Ethylene Modulates Rice Root Plasticity under Abiotic Stresses
Abstract
:1. Introduction
2. Improving Root System Contributes to Increase Rice Yield and Stress Resistance
3. Ethylene Confers Diverse Roles in Rice Root Development
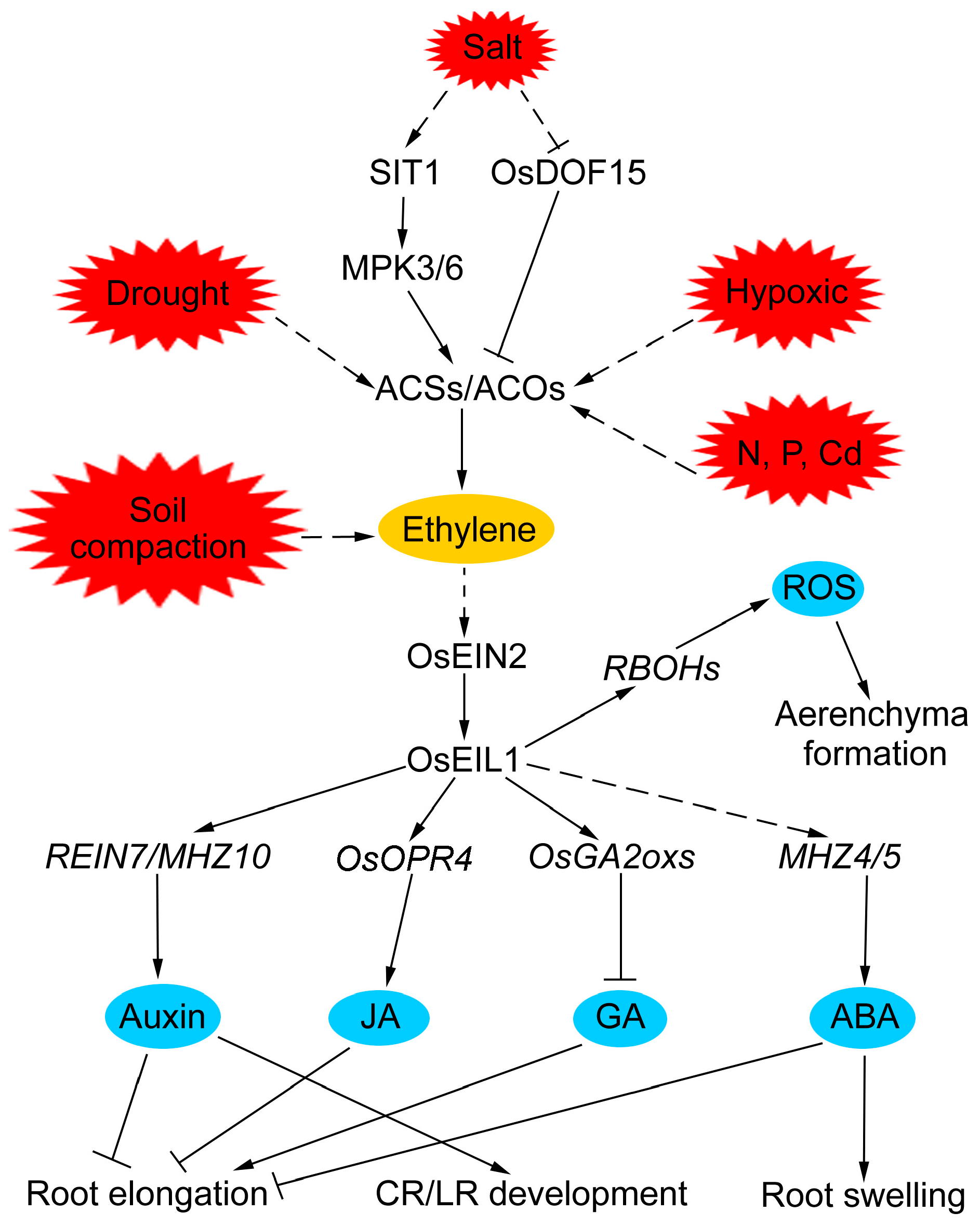
4. Ethylene Regulates Root Development under Abiotic Stresses
4.1. Salinity
4.2. Drought
4.3. Soil Compaction
4.4. Hypoxic Conditions
4.5. Nutrients
4.6. Heavy Metals
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Oort, P.A.J.; Zwart, S.J. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Glob. Chang. Biol. 2018, 24, 1029–1045. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Butardo, V.M., Jr.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kavi Kishor, P.B. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Hussain, S.; Yang, S.; Li, R.K.; Liu, S.L.; Chen, Y.L.; Wei, H.H.; Dai, Q.G.; Hou, H.Y. Study on the effect of salt stress on yield and grain quality among different rice varieties. Front. Plant Sci. 2022, 13, 918460. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, W.; Xing, L.; Zhu, J.K.; Persson, S.; Zhao, Y. Root twisting drives halotropism via stress-induced microtubule reorientation. Dev. Cell 2022, 57, 2412–2425. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Seomun, S.; Choi, Y.D.; Jang, G. Root development and stress tolerance in rice: The key to improving stress tolerance without yield penalties. Int. J. Mol. Sci. 2020, 21, 1807. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Villordon, A.Q.; Ginzberg, I.; Firon, N. Root architecture and root and tuber crop productivity. Trends Plant Sci. 2014, 19, 419–425. [Google Scholar] [CrossRef]
- Gewin, V. Food: An underground revolution. Nature 2010, 466, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; He, L.; Huang, R. The coordination of ethylene and other hormones in primary root development. Front. Plant Sci. 2019, 10, 874. [Google Scholar] [CrossRef]
- Pandey, B.K.; Huang, G.Q.; Bhosale, R.; Hartman, S.; Sturrock, C.J.; Jose, L.; Martin, O.C.; Karady, M.; Voesenek, L.A.C.J.; Ljung, K.; et al. Plant roots sense soil compaction through restricted ethylene diffusion. Science 2021, 371, 276–280. [Google Scholar] [CrossRef]
- Zou, X.; Liu, L.; Hu, Z.B.; Wang, X.K.; Zhu, Y.C.; Zhang, J.L.; Li, X.F.; Kang, Z.Y.; Lin, Y.J.; Yin, C.X. Salt-induced inhibition of rice seminal root growth is mediated by ethylene-jasmonate interaction. J. Exp. Bot. 2021, 72, 5656–5672. [Google Scholar] [CrossRef]
- Okamoto, T.; Tsurumi, S.; Shibasaki, K.; Obana, Y.; Takaji, H.; Oono, Y.; Rahman, A. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiol. 2008, 146, 1651–1662. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher-plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice and Arabidopsis: New regulators and mechanisms. J. Integr. Plant Biol. 2021, 63, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, K.X.; Ma, B.; Yin, C.C.; Hu, Y.; Tao, J.J.; Huang, Y.H.; Cao, W.Q.; Chen, H.; Yang, C.; et al. Histidine kinase MHZ1/OsHK1 interacts with ethylene receptors to regulate root growth in rice. Nat. Commun. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhou, Y.; Chen, H.; He, S.J.; Huang, Y.H.; Zhao, H.; Lu, X.; Zhang, W.K.; Pang, J.H.; Chen, S.Y.; et al. Membrane protein MHZ3 stabilizes OsEIN2 in rice by interacting with its Nramp-like domain. Proc. Natl. Acad. Sci. USA 2018, 115, 2520–2525. [Google Scholar] [CrossRef]
- Huang, Y.H.; Han, J.Q.; Ma, B.; Cao, W.Q.; Li, X.K.; Xiong, Q.; Zhao, H.; Zhao, R.; Zhang, X.; Zhou, Y.; et al. A translational regulator MHZ9 modulates ethylene signaling in rice. Nat. Commun. 2023, 14, 4674. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, B.; Duan, K.X.; Li, X.K.; Lu, X.; Yin, C.C.; Tao, J.J.; Wei, W.; Zhang, W.K.; Xin, P.Y.; et al. The GDSL lipase MHZ11 modulates ethylene signaling in rice roots. Plant Cell 2020, 32, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Sauter, M. Polar auxin transport determines adventitious root emergence and growth in rice. Front. Plant Sci. 2019, 10, 444. [Google Scholar] [CrossRef]
- Ma, B.; He, S.J.; Duan, K.X.; Yin, C.C.; Chen, H.; Yang, C.; Xiong, Q.; Song, Q.X.; Lu, X.; Chen, H.W.; et al. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol. Plant 2013, 6, 1830–1848. [Google Scholar] [CrossRef]
- Tao, J.J.; Chen, H.W.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. The role of ethylene in plants under salinity stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Cui, F.; You, L.; Li, X.; Xiong, L. A homolog of ETHYLENE OVERPRODUCER, OsETOL1, differentially modulates drought and submergence tolerance in rice. Plant J. 2014, 78, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Quan, R.; Pan, X.; Wan, L.; Huang, R. EAR motif mutation of rice OsERF3 alters the regulation of ethylene biosynthesis and drought tolerance. Planta 2013, 237, 1443–1451. [Google Scholar] [CrossRef]
- Yang, C.; Ma, B.; He, S.J.; Xiong, Q.; Duan, K.X.; Yin, C.C.; Chen, H.; Lu, X.; Chen, S.Y.; Zhang, J.S. MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol. 2015, 169, 148–165. [Google Scholar] [CrossRef]
- Coudert, Y.; Perin, C.; Courtois, B.; Khong, N.G.; Gantet, P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010, 15, 219–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, S.F.; Song, Y.L.; Huang, Y.L.; Zhou, S.L.; Liu, X.Y.; Zhou, D.X. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 2015, 27, 2469–2483. [Google Scholar] [CrossRef]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T.; et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef]
- Steele, K.A.; Price, A.H.; Witcombe, J.R.; Shrestha, R.; Singh, B.N.; Gibbons, J.M.; Virk, D.S. QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theor. Appl. Genet. 2013, 126, 101–108. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular mechanisms of root development in rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, S.F.; Yu, X.B.; Yu, J.; He, X.W.; Zhang, S.L.; Shou, H.X.; Wu, P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005, 43, 47–56. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.F.; Dai, M.Q.; Huang, L.M.; Zhou, D.X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Li, Q.; Jiao, L.; Xiang, Y.; Deng, Q.; Zhou, D.X.; Zhao, Y. WOX11 and CRL1 act synergistically to promote crown root development by maintaining cytokinin homeostasis in rice. New Phytol. 2023, 237, 204–216. [Google Scholar] [CrossRef]
- Cheng, S.; Zhou, D.X.; Zhao, Y. WUSCHEL-related homeobox gene WOX11 increases rice drought resistance by controlling root hair formation and root system development. Plant Signal. Behav. 2016, 11, e1130198. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, Y.; Zhao, Z.K.; Liu, W.; Jiang, C.H.; Li, J.J.; Zhang, Z.Y.; Zhang, H.L.; Zhang, Y.G.; Wang, X.N.; et al. RRS1 shapes robust root system to enhance drought resistance in rice. New Phytol. 2023, 238, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Wu, J.; Yue, X.; Wang, M.; Sun, L.; Di, D.; Kronzucker, H.J.; Shi, W. OsEIL1 protects rice growth under NH4+ nutrition by regulating OsVTC1-3-dependent N-glycosylation and root NH4+ efflux. Plant Cell Environ. 2022, 45, 1537–1553. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Lv, W.; Yu, X.; Zhang, Z. Ethylene positively regulates Cd tolerance via reactive oxygen species scavenging and apoplastic transport barrier formation in rice. Environ. Pollut. 2022, 302, 119063. [Google Scholar] [CrossRef]
- Taylor, I.; Lehner, K.; McCaskey, E.; Nirmal, N.; Ozkan-Aydin, Y.; Murray-Cooper, M.; Jain, R.; Hawkes, E.W.; Ronald, P.C.; Goldman, D.I.; et al. Mechanism and function of root circumnutation. Proc. Natl. Acad. Sci. USA 2021, 118, e2018940118. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D.X. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef]
- Huang, G.; Kilic, A.; Karady, M.; Zhang, J.; Mehra, P.; Song, X.; Sturrock, C.J.; Zhu, W.; Qin, H.; Hartman, S.; et al. Ethylene inhibits rice root elongation in compacted soil via ABA- and auxin-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2201072119. [Google Scholar] [CrossRef]
- Ma, B.; Yin, C.C.; He, S.J.; Lu, X.; Zhang, W.K.; Lu, T.G.; Chen, S.Y.; Zhang, J.S. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet. 2014, 10, e1004701. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.C.; Ma, B.; Collinge, D.P.; Pogson, B.J.; He, S.J.; Xiong, Q.; Duan, K.X.; Chen, H.; Yang, C.; Lu, X.; et al. Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 2015, 27, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, B.; Tao, J.J.; Yin, C.C.; Hu, Y.; Huang, Y.H.; Wei, W.; Xin, P.Y.; Chu, J.F.; Zhang, W.K.; et al. Rice EIL1 interacts with OsIAAs to regulate auxin biosynthesis mediated by the tryptophan aminotransferase MHZ10/OsTAR2 during root ethylene responses. Plant Cell 2022, 34, 4366–4387. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2023, 191, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, Z.; Wang, J.; Chen, X.; Wei, P.; Huang, R. The activation of OsEIL1 on YUC8 transcription and auxin biosynthesis is required for ethylene-inhibited root elongation in rice early seedling development. PLoS Genet. 2017, 13, e1006955. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Chen, X.; Wang, F.; Peng, P.; Zhou, Y.; Miao, Y.; Zhang, Y.; Gao, Y.; Qi, Y.; et al. Rice OsDOF15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol. 2019, 223, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Wang, G.; Zhao, J.L.; Zhang, L.Q.; Ai, L.F.; Han, Y.F.; Sun, D.Y.; Zhang, S.W.; Sun, Y. The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell 2014, 26, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xiong, W.; Yin, C.; Xie, X.; Jin, Y.J.; Zhang, S.; Yang, B.; Ye, G.; Chen, S.; Luan, W.J. Overexpression of OsARD1 improves submergence, drought, and salt tolerances of seedling through the enhancement of ethylene synthesis in rice. Front. Plant Sci. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.H.; Do Choi, Y.; Kim, J.K. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016, 172, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2013, 11, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Redillas, M.C.; Jeong, J.S.; Kim, Y.S.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 2012, 10, 792–805. [Google Scholar] [CrossRef]
- Lee, D.K.; Chung, P.J.; Jeong, J.S.; Jang, G.; Bang, S.W.; Jung, H.; Kim, Y.S.; Ha, S.H.; Choi, Y.D.; Kim, J.K. The rice OsNAC6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol. J. 2017, 15, 754–764. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Yin, C.C.; Zhao, H.; Ma, B.; Chen, S.Y.; Zhang, J.S. Diverse roles of ethylene in regulating agronomic traits in rice. Front. Plant Sci. 2017, 8, 1676. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; Scheres, B. Root development-two meristems for the price of one? Curr. Top. Dev. Biol. 2010, 91, 67–102. [Google Scholar] [PubMed]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorska, R.; Beeckman, T.; Friml, J.; Benkova, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Perilli, S.; Di Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2012, 15, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, S.; Shen, C.; Zhang, S.; Chen, Y.; Xu, Y.; Liu, Y.; Wu, Y.; Jiang, D. OsARF12, a transcription activator on auxin response gene, regulates root elongation and affects iron accumulation in rice (Oryza sativa). New Phytol. 2012, 193, 109–120. [Google Scholar] [CrossRef]
- Street, I.H.; Aman, S.; Zubo, Y.; Ramzan, A.; Wang, X.; Shakeel, S.N.; Kieber, J.J.; Schaller, G.E. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol. 2015, 169, 338–350. [Google Scholar] [CrossRef]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.X.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Sauter, M. Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiol. 2018, 176, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, B.; Zhou, Y.; He, S.J.; Tang, S.Y.; Lu, X.; Xie, Q.; Chen, S.Y.; Zhang, J.S. E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc. Natl. Acad. Sci. USA 2018, 115, 4513–4518. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Xing, J.; Yan, L.; Wang, R.; Zhao, Y. The YUCCA-auxin-WOX11 module controls crown root development in rice. Front. Plant Sci. 2018, 9, 523. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Costa, L.C.; Avila, R.T.; Pimenta, T.M.; Teixeira, L.S.; Brito, F.A.L.; Zsogon, A.; Araujo, W.L.; Ribeiro, D.M. Selenium downregulates auxin and ethylene biosynthesis in rice seedlings to modify primary metabolism and root architecture. Planta 2019, 250, 333–345. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Yang, W.; Pasriga, R.; Wu, Y.; Hong, W.J.; Bureau, C.; Wi, S.J.; Zhang, T.; Wang, R.; et al. Homeobox transcription factor OsZHD2 promotes root meristem activity in rice by inducing ethylene biosynthesis. J. Exp. Bot. 2020, 71, 5348–5364. [Google Scholar] [CrossRef]
- Nam, M.H.; Bang, E.; Kwon, T.Y.; Kim, Y.; Kim, E.H.; Cho, K.; Park, W.J.; Kim, B.G.; Yoon, I.S. Metabolite profiling of diverse rice germplasm and identification of conserved metabolic markers of rice roots in response to long-term mild salinity stress. Int. J. Mol. Sci. 2015, 16, 21959–21974. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Li, Y.; Quan, R.; Wang, J.; Huang, R.; Qin, H. Salt stress promotes abscisic acid accumulation to affect cell proliferation and expansion of primary roots in rice. Int. J. Mol. Sci. 2021, 22, 10892. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, D.; Zhou, S.; Gu, J.; Wang, F.; Dong, J.; Huang, R. The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 2017, 254, 401–408. [Google Scholar] [CrossRef]
- Pandey, B.K.; Bennett, M.J. Uncovering root compaction response mechanisms: New insights and opportunities. J. Exp. Bot. 2024, 75, 578–583. [Google Scholar] [CrossRef]
- Gunawardena, A.H.; Pearce, D.M.; Jackson, M.B.; Hawes, C.R.; Evans, D.E. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 2001, 212, 205–214. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Cox, M.C.; Voesenek, L.A. Root aeration in rice (Oryza sativa): Evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol. 2006, 170, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Cao, J.D.; Meng, F.W.; Yu, Y.Q.; Huang, J.K.; Jiang, L.; Liu, M.X.; Zhang, Z.G.; Chen, X.W.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [PubMed]
- Yamauchi, T.; Tanaka, A.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. A role for auxin in ethylene-dependent inducible aerenchyma formation in rice roots. Plants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Zhao, X.Y.; Xuan, W.; Wang, P.; Zhao, F.J. Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade. Mol. Plant 2023, 16, 1678–1694. [Google Scholar] [CrossRef]
- Peret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, M.; Jia, L.; Xie, Y.; Wang, Z.; Xuan, W. Ammonium transporters cooperatively regulate rice crown root formation responding to ammonium nitrogen. J. Exp. Bot. 2022, 73, 3671–3685. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, D. The plasticity of root systems in response to external phosphate. Int. J. Mol. Sci. 2020, 21, 5955. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhu, C.Q.; Zhao, X.S.; Zheng, S.J.; Shen, R.F. Ethylene is involved in root phosphorus remobilization in rice (Oryza sativa) by regulating cell-wall pectin and enhancing phosphate translocation to shoots. Ann. Bot. 2016, 118, 645–653. [Google Scholar] [CrossRef]
- Song, L.; Yu, H.; Dong, J.; Che, X.; Jiao, Y.; Liu, D. The molecular mechanism of ethylene-mediated root hair development induced by phosphate starvation. PLoS Genet. 2016, 12, e1006194. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Z.; Zhang, C.; Yoon, G.M. Editing of the OsACS locus alters phosphate deficiency-induced adaptive responses in rice seedlings. J. Exp. Bot. 2019, 70, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Betti, C.; Della Rovere, F.; Piacentini, D.; Fattorini, L.; Falasca, G.; Altamura, M.M. Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids. Biomolecules 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inze, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]

| Gene Name | Gene Function | Reference |
|---|---|---|
| OsEIL1 | Regulates ethylene response of roots and salt tolerance in rice, maintains rice growth under NH4+ by stabilizing protein N-glycosylation and reducing root NH4+ efflux | [27,41] |
| OsEIN2 | Regulates ethylene response of roots and salt tolerance in rice, positively regulates Cd tolerance in rice roots | [22,27,42] |
| MHZ1/OsHK1 | Positively modulates root ethylene responses and root circumnutation | [17,43] |
| OsWOX11 | promotes AR development by modulating cell proliferation in AR meristem, enhances drought tolerance by controlling root hair formation and root system development | [37,39,44] |
| OsERF3 | Regulates AR development through interacting with OsWOX11, regulates drought tolerance in rice through modulating ethylene biosynthesis | [26,29] |
| OsDERF1 | Negatively modulates ethylene synthesis and drought tolerance in rice by directly activating OsERF3 and OsAP2-39 | [45] |
| MHZ4 | Regulates ethylene response of roots and the ability of root to penetrate compacted soil | [46,47] |
| MHZ5 | Regulates ethylene response of roots and the ability of root to penetrate compacted soil | [46,48] |
| MHZ10/TAA1 | Regulates ethylene response of roots and the ability of root to penetrate compacted soil | [49,50] |
| REIN7/YUC8 | Regulates ethylene response of roots and the ability of root to penetrate compacted soil | [46,51] |
| OsDOF15 | Regulates root growth under salt stress by modulating ethylene biosynthesis | [52] |
| OsSIT1 | Specially expressed in root epidermal cells, regulates salt tolerance by promoting ethylene and ROS production in rice | [53] |
| OsARD1 | Improves root growth under drought stress and increases submergence, salt, and drought tolerance in rice | [54] |
| RBOHH | Regulates aerenchyma formation in rice roots under oxygen-deficient conditions | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Xiao, M.; Li, Y.; Huang, R. Ethylene Modulates Rice Root Plasticity under Abiotic Stresses. Plants 2024, 13, 432. https://doi.org/10.3390/plants13030432
Qin H, Xiao M, Li Y, Huang R. Ethylene Modulates Rice Root Plasticity under Abiotic Stresses. Plants. 2024; 13(3):432. https://doi.org/10.3390/plants13030432
Chicago/Turabian StyleQin, Hua, Minggang Xiao, Yuxiang Li, and Rongfeng Huang. 2024. "Ethylene Modulates Rice Root Plasticity under Abiotic Stresses" Plants 13, no. 3: 432. https://doi.org/10.3390/plants13030432





