Ferula communis Root Extract: In Vitro Evaluation of the Potential Additive Effect with Chemotherapy Tamoxifen in Breast Cancer (MCF-7) Cells Part II
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedure
2.2. Free Radical Scavenging Activity
2.3. Reducing Power Assay
2.4. Ferrous Ions (Fe 2+) Chelating Activity
2.5. Oxygen Radical Absorbance Capacity (ORAC) Assay
- (1)
- Addition of fluorescein solution (20 μM in PBS);
- (2)
- Addition of the different Trolox concentrations (6.25, 12.5, 25, 50, 100 μM) and the sample to be tested (FER-E, 2mg/mL);
- (3)
- Addition of the buffer PBS as a negative control;
- (4)
- Addition of the AAPH (31.7 μM in PBS) to all wells.
2.6. Measurement of In Vitro Reactive Oxygen Species
2.7. Cell Cultures and Treatments
2.8. Proliferation Assay and Cytotoxicity Study
2.9. Cell Lysis and Immunoblot Analysis
3. Results
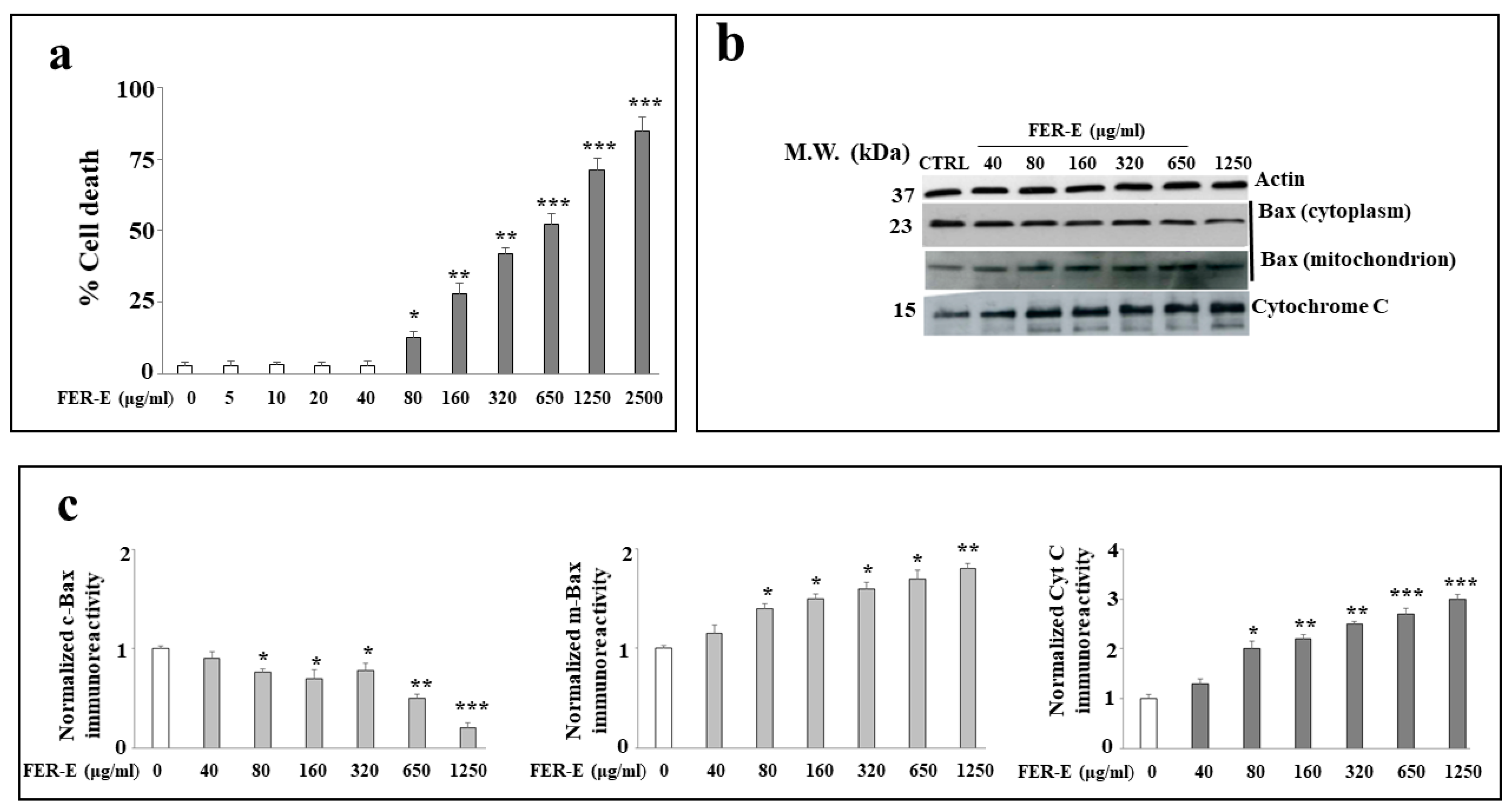
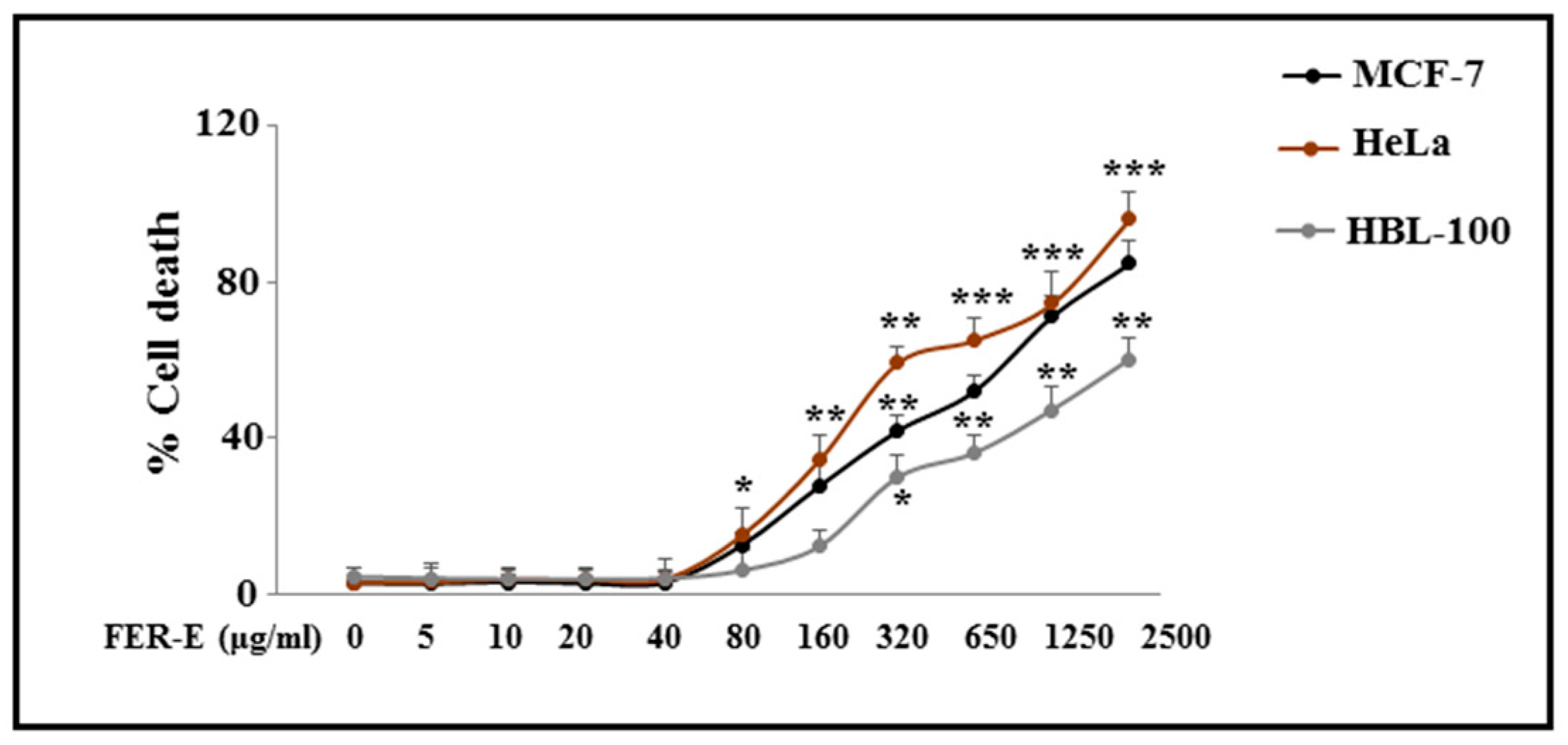
3.1. Cytotoxicity Induced by FER-E
3.2. Antioxidant Activity
3.3. Measuring the Oxidative Effect of FER-E on MCF-7 Cells
3.4. Additive Effect of FER-E and TAM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekiert, H.M.; Szopa, A. Biological Activities of Natural Products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef]
- Hadidi, L.; Babou, L.; Zaidi, F.; Valentão, P.; Andrade, P.B.; Grosso, C. Quercus ilex L.: How season, Plant Organ and Extraction Procedure Can Influence Chemistry and Bioactivities. Chem. Biodivers. 2017, 14, e1600187. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [PubMed]
- Zhou, Y.; Xin, F.; Zhang, G.; Qu, H.; Yang, D.; Han, X. Recent Advances on Bioactive Constituents in Ferula. Drug Dev. Res. 2017, 78, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, S. Flora d’Italia, 1st ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Schauer, T.; Caspari, C.; Rambelli, A. Guida All’identificazione Delle Piante; Zanichelli: Bologna, Italy, 1987. [Google Scholar]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050–1056. [Google Scholar] [PubMed]
- Maggi, F.; Papa, F.; Dall’Acqua, S.; Nicoletti, M. Chemical analysis of essential oils from different parts of Ferula communis L. growing in central Italy. Nat. Prod.Res. 2016, 30, 806–813. [Google Scholar] [CrossRef]
- Gault, G.; Lefebvre, S.; Benoit, E.; Lattard, V.; Grancher, D. Variability of ferulenol and ferprenin concentration in French giant fennel (Ferula sp.) leaves. Toxicon 2019, 165, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of Ferula persica Boiss.: A review. Iran. J. Basic Med. Sci. 2017, 20, 1. [Google Scholar]
- Iranshahi, M.; Ghiadi, M.; Sahebkar, A.; Rahimi, A.; Bassarello, C.; Piacente, S. Badrakemonin, a new eremophilane-type sesquiterpene from the roots of Ferula badrakema Kos.-Pol. Iran. J.Pharm. Res. 2009, 8, 275–279. [Google Scholar]
- Iranshahi, M.; Masullo, M.; Asili, A.; Hamedzadeh, A.; Jahanbin, B.; Festa, M.; Capasso, A.; Piacente, S. Sesquiterpene coumarins from Ferula gumosa. J. Nat. Prod. 2010, 73, 1958–1962. [Google Scholar] [CrossRef]
- Kasaian, J.; Iranshahy, M.; Masullo, M.; Piacente, S.; Ebrahimi, F.; Iranshahi, M. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J. Asian Nat. Prod. Res. 2014, 16, 248–253. [Google Scholar] [CrossRef]
- Matin, M.M.; Nakhaeizadeh, H.; Bahrami, A.R.; Iranshahi, M.; Arghiani, N.; Rassouli, F.B. Ferutinin, an apoptosis inducing terpenoid from Ferula ovina. Asian Pac. J. Cancer Prev. 2014, 15, 2123–2128. [Google Scholar] [CrossRef]
- Poli, F.; Appendino, G.; Sacchetti, G.; Ballero, M.; Maggiano, N.; Ranelletti, F.O. Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phytother. Res. 2005, 19, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity in vitro (O/W Emulsion Systems) along with Their in vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Rahali, F.Z.; Lamine, M.; Gargouri, M.; Rebey, I.B.; Hammami, M.; Sellami, I.H. Metabolite profiles of essential oils and molecular markers analysis to explore the biodiversity of Ferula communis: Towards conservation of the endemic giant fennel. Phytochemistry 2016, 124, 58–67. [Google Scholar] [CrossRef]
- Monti, M.; Pinotti, M.; Appendino, G.; Dallocchio, F.; Bellini, T.; Antognoni, F.; Poli, F.; Bernardi, F. Characterization of anti-coagulant properties of prenylated coumarin ferulenol. Biochim. Biophys. Acta. 2007, 1770, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Musolino, V.; Guarnieri, L.; Macrì, R.; Coppoletta, A.R.; Cardamone, A.; Serra, M.; Gliozzi, M.; Bava, I.; Lupia, C.; et al. Ferula communis L. (Apiaceae) Root Acetone-Water Extract: Phytochemical Analysis, Cytotoxicity and In Vitro Evaluation of Estrogenic Properties. Plants 2022, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Alashim, A.M.; Taiwo, K.A.; Oyedele, D.J.; Adebooye, O.C.; Aluko, R.E. Polyphenol composition and antioxidant properties of vegetable leaf-fortified bread. J. Food Biochem. 2019, 43, e12625. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Tow, W.W.; Hamauzu, Y.; Katayama, S.; Tanaka, S.; Nakamura, S. Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-products. Food Chem. 2015, 167, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Shitzuo, T.; Yoshiaki, S.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acid on linoleic acid peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Russo, R.; Navarra, M.; Maiuolo, J.; Rotiroti, D.; Bagetta, G.; Corasaniti, M.T. 17beta-estradiol protects SH-SY5Y Cells against HIV-1 gp120-induced cell death: Evidence for a role of estrogen receptors. Neurotoxicology 2005, 26, 905–913. [Google Scholar] [CrossRef]
- Fan, P.; Yang, D.; Wu, J.; Yang, Y.; Guo, X.; Tu, J.; Zhang, D. Cell-cycle-dependences of membrane permeability and viability observed for HeLa cells undergoing multi-bubble-cell interactions. Ultrason. Sonochemistry 2019, 53, 178–186. [Google Scholar] [CrossRef]
- Wu, S.; Wei, X.; Jiang, J.; Shang, L.; Hao, W. Effects of bisphenol A on the proliferation and cell cycle of HBL-100 cells. Food Chem. Toxicol. 2012, 50, 3100–3105. [Google Scholar] [CrossRef]
- Sonigra, P.; Meena, M. Metabolic Profile, Bioactivities, and Variations in the Chemical Constituents of Essential Oils of the Ferula Genus (Apiaceae). Front. Pharmacol. 2021, 11, 608649. [Google Scholar] [CrossRef]
- Maggi, F.; Cecchini, C.; Cresci, A.; Coman, M.M.; Tirillini, B.; Sagratini, G.; Papa, F. Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy). Fitoterapia 2009, 80, 68–72. [Google Scholar] [CrossRef]
- La Rosa, A.; Cornara, L.; Saitta, A.; Salam, A.M.; Grammatico, S.; Caputo, M.; La Mantia, T.; Quave, C.L. Ethnobotany of the Aegadian Islands: Safeguarding biocultural refugia in the Mediterranean. J.Ethnobiol. Ethnomed. 2021, 17, 47. [Google Scholar] [CrossRef]
- Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules 2020, 25, 5768. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macri, R.; Oppedisano, F.; Scarano, F.; Zito, M.C.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Concepción, M.; Boronat, A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr. Opin. Plant Biol. 2015, 25, 17–22. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E.; Loi, M.; Sollai, F.; Ballero, M.; Pintus, M.; Rescigno, A. Isolation and characterization of polyphenol oxidase from Sardinian poisonous and non-poisonous chemotypes of Ferula communis (L.). Phytochemistry 2013, 90, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, D.; Mahdavi, M.; Dehghan, G.; Charoudeh, H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017, 4, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Tanzadehpanah, H.; Mahaki, H.; Samadi, P.; Karimi, J.; Moghadam, N.H.; Salehzadeh, S.; Dastan, D.; Saidijam, M. Anticancer activity, calf thymus DNA and human serum albumin binding properties of Farnesiferol C from Ferula pseudalliacea. J. Biomol. Struct. Dyn. 2019, 37, 2789–2800. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef]
- Hammam, A.M.M.; Elmotayam, A.K.; Elnattat, W.M.; Ali, G.A.; Madbouly, A.E.M.; El Khatteb, R.M.; Abdelhameed, M.F.; Ali, A.H.; Qasim, S.; Ahmed, S.R. Assessment of Ferula hermonis Boiss fertility effects in immature female rats supported by quantification of ferutinin via HPLC and molecular docking. J. Ethnopharmacol. 2022, 289, 115062. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Mahdipour, E.; Iranshahi, M.; Jamialahmadi, K. Osteogenic induction of menstrual blood mesenchymal stem cell by different Ferula species extracts. Avicenna J. Phytomed. 2021, 11, 281–291. [Google Scholar]
- Ferretti, M.; Cavani, F.; Manni, P.; Carnevale, G.; Bertoni, L.; Zavatti, M.; Palumbo, C. Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. J. Histol. Histopathol. 2014, 29, 1027–1037. [Google Scholar]
- Arghiani, N.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Sazgarnia, A.; Rassouli, F.B. Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells, in vitro and in vivo. Life Sci. 2014, 109, 87–94. [Google Scholar] [CrossRef]
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todt, F.; Cakir, Z.; Reichenbach, F.; Emschermann, F.; Lauterwasser, J.; Kaiser, A.; Ichim, G.; Tait, S.W.; Frank, S.; Langer, H.F.; et al. Differential retrotranslocationof mitochondrial Bax and Bak. EMBO J 2015, 34, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, J.F.; Billen, L.P.; Bindner, S.; Shamas-Din, A.; Fradin, C.; Leber, B.; Andrews, D.W. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 2008, 135, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Ugarte-Uribe, B.; García-Sáez, A.J. Apoptotic foci at mitochondria: In and around Bax pores. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shagufta Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Brauch, H.; Jordan, V.C. Targeting of tamoxifen to enhance antitumour action for the treatment and prevention of breast cancer: The ‘personalised; approach? Eur. J. Cancer. 2009, 45, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Moon, Z.; Moss-Morris, R.; Hunter, M.S.; Hughes, L.D. More than just side-effects: The role of clinical and psychosocial factors in non-adherence to tamoxifen. Br. J. Health Psychol. 2017, 22, 998–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Shi, X.; Xu, Y.; Wan, J.; Wei, S.; Zhu, R. Tamoxifen promotes apoptosis and inhibits invasion in estrogen-positive breast cancer MCF-7 cells. Mol. Med. Rep. 2017, 16, 478–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Miceli, N.; Davì, F.; Bava, I.; Tucci, L.; Ragusa, S.; Taviano, M.F.; Musolino, V.; Gliozzi, M.; Carresi, C.; et al. Ferula communis Root Extract: In Vitro Evaluation of the Potential Additive Effect with Chemotherapy Tamoxifen in Breast Cancer (MCF-7) Cells Part II. Plants 2023, 12, 1194. https://doi.org/10.3390/plants12051194
Maiuolo J, Miceli N, Davì F, Bava I, Tucci L, Ragusa S, Taviano MF, Musolino V, Gliozzi M, Carresi C, et al. Ferula communis Root Extract: In Vitro Evaluation of the Potential Additive Effect with Chemotherapy Tamoxifen in Breast Cancer (MCF-7) Cells Part II. Plants. 2023; 12(5):1194. https://doi.org/10.3390/plants12051194
Chicago/Turabian StyleMaiuolo, Jessica, Natalizia Miceli, Federica Davì, Irene Bava, Luigi Tucci, Salvatore Ragusa, Maria Fernanda Taviano, Vincenzo Musolino, Micaela Gliozzi, Cristina Carresi, and et al. 2023. "Ferula communis Root Extract: In Vitro Evaluation of the Potential Additive Effect with Chemotherapy Tamoxifen in Breast Cancer (MCF-7) Cells Part II" Plants 12, no. 5: 1194. https://doi.org/10.3390/plants12051194






