Screening of Sugarcane Proteins Associated with Defense against Leifsonia xyli subsp. xyli, Agent of Ratoon Stunting Disease
Abstract
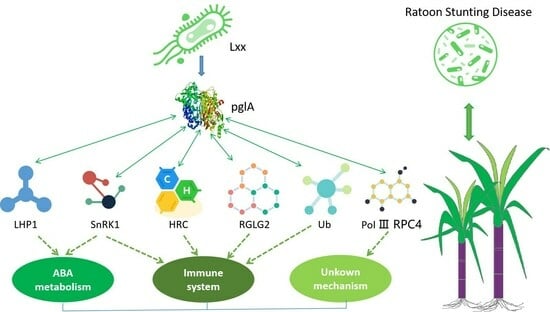
:1. Introduction
2. Results
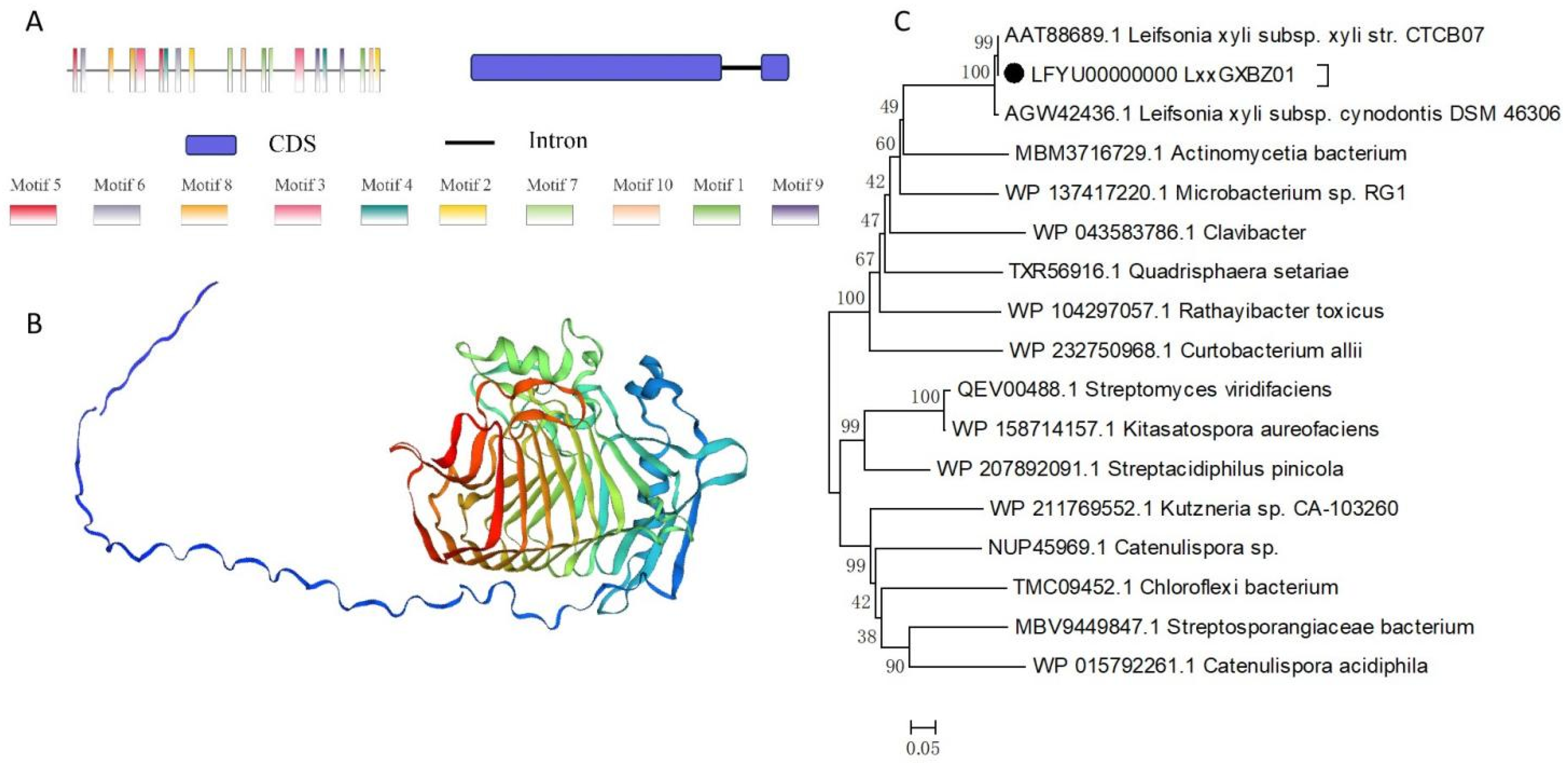
2.1. Characteristics of pglA Gene and Protein
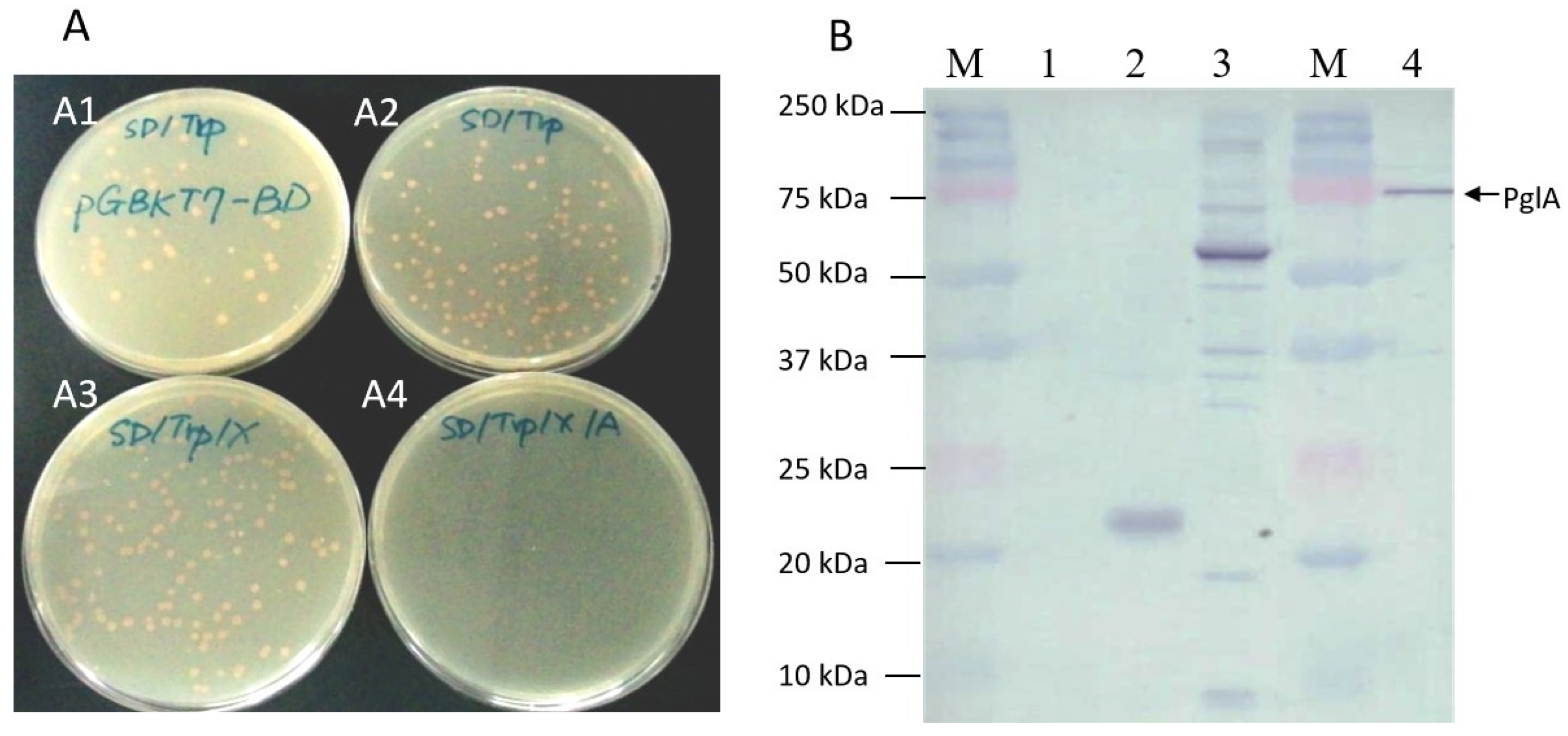
2.2. Toxicity, Autoactivation and Protein Expression of pglA Bait
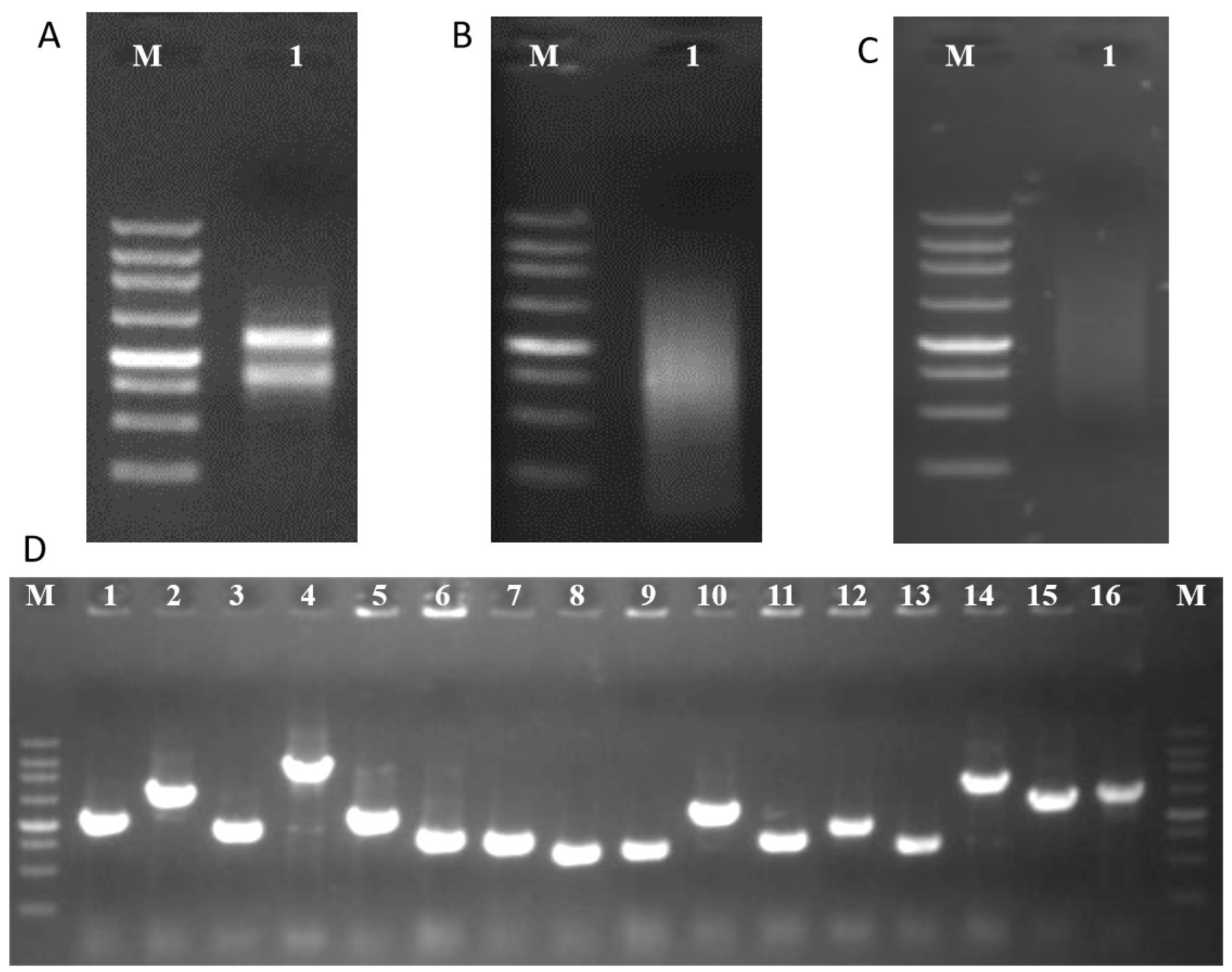
2.3. Construction of Y2H Library
2.4. Screening of pglA-Interacting Proteins
2.5. Identification of Proteins Interacting with pglA
2.6. Re-Test of Proteins Interaction
2.7. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Sequence Characteristics of the pglA Gene
4.2. Construction, Toxicity Testing, Autoactivation and Expression of pglA Bait
4.3. Construction of Y2H Library
4.4. Screening of pglA-Interacting Proteins
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, Y.W.; Gao, X.N.; Zeng, Q.Y.; Zheng, Z.; Wu, C.W.; Yang, R.Z.; Feng, X.M. Sugarcane breeding, germplasm development and related molecular research in China. Sugar Tech 2022, 24, 73–85. [Google Scholar] [CrossRef]
- Quecine, M.C.; Silva, T.M.; Carvalho, G.; Saito, S.; Mondin, M.; Teixeira-Silva, N.S.; Camargo, L.E.A.; Monteiro-Vitorello, C.B. A stable Leifsonia xyli subsp. xyli GFP-tagged strain reveals a new colonization niche in sugarcane tissues. Plant Pathol. 2016, 65, 154–162. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Cia, M.C.; Batista-de-Andrade-Granato, A.; Muniz, L.F.; Appezzato-da-Glória, B.; Camargo, L.E.A. Histopathology of the shoot apex of sugarcane colonized by Leifsonia xyli subsp. xyli. Phytopathology 2022, 12, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Chen, M.H.; Liang, Y.J.; Xing, Y.X.; Comstock, J.C.; Yang, L.T.; Li, Y.R. Morphological and physiological responses of sugarcane to Leifsonia xyli subsp. xyli infection. Plant Dis. 2016, 100, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Liang, Y.J.; Song, X.P.; Wang, Z.P.; Zhang, B.Q.; Lei, J.C.; Yang, R.L.; Yan, M.X. Changes in gene expression levels and chloroplast anatomy induced by Leifsonia xyli subsp. xyli in sugarcane. J. Plant Interact. 2021, 16, 564–574. [Google Scholar] [CrossRef]
- Zhu, K.; Yuan, D.; Zhang, X.Q.; Yang, L.T.; Li, Y.R. The physiological characteristics and associated gene expression of sugar cane inoculated with Leifsonia xyli subsp. xyli. J. Phytopathol. 2018, 166, 44–52. [Google Scholar] [CrossRef]
- Chakraborty, M.; Ford, R.; Soda, N.; Strachan, S.; Ngo, C.N.; Bhuiyan, S.A.; Shiddiky, M. Ratoon Stunting Disease (RSD) of sugarcane: A review emphasizing detection strategies and challenges. Phytopathology 2023. [Google Scholar] [CrossRef]
- Li, W.F.; Shen, K.; Huang, Y.K.; Wang, X.Y.; Yin, J.; Luo, Z.M.; Zhang, R.Y.; Shan, H.L. Incidence of sugarcane ratoon stunting disease in the major cane-growing regions of China. Crop Prot. 2014, 60, 44–47. [Google Scholar] [CrossRef]
- Carvalho, G.; Da Silva, T.G.E.R.; Munho, A.T.; Monteiro-Vitorello, C.B.; Azevedo, R.A.; Melotto, M.; Camargo, L.E.A. Development of a qPCR for Leifsonia xyli subsp. xyli and quantification of the effects of heat treatment of sugarcane cuttings on Lxx. Crop Prot. 2016, 80, 51–55. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Du, H.; Fang, C.; Li, Y.; Kong, F.; Liu, B. Understandings and future challenges in soybean functional genomics and molecular breeding. J. Integr. Plant Biol. 2023, 5, 468–495. [Google Scholar] [CrossRef]
- Collmer, A.; Keen, N.T. The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 1986, 24, 383–409. [Google Scholar] [CrossRef]
- Roper, M.C.; Greve, L.C.; Warren, J.G.; Labavitch, J.M.; Kirkpatrick, B.C. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol. Plant-Microbe Interact. 2007, 20, 411–419. [Google Scholar] [CrossRef]
- Denny, T.P.; Carney, B.F.; Schell, M.A. Inactivation of multiple virulence genes reduces the ability of Pseudomonas solanacearum to cause wilt symptoms. Mol. Plant-Microbe Interact. 1990, 3, 293–300. [Google Scholar] [CrossRef]
- Warren, J.G.; Lincoln, E.; Kirkpatrick, B.C. Insights into the activity and substrate binding of Xylella fastidiosa polygalacturonase by modification of a unique QMK amino acid motif using protein chimeras. PLoS ONE 2015, 10, e0142694. [Google Scholar] [CrossRef]
- Tayi, L.; Maku, R.V.; Patel, H.K.; Sonti, R.V. Identification of pectin degrading enzymes secreted by Xanthomonas oryzae pv. oryzae and determination of their role in virulence on rice. PLoS ONE 2016, 11, e0166396. [Google Scholar] [CrossRef]
- Bashi, Z.D.; Rimmer, S.R.; Khachatourians, G.G.; Hegedus, D.D. Brassica napus polygalacturonase inhibitor proteins inhibit Sclerotinia sclerotiorum polygalacturonase enzymatic and necrotizing activities and delay symptoms in transgenic plants. Can. J. Microbiol. 2013, 59, 79–86. [Google Scholar] [CrossRef]
- Liu, N.N.; Ma, X.W.; Sun, Y.; Hou, Y.X.; Zhang, X.Y.; Li, F.G. Necrotizing activity of Verticillium dahliae and Fusarium oxysporum f. sp. vasinfectum endopolygalacturonases in cotton. Plant Dis. 2017, 101, 1128–1138. [Google Scholar] [CrossRef]
- Monteiro-Vitorello, C.B.; Camargo, L.E.; Van-Sluys, M.A.; Kitajima, J.P.; Truffi, D.; Do-Amaral, A.M.; Harakava, R.; de-Oliveira, J.C.F.; Wood, D.; de-Oliveira, M.C.; et al. The genome sequence of the gram-positive sugarcane pathogen Leifsonia xyli subsp. xyli. Mol. Plant-Microbe Interact. 2004, 17, 827–836. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, L.; Cao, G.; Zhang, M.Q.; Guo, Y. Draft genome sequence of Leifsonia xyli subsp. xyli strain gdw1. Genome Announc. 2016, 4, e01128-16. [Google Scholar] [CrossRef]
- Vidal, M.; Fields, S. The yeast two-hybrid assay: Still finding connections after 25 years. Nat. Methods 2014, 11, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, P.; Ponnusamy, K.; Chakraborty, S. A geminivirus betasatellite encoded βC1 protein interacts with PsbP and subverts PsbP-mediated antiviral defence in plants. Mol. Plant Pathol. 2019, 20, 943–960. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Liu, X.; Zhang, X.; Jiang, P.; Ma, H. Yeast two-hybrid screening for proteins that interact with PFT in wheat. Sci. Rep. 2019, 9, 15521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yang, L.T.; Li, C.X.; Lakshmanan, P.; Xing, Y.X.; Li, Y.R. A transcriptomic analysis of sugarcane response to Leifsonia xyli subsp. xyli infection. PLoS ONE 2021, 16, e0245613. [Google Scholar] [CrossRef]
- Stynen, B.; Tournu, H.; Tavernier, J.; Van-Dijck, P. Diversity in genetic in vivo methods for protein-protein interaction studies: From the yeast two-hybrid system to the mammalian split-luciferase system. Microbiol. Mol. Biol. Rev. 2012, 76, 331–382. [Google Scholar] [CrossRef]
- Gao, J.; Jing, J.; Yu, C.J.; Chen, J. Construction of a high-quality yeast two-hybrid library and its application in identification of interacting proteins with brn1 in Curvularia lunata. Plant Pathol. J. 2015, 31, 108–114. [Google Scholar] [CrossRef]
- Holcroft, J.; Ganss, B. Identification of amelotin-and ODAM-interacting enamel matrix proteins using the yeast two-hybrid system. Eur. J. Oral Sci. 2011, 119, 301–306. [Google Scholar] [CrossRef]
- Zhang, S. Screening and verification for proteins that interact with leucine aminopeptidase of Taenia pisiformis using a yeast two-hybrid system. Parasitol. Res. 2019, 118, 3387–3398. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Latrasse, D.; Rodriguez-Granados, N.Y.; Huang, Y.; Manza-Mianza, D.; Brik-Chaouche, R.; Jaouannet, M. The Polycomb protein LHP 1 regulates Arabidopsis thaliana stress responses through the repression of the MYC2-dependent branch of immunity. Plant J. 2019, 100, 1118–1131. [Google Scholar] [CrossRef]
- Comstock, J.C. Ratoon stunting disease. Sugar Tech 2002, 4, 1–6. [Google Scholar] [CrossRef]
- Mansilla, N.; Ferrero, L.; Ariel, F.D.; Lucero, L.E. The Potential Use of the Epigenetic Remodeler LIKE HETEROCHROMATIN PROTEIN 1 (LHP1) as a Tool for Crop Improvement. Horticulture 2023, 9, 199. [Google Scholar] [CrossRef]
- Ramon, M.; Ruelens, P.L.Y.; Sheen, J.; Geuten, K.; Rolland, F. The hybrid four-CBS-domain KIN βγ subunit functions as the canonical γ subunit of the plant energy sensor SnRK1. Plant J. 2013, 75, 11–25. [Google Scholar] [CrossRef]
- Emanuelle, S.; Doblin, M.S.; Stapleton, D.I.; Bacic, A.; Gooley, P.R. Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci. 2016, 21, 341–353. [Google Scholar] [CrossRef]
- Hulsmans, S.; Rodriguez, M.; De Coninck, B.; Rolland, F. The SnRK1 energy sensor in plant biotic interactions. Trends Plant Sci. 2016, 21, 648–661. [Google Scholar] [CrossRef]
- Belda-Palazon, B.; Adamo, M.; Valerio, C.; Ferreira, L.J.; Confraria, A.; Reis-Barata, D.; Rodrigues, A.; Meyer, C.; Rodriguez, P.L.; Baena-González, E. A dual function of SnRK2 kinases in the regulation of SnRK1 and plant growth. Nat. Plants 2020, 6, 345–1353. [Google Scholar] [CrossRef]
- Han, X.; Zhang, L.; Zhao, L.; Xue, P.; Qi, T.; Zhang, C.; Yuan, H.; Zhou, L.; Wang, D.; Qiu, J.; et al. SnRK1 phosphorylates and destabilizes WRKY3 to enhance barley immunity to powdery mildew. Plant Commun. 2020, 1, 100083. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Kushalappa, A.C.; Hegde, N.G.; Gunnaiah, R.; Sathe, A.; Yogendra, K.N.; Ajjamada, L. Apoptotic-like PCD inducing HRC gene when silenced enhances multiple disease resistance in plants. Sci. Rep. 2022, 12, 20402. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Mao, H.; Jin, H.; Sun, Y.; Qi, Z. A Leymus chinensis histidine-rich Ca2+-binding protein binds Ca2+/Zn2+ and suppresses abscisic acid signaling in Arabidopsis. J. Plant Physiol. 2020, 252, 153209. [Google Scholar] [CrossRef]
- Serrano, I.; Campos, L.; Rivas, S. Roles of E3 ubiquitin-ligases in nuclear protein homeostasis during plant stress responses. Front. Plant Sci. 2018, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Sadanandom, A.; Bailey, M.; Ewan, R.; Lee, J.; Nelis, S. The ubiquitin-proteasome system: Central modifier of plant signalling. New Phytol. 2012, 196, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Froidure, S.; Canonne, J.; Ben Khaled, S.; Khafif, M.; Pouzet, C.; Jauneau, A.; Roby, D.; Rivas, S. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defense. Nat. Commun. 2013, 4, 1476. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, W.; Wang, J.; Wang, L.; Yao, W.; Yang, Y.; Xu, Y.; Ma, F.; Du, Y.; Wang, Y. The Chinese wild grapevine (Vitis pseudoreticulata) E3 ubiquitin ligase Erysiphe necator-induced RING finger protein 1 (EIRP1) activates plant defense responses by inducing proteolysis of the VpWRKY11 transcription factor. New Phytol. 2013, 200, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Hsieh, E.J.; Chen, J.H.; Chen, H.Y.; Lin, T.P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012, 158, 363–375. [Google Scholar] [CrossRef]
- Dieci, G.; Bosio, M.C.; Fermi, B.; Ferrari, R. Transcription reinitiation by RNA polymerase III. Biochim. Biophys. Acta. 2013, 1829, 331–341. [Google Scholar] [CrossRef]
- Arimbasseri, A.G.; Maraia, R.J.T. RNA polymerase III advances: Structural and tRNA functional views. Trends Biochem. Sci. 2016, 41, 546–559. [Google Scholar] [CrossRef]
- Wang, Q.; Daiß, J.L.; Xu, Y.; Engel, C. Snapshots of RNA polymerase III in action-A mini review. Gene 2022, 821, 146282. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Yamagata, Y.; Shigematsu, Y.; Watanabe, M.; Miyazaki, Y.; Doi, K.; Tashiro, K.; Kuhara, S.; Kanamori, H.; Wu, J.; et al. Duplication and loss of function of genes encoding RNA polymerase III subunit C4 causes hybrid incompatibility in rice. G3-Genes Genomes Genet. 2017, 7, 2565–2575. [Google Scholar] [CrossRef]
- Nemchinov, L.G.; Boutanaev, A.M.; Postnikova, O.A. Virus-induced gene silencing of the RPC5-like subunit of RNA polymerase III caused pleiotropic effects in Nicotiana benthamiana. Sci. Rep. 2016, 6, 27785. [Google Scholar] [CrossRef]
- Park, J.L.; Lee, Y.S.; Kunkeaw, N.; Kim, S.Y.; Kim, I.H.; Lee, Y.S. Epigenetic regulation of noncoding RNA transcription by mammalian RNA polymerase III. Epigenomics 2017, 9, 171–187. [Google Scholar] [CrossRef] [PubMed]


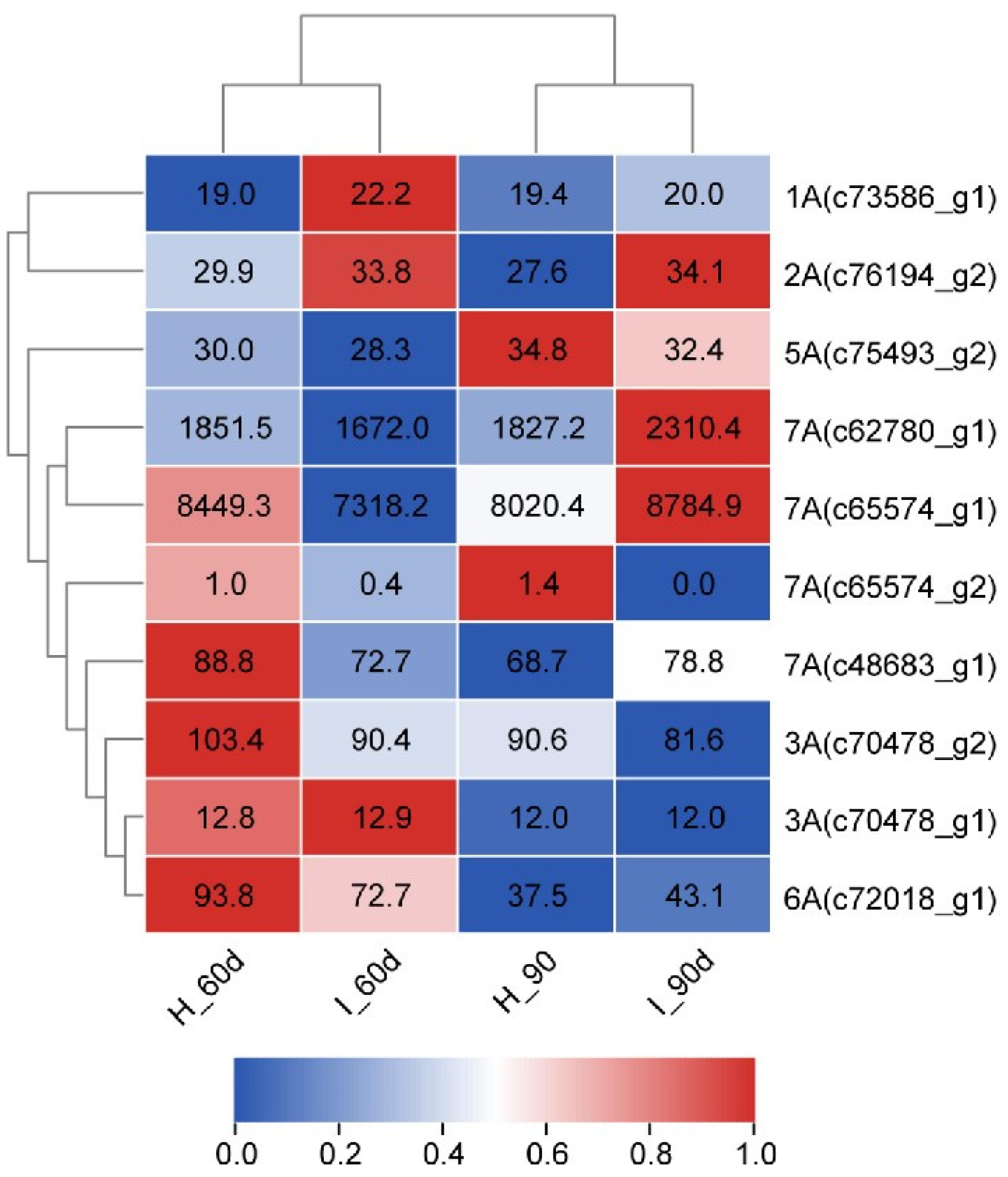
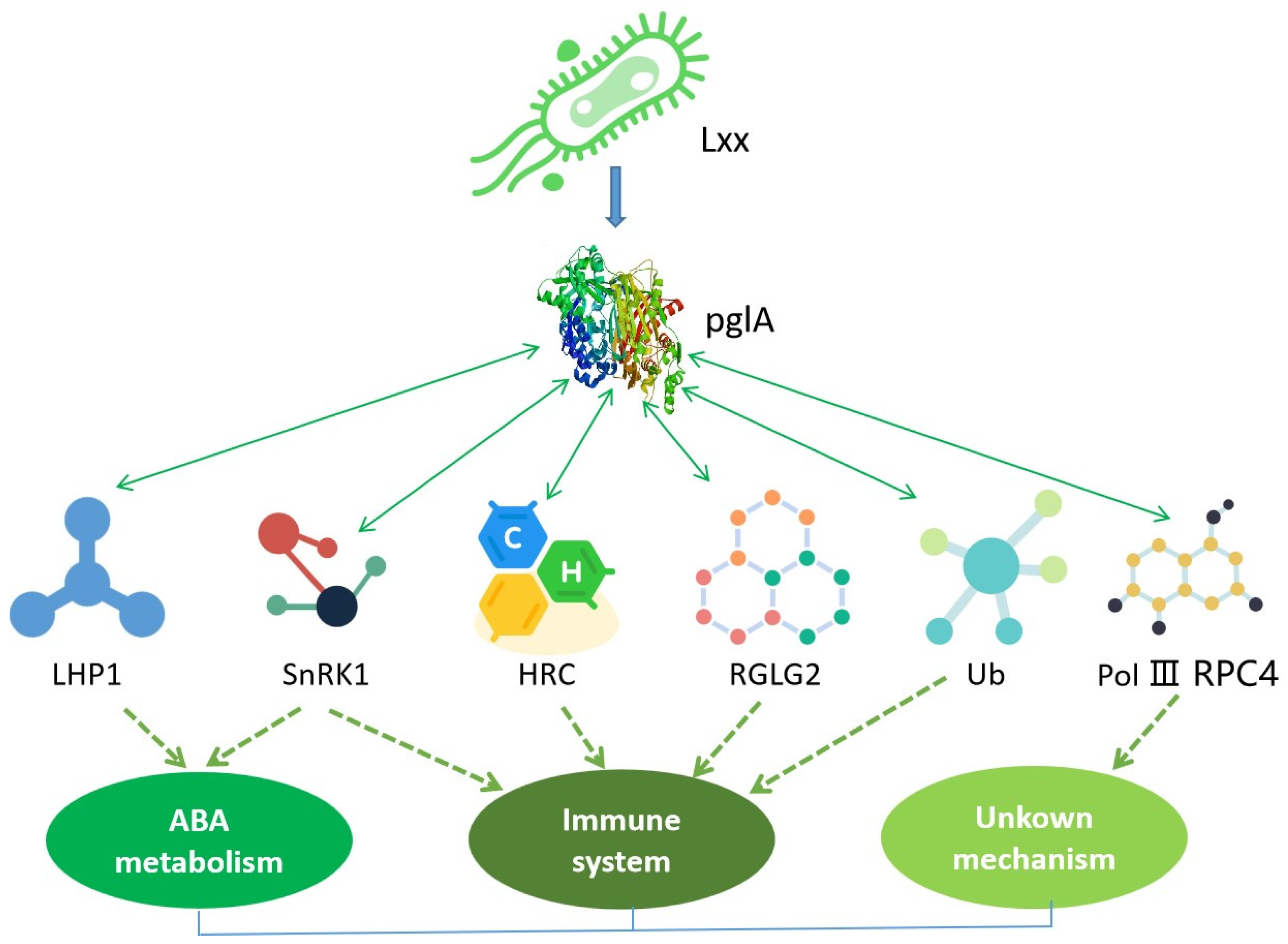
| No. | Gene Name | UniProtKB Name | Protein Name | Homology Function in Sorghum bicolor |
|---|---|---|---|---|
| 1A | AtLHP1 | A0A1P8B9G6_ARATH | Like heterochromatin protein (LHP1) | Probable chromo domain protein LHP1 |
| 2A | KINB1 | KINB1_ARATH | SNF1-related protein kinase regulatory subunit beta-1 | SNF1-related protein kinase regulatory subunit beta-1 |
| 3A | Hrc | A0A8I5ZZ96 | Histidine-rich calcium-binding protein | Sarcoplasmic reticulum histidine-rich calcium-binding protein |
| 4A | / | / | / | Yeast vector pDEST-GADT7 |
| 5A | RPC4 | RPC4_YEAST | DNA-directed RNA polymerase III subunit RPC4 | DNA-directed RNA polymerase III subunit RPC4 |
| 6A | RGLG2 | RGLG2_ARATH | E3 ubiquitin-protein ligase RGLG2 | E3 ubiquitin-protein ligase RGLG2 |
| 7A | UBC | UBC_HUMAN | Polyubiquitin-C | Polyubiquitin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Q.; Liang, Y.-J.; Zhang, B.-Q.; Yan, M.-X.; Wang, Z.-P.; Huang, D.-M.; Huang, Y.-X.; Lei, J.-C.; Song, X.-P.; Huang, D.-L. Screening of Sugarcane Proteins Associated with Defense against Leifsonia xyli subsp. xyli, Agent of Ratoon Stunting Disease. Plants 2024, 13, 448. https://doi.org/10.3390/plants13030448
Zhang X-Q, Liang Y-J, Zhang B-Q, Yan M-X, Wang Z-P, Huang D-M, Huang Y-X, Lei J-C, Song X-P, Huang D-L. Screening of Sugarcane Proteins Associated with Defense against Leifsonia xyli subsp. xyli, Agent of Ratoon Stunting Disease. Plants. 2024; 13(3):448. https://doi.org/10.3390/plants13030448
Chicago/Turabian StyleZhang, Xiao-Qiu, Yong-Jian Liang, Bao-Qing Zhang, Mei-Xin Yan, Ze-Ping Wang, Dong-Mei Huang, Yu-Xin Huang, Jing-Chao Lei, Xiu-Peng Song, and Dong-Liang Huang. 2024. "Screening of Sugarcane Proteins Associated with Defense against Leifsonia xyli subsp. xyli, Agent of Ratoon Stunting Disease" Plants 13, no. 3: 448. https://doi.org/10.3390/plants13030448