity against S. aureus and K. pneumonia | [49,52] |
| Turnera diffusa | Leaves | Ethanol/distilled water/glycerol (63/27/10) | MIC 300 µg/mL | Flavonoids, phenolic acids and derivatives, cyanogenic glycosides, fatty acids, alkaloids, and sugars conjugates. | Unspecified | Activity against S. aureus | [57,59] |
| Fouquieria splendens | Leaves | Methanol extract | (C33-A IC50: 9.06 µg/mL; HeLa IC50: 74.7 µg/mL | Phenolic compounds, ellagic acid, kaempferol-3-β-glucoside. | Unspecified | Anti-proliferative effect specifically against cervical cancer cell lines, particularly HPV negative cells | [72] |
| Scientific Name | Family | Common Name | Parts Used | Form of Use | Ailment or Symptoms | References |
|---|---|---|---|---|---|---|
| Bougainvillea glabra | Nyctaginaceae | Bugambilia | Leaves/Flower | Infusion/oral | Asthma/Cough/Bronchitis | [132,133] |
| B. spectabilis | Nyctaginaceae | Bugambilia | Leaves/Flower | Infusion/oral | Snoring or lung pain/Flu/Bronchitis | [133] |
| Lippia berlandieri | Verbenaceae | Oregano | Whole plant | Infusion/oral | Cough | [132] |
| Ruta chalepensis | Rutaceae | Ruda | Whole plant | Infusion/oral | Cough | [132] |
| Mentha spicata | Lamiaceae | Hierbabuena/menta | Whole plant | Infusion/oral | Bronchitis/Cough | [129,132] |
| Foeniculum vulgare | Apiaceae | Hinojo | Whole plant | Infusion/oral | Bronchitis | [132] |
| Schinus molle | Anacardiaceae | Pirul | Leaves | Infusion/topical | Cough | [132] |
| Allium cepa | Alliaceae | Cebolla | Bulb | Bulb/bulb infusion | Tuberculosis/Cough | [132] |
| Prosopis juliflora | Fabaceae | Mesquite | Bark/leaves | Paste/poultice/Gum/Smoke/Decoction/Infusion/Maceration/Baths | Respiratory disorders/Asthma/Antibacterial capacity against Gram-negative bacteria | [134,135] |
| Prosopis spp. | Fabaceae | Mesquite | Bark/Resin from the trunk/Seed pods | Resin from the trunk/Seed pods/leaves. | Spasmolytic/Bronchodilator/Vasodilator/Asthma/Sore throat/Antibacterial | [134,135,136] |
| Larrea tridentata | Zygophyllaceae | Gobernadora, hediondilla or guamis, creosote bush | Leaves | Infusion/Oral | Cold virus infections e.g influenza virus /Sinusitis/Lung cancer | [117,118] |
| Jatropha dioica | Euphorbiaceae | Sangre de drago or Sangregrado | Root/Stem | Infusion/Decoctions/Oral | Antimicrobial/asthma/Influenza type A virus | [118] |
| Turnera diffusa | Turneraceae | Damiana, hierba del venado, hierba de la pastora | Leaves | Infusion/Decoctions/Oral | Bronchitis/Cough/Pulmonar/Respiratory diseases/Expectorant | [47,118] |
| Opuntia ficus-indica | Cactaceae | Nopal, penca, tunera. | Dry flowers | Infusion/Decoctions/Oral | Antiviral, RSV. Inhibitory effect against pneumonia pathogens. | [137] |
| Fouquieria splendens | Fouquieriaceae | Ocotillo | Fresh flowers/Seeds | Infusion/Oral | Cough/Sore throat | [138] |
| Chaenopodium ambrosioides | Chenopodiaceae | Epazote | Flowers/Leaves/Stems | Infusion/Oral | Flu/cold/asthma and pectoral complaints | [129] |
| Flourensia cernua | Asteraceae | Hojasen | Leaves/Stems | Infusion/Oral | Expectorant/Bactericidal compounds against multidrug-resistant M. tuberculosis | [129] |
| Lavandula angustifolia | Lamiaceae | Lavanda | Flowers/Leaves/Stems | Infusion/Oral | Infection by K. pneumoniae | [106] |
| Gnaphalium oxyphyllum | Asteraceae | Gordolobo | Flowers/Leaves/Stems | Infusion/Oral | Cough/Chest pain/Bronchitis/Sore throat/Gripe/Asthma/Cancer | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila-Rangel, I.E.; Charles-Rodríguez, A.V.; López-Romero, J.C.; Flores-López, M.L. Plants from Arid and Semi-Arid Zones of Mexico Used to Treat Respiratory Diseases: A Review. Plants 2024, 13, 792. https://doi.org/10.3390/plants13060792
Dávila-Rangel IE, Charles-Rodríguez AV, López-Romero JC, Flores-López ML. Plants from Arid and Semi-Arid Zones of Mexico Used to Treat Respiratory Diseases: A Review. Plants. 2024; 13(6):792. https://doi.org/10.3390/plants13060792
Chicago/Turabian StyleDávila-Rangel, Irma E., Ana V. Charles-Rodríguez, Julio C. López-Romero, and María L. Flores-López. 2024. "Plants from Arid and Semi-Arid Zones of Mexico Used to Treat Respiratory Diseases: A Review" Plants 13, no. 6: 792. https://doi.org/10.3390/plants13060792