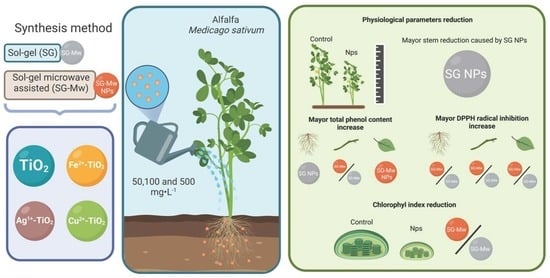
Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2: Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization of TiO2 Materials
2.2. Morphological Data
2.3. Total Phenol and Flavonoid Quantification
2.4. Antioxidant Activity
2.5. Chlorophyll Index
2.6. Two-Way ANOVA
3. Discussion
4. Materials and Methods
4.1. Titanium Dioxide and Doped TiO2 Materials Synthesis and Characterization
4.2. Nanoparticle Crystallite Size
4.3. Plant Harvest and Growth Parameters
4.4. Total Phenol and Flavonoid Quantification
4.4.1. Antioxidant Activity
4.4.2. Chlorophyll Index
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Tarrahi, R.; Mahjouri, S.; Khataee, A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Ecotoxicol. Environ./Saf. 2021, 208, 111697. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Anjum, S. Nano-Elicitation as an Effective and Emerging Strategy for In Vitro Production of Industrially Important Flavonoids. Appl. Sci. 2021, 11, 1694. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.D.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Zhang, W.; Long, J.; Geng, J.; Li, J.; Wei, Z. Impact of Titanium Dioxide Nanoparticles on Cd Phytotoxicity and Bioaccumulation in Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2020, 17, 2979. [Google Scholar] [CrossRef] [PubMed]
- Chutipaijit, S.; Sutjaritvorakul, T. Enhancements of growth and metabolites of indica rice callus (Oryza sativa L. cv. pathumthani1) using TiO2 nanoparticles (NANO-TiO2). Dig. J. Nanomater. Biostruct. 2020, 15, 483–489. [Google Scholar]
- Hatami, M.; Badi, H.N.; Ghorbanpour, M. Nano-elicitation of secondary pharmaceutical metabolites in plant cells: A review. J. Med. Plants 2019, 18, 6–36. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.M.A.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynthetica 2019, 57, 599–606. [Google Scholar] [CrossRef]
- Hedayati, A.; Naseri, F.; Nourozi, E.; Hosseini, B.; Honari, H.; Hemmaty, S. Response of Saponaria officinalis L. hairy roots to the application of TiO2 nanoparticles in terms of production of valuable polyphenolic compounds and SO6 protein. Plant Physiol. Biochem. 2022, 178, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Gohari, G.; Esmaielpour, B.; Panahirad, S.; Milani, M.H.; Kulak, M.; Janda, T. Melatonin and TiO2 NPs Application-Induced Changes in Growth, Photosynthesis, Antioxidant Enzymes Activities and Secondary Metabolites in Stevia (Stevia rebaudiana Bertoni) Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 1–18. [Google Scholar] [CrossRef]
- Zare, A.S.; Ganjeali, A.; Kakhki, M.R.V.; Cheniany, M.; Mashreghi, M. Plant elicitation and TiO2 nanoparticles application as an effective strategy for improving the growth, biochemical properties, and essential oil of peppermint. Physiol. Mol. Biol. Plants 2022, 28, 1391–1406. [Google Scholar] [CrossRef]
- Mustafa, N.; Raja, N.I.; Ilyas, N.; Abasi, F.; Ahmad, M.S.; Ehsan, M.; Mehak, A.; Badshah, I.; Proćków, J. Exogenous Application of Green Titanium Dioxide Nanoparticles (TiO2 NPs) to Improve the Germination, Physiochemical, and Yield Parameters of Wheat Plants under Salinity Stress. Molecules 2022, 27, 4884. [Google Scholar] [CrossRef]
- Hernandez, R.; Hernández-Reséndiz, J.R.; Cruz-Ramírez, M.; Velázquez-Castillo, R.; Escobar-Alarcón, L.; Ortiz-Frade, L.; Esquivel, K. Au-TiO2 Synthesized by a Microwave-and Sonochemistry-Assisted Sol-Gel Method: Characterization and Application as Photocatalyst. Catalysts 2020, 10, 1052. [Google Scholar] [CrossRef]
- Tan, W.; Du, W.; Barrios, A.C.; Armendariz, R., Jr.; Zuverza-Mena, N.; Ji, Z.; Chang, C.H.; Zink, J.I.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; et al. Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ. Pollut. 2017, 222, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Du, W.; Darrouzet-Nardi, A.J.; Hernandez-Viezcas, J.A.; Ye, Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of the exposure of TiO2 nanoparticles on basil (Ocimum basilicum) for two generations. Sci. Total Environ. 2018, 636, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.; Siracusa, G.; Giorgetti, L.; Di Gregorio, S.; Castiglione, M.R.; Spanò, C.; Muccifora, S.; Bottega, S.; Pini, R.; Tassi, E. TiO2 nanoparticles in a biosolid-amended soil and their implication in soil nutrients, microorganisms and Pisum sativum nutrition. Ecotoxicol. Environ. Saf. 2020, 190, 110095. [Google Scholar] [CrossRef]
- Gordillo-Delgado, F.; Zuluaga-Acosta, J.; Restrepo-Guerrero, G. Effect of the suspension of Ag-incorporated TiO2 nanoparticles (Ag-TiO2 NPs) on certain growth, physiology and phytotoxicity parameters in spinach seedlings. PLoS ONE 2020, 15, e0244511. [Google Scholar] [CrossRef]
- Kole, C.; Kumar, D.S.; Khodakovskaya, M.V. Plant Nanotechnology: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Tang, Y.; He, R.; Zhao, J.; Nie, G.; Xu, L.; Xing, B. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ. Pollut. 2016, 212, 605–614. [Google Scholar] [CrossRef]
- Karpiński, W.C.S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- García López, J.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Null 2019, 47, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Miras-Moreno, B.; Chehregani Rad, A.; Pii, Y.; Mimmo, T.; Cesco, S.; Lucini, L. Relatively Low Dosages of CeO2 Nanoparticles in the Solid Medium Induce Adjustments in the Secondary Metabolism and Ionomic Balance of Bean (Phaseolus vulgaris L.) Roots and Leaves. J. Agric. Food Chem. 2020, 68, 67–76. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Wrona, O.; Górecki, R.; Buszewski, B. Medicago sativa as a source of secondary metabolites for agriculture and pharmaceutical industry. Phytochem. Lett. 2017, 20, 520–539. [Google Scholar] [CrossRef]
- Hu, J.; Wu, X.; Wu, F.; Chen, W.; Zhang, X.; White, J.C.; Li, J.; Wan, Y.; Liu, J.; Wang, X. TiO2 nanoparticle exposure on lettuce (Lactuca sativa L.): Dose-dependent deterioration of nutritional quality. Environ. Sci. Nano 2020, 7, 501–513. [Google Scholar] [CrossRef]
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Park, T.J.; Kallerhoff, J.; Arshad, M. Dose-dependent physiological responses of Triticum aestivum L. to soil applied TiO2 nanoparticles: Alterations in chlorophyll content, H2O2 production, and genotoxicity. Agric. Ecosyst. Environ. 2018, 255, 95–101. [Google Scholar] [CrossRef]
- Daghan, H. Effects of TiO2 nanoparticles on maize (Zea mays L.) growth, chlorophyll content and nutrient uptake. Appl. Ecol. Environ. Res. 2018, 16, 6873–6883. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 35. [Google Scholar] [CrossRef]
- Satti, S.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.U.R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Silva, S.; Craveiro, S.C.; Oliveira, H.; Calado, A.J.; Pinto, R.J.; Silva, A.M.; Santos, C. Wheat chronic exposure to TiO2-nanoparticles: Cyto-and genotoxic approach. Plant Physiol. Biochem. 2017, 121, 89–98. [Google Scholar] [CrossRef]
- Levresse, G.; Cruzabeyro-Lopez, J.; Trillta, J.; Perez-Enriquez, R.J.; Kotzarenko, A.; Carrillo-Chavez, A. Centro de Geociencias, Meteostacion, UNAM. Available online: http://132.248.185.112/CurrentMonitorHTML/Medicion_actual.html (accessed on 15 September 2022).
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Meneses-Reyes, A.E.; Jimenez-Garcia, S.N.; Mercado Luna, A.; García Trejo, J.F.; Contreras-Medina, L.M.; Feregrino-Perez, A.A. Polyphenol Content and Antioxidant Activity of Stevia and Peppermint as a Result of Organic and Conventional Fertilization. J. Food Qual. 2021, 2021, 6620446. [Google Scholar] [CrossRef]
- Parit, S.B.; Dawkar, V.V.; Tanpure, R.S.; Pai, S.R.; Chougale, A.D. Nutritional Quality and Antioxidant Activity of Wheatgrass (Triticum aestivum) Unwrap by Proteome Profiling and DPPH and FRAP assays. J. Food Sci. 2018, 83, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Doğaroğlu, Z.G.; Ece, F.; Çiftci, B.N.; Yıldırımcan, S.; Erat, S. Evaluation of stress factor on wheat (Triticum aestivum): The effect of ZnO and Ni-doped ZnO nanoparticles. Null 2021, 1–17. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef] [Green Version]









| Material | Scherrer (nm) | Williamson-Hall (nm) | ||
|---|---|---|---|---|
| SG | Mw-SG | SG | Mw-SG | |
| TiO2 | 8.65 | 8.04 | 19.80 | 12.49 |
| Ag-TiO2 | 9.62 | 7.42 | 14.00 | 12.27 |
| Fe-TiO2 | 9.28 | 6.81 | 18.73 | 11.45 |
| Cu-TiO2 | 8.75 | 7.69 | 16.31 | 12.27 |
| Leaves | Stem | Root | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid 1 (mg/g) | SD (±) | % | Gallic Acid 1 (mg/g) | SD (±) | % | Gallic Acid 1 (mg/g) | SD (±) | % | |
| 50 ppm | |||||||||
| Control | 49.21 A | 6.82 | - | 25.40 A | 6.73 | - | 21.52 A | 4.10 | - |
| TiO2 | 58.21 A | 6.26 | 18.28 | 35.13 A | 2.46 | 38.30 | 36.78 B | 2.22 | 70.91 |
| Ag-TiO2 | 64.75 A | 6.31 | 31.57 | 29.9 A | 2.78 | 17.78 | 26.39 A | 4.70 | 22.63 |
| Fe-TiO2 | 62.00 A | 6.56 | 25.99 | 31.42 A | 3.37 | 23.70 | 25.94 A | 1.58 | 20.53 |
| Cu-TiO2 | 59.18 A | 7.59 | 20.26 | 35.90 A | 6.40 | 41.33 | 24.91 A | 1.29 | 15.75 |
| 100 ppm | |||||||||
| TiO2 | 84.08 B | 5.80 | 70.85 | 49.01 B | 2.83 | 92.95 | 34.65 B | 8.12 | 61.01 |
| Ag-TiO2 | 59.36 A | 5.50 | 20.62 | 43.72 B | 7.87 | 72.12 | 34.05 B | 6.37 | 58.22 |
| Fe-TiO2 | 49.99 A | 1.87 | 1.58 | 34.77 A | 6.24 | 36.88 | 27.43 A | 1.69 | 27.46 |
| Cu-TiO2 | 63.20 A | 9.24 | 28.42 | 42.98 B | 5.72 | 69.21 | 33.21 B | 3.72 | 54.32 |
| 500 ppm | |||||||||
| TiO2 | 67.03 B | 11.27 | 36.21 | 49.86 B | 9.08 | 96.29 | 32.47 B | 2.73 | 50.88 |
| Ag-TiO2 | 52.26 A | 4.49 | 6.19 | 37.72 A | 3.95 | 48.50 | 24.65 A | 5.91 | 14.54 |
| Fe-TiO2 | 59.02 A | 1.16 | 19.93 | 37.59 A | 5.72 | 47.99 | 25.14 A | 3.14 | 16.82 |
| Cu-TiO2 | 53.62 A | 8.24 | 8.96 | 38.24 A | 3.42 | 50.55 | 34.44 B | 1.66 | 60.03 |
| Leaves | Stem | Root | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid 1 (mg/g) | SD (±) | % | Gallic Acid 1 (mg/g) | SD (±) | % | Gallic Acid 1 (mg/g) | SD (±) | % | |
| 50 ppm | |||||||||
| Control | 45.72 A | 4.01 | - | 20.48 A | 2.38 | - | 21.71 A | 7.76 | - |
| TiO2 | 60.71 B | 7.34 | 32.78 | 28.27 A | 1.92 | 38.05 | 29.92 A | 5.02 | 37.81 |
| Ag-TiO2 | 64.08 B | 9.87 | 40.16 | 24.57 A | 2.57 | 19.97 | 22.28 A | 1.14 | 2.62 |
| Fe-TiO2 | 59.83 B | 3.45 | 30.86 | 30.57 B | 9.08 | 49.26 | 18.09 A | 3.87 | 6.33 |
| Cu-TiO2 | 54.96 A | 13.89 | 20.20 | 32.46 B | 5.85 | 58.49 | 29.08 A | 3.54 | 33.94 |
| 100 ppm | |||||||||
| TiO2 | 67.55 B | 5.11 | 47.74 | 38.26 B | 7.02 | 86.84 | 21.80 A | 5.39 | 0.41 |
| Ag-TiO2 | 58.83 B | 7.19 | 28.68 | 28.09 A | 3.14 | 37.16 | 27.28 A | 4.90 | 25.65 |
| Fe-TiO2 | 65.81 B | 4.58 | 43.95 | 34.31 B | 6.51 | 67.53 | 22.32 A | 6.18 | 2.80 |
| Cu-TiO2 | 72.76 B | 5.93 | 59.14 | 28.68 A | 7.47 | 40.03 | 37.00 B | 8.77 | 70.42 |
| 500 ppm | |||||||||
| TiO2 | 69.45 B | 3.10 | 51.90 | 26.99 A | 1.71 | 31.78 | 25.82 A | 2.08 | 18.93 |
| Ag-TiO2 | 58.31 B | 4.88 | 27.53 | 30.01 B | 3.98 | 46.53 | 25.64 A | 4.60 | 18.10 |
| Fe-TiO2 | 73.85 B | 8.84 | 61.53 | 35.12 B | 3.16 | 71.48 | 29.94 A | 6.61 | 37.89 |
| Cu-TiO2 | 65.67 B | 5.88 | 43.63 | 46.09 B | 8.34 | 125.04 | 37.77 B | 6.44 | 73.97 |
| Leaves | Stem | Root | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IHB 1 % | SD (±) | % | IHB 1 % | SD (±) | % | IHB 1 % | SD (±) | % | |
| 50 ppm | |||||||||
| Control | 30.66 A | 4.08 | - | 25.65 A | 2.19 | 12.63 A | 0.92 | - | |
| TiO2 | 43.47 B | 1.54 | 41.64 | 39.28 B | 2.02 | 53.31 | 19.85 B | 3.33 | 57.16 |
| Ag-TiO2 | 43.88 A | 2.69 | 42.97 | 41.15 B | 4.10 | 60.61 | 17.41 A | 3.19 | 37.84 |
| Fe-TiO2 | 39.86 A | 4.27 | 29.87 | 29.65 A | 1.04 | 15.72 | 23.97 B | 1.58 | 89.78 |
| Cu-TiO2 | 38.19 A | 1.97 | 24.43 | 33.68 A | 4.17 | 31.45 | 17.75 A | 5.32 | 40.53 |
| 100 ppm | |||||||||
| TiO2 | 46.28 B | 11.44 | 50.79 | 43.76 B | 1.26 | 70.80 | 22.60 B | 0.45 | 78.93 |
| Ag-TiO2 | 40.47 A | 4.34 | 31.86 | 32.07 A | 2.67 | 25.17 | 21.13 B | 2.36 | 67.30 |
| Fe-TiO2 | 35.32 A | 0.54 | 15.08 | 28.01 A | 1.50 | 9.32 | 25.78 B | 1.37 | 104.11 |
| Cu-TiO2 | 37.93 A | 1.70 | 23.59 | 30.15 A | 5.69 | 17.68 | 15.73 A | 2.56 | 24.54 |
| 500 ppm | |||||||||
| TiO2 | 40.80 A | 6.19 | 32.94 | 40.41 B | 9.60 | 57.72 | 20.84 B | 2.14 | 65.00 |
| Ag-TiO2 | 41.06 A | 6.27 | 33.79 | 32.50 A | 6.51 | 26.85 | 21.20 B | 4.04 | 67.85 |
| Fe-TiO2 | 39.10 A | 1.52 | 27.40 | 32.21 A | 2.59 | 25.72 | 24.54 B | 2.99 | 94.29 |
| Cu-TiO2 | 34.98 A | 4.58 | 13.97 | 28.33 A | 2.42 | 10.57 | 21.38 B | 2.53 | 69.27 |
| Leaves | Stem | Root | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IHB 1 % | SD (±) | % | IHB 1 % | SD (±) | % | IHB 1 % | SD (±) | % | |
| 50 ppm | |||||||||
| Control | 33.93 A | 1.78 | - | 23.24 A | 2.58 | - | 20.40 A | 1.71 | - |
| TiO2 | 55.88 B | 1.80 | 64.69 | 36.94 B | 1.39 | 58.95 | 45.47 B | 6.22 | 122.89 |
| Ag-TiO2 | 49.05 B | 4.24 | 44.55 | 42.09 B | 1.46 | 81.11 | 26.20 A | 2.72 | 28.43 |
| Fe-TiO2 | 44.55 B | 1.63 | 31.29 | 42.80 B | 1.53 | 84.16 | 33.80 B | 1.36 | 65.68 |
| Cu-TiO2 | 55.91 B | 3.43 | 64.77 | 42.75 B | 4.54 | 83.95 | 38.80 B | 1.68 | 90.19 |
| 100 ppm | |||||||||
| TiO2 | 47.49 B | 2.96 | 40.84 | 37.36 B | 1.29 | 60.76 | 44.06 B | 3.86 | 115.98 |
| Ag-TiO2 | 54.14 B | 4.31 | 59.56 | 44.35 B | 4.08 | 90.83 | 39.14 B | 2.40 | 91.86 |
| Fe-TiO2 | 43.78 B | 2.87 | 29.03 | 41.83 B | 2.20 | 79.99 | 45.12 B | 1.98 | 121.13 |
| Cu-TiO2 | 46.74 B | 5.51 | 37.75 | 31.96 B | 2.43 | 37.55 | 35.48 B | 7.06 | 73.92 |
| 500 ppm | |||||||||
| TiO2 | 46.67 B | 0.91 | 37.54 | 34.05 B | 1.36 | 46.51 | 42.52 B | 5.22 | 108.43 |
| Ag-TiO2 | 47.65 B | 2.19 | 40.42 | 42.17 B | 1.41 | 81.45 | 38.17 B | 3.79 | 87.10 |
| Fe-TiO2 | 46.55 B | 3.56 | 37.20 | 42.07 B | 2.65 | 81.04 | 37.28 B | 5.50 | 83.82 |
| Cu-TiO2 | 53.19 B | 2.64 | 56.76 | 47.25 B | 2.11 | 103.31 | 37.66 B | 4.32 | 84.60 |
| Factors | ANOVA | SS | DF | MS | F | p Value |
|---|---|---|---|---|---|---|
| Synthesis–Dopant (Control) | Synthesis:dopant | 0.3898 | 4 | 0.09745 | 2.980 | 0.0191 * |
| Synthesis | 0.1639 | 1 | 0.1639 | 5.012 | 0.0257 * | |
| Dopant | 1.736 | 4 | 0.4341 | 13.28 | 0.0001 *** | |
| Residual | 12.82 | 392 | 0.03270 | |||
| Synthesis–Dopant (No control) | Synthesis:dopant | 0.3703 | 3 | 0.1234 | 3.648 | 0.0129 * |
| Synthesis | 0.2614 | 1 | 0.2614 | 7.726 | 0.0057 ** | |
| Dopant | 0.2686 | 3 | 0.08955 | 2.647 | 0.0489 * | |
| Residual | 12.11 | 358 | 0.03383 |
| Attribute | ANOVA | SS | DF | MS | F | p Value |
|---|---|---|---|---|---|---|
| Synthesis–Dopant (Control) | Synthesis:dopant | 0.2995 | 4 | 0.07489 | 2.190 | 0.0750 ns |
| Synthesis | 0.3779 | 1 | 0.3779 | 11.05 | 0.0012 ** | |
| Dopant | 1.167 | 4 | 0.2917 | 8.529 | 0.0001 *** | |
| Residual | 3.659 | 107 | 0.03419 | |||
| Attribute | ANOVA table | SS | DF | MS | F | pvalue |
| Synthesis–Dopant (No control) | Synthesis:dopant | 0.2487 | 3 | 0.08289 | 2.314 | 0.0805 ns |
| Synthesis | 0.6614 | 1 | 0.6614 | 18.47 | 0.0001 *** | |
| Dopante | 0.3924 | 3 | 0.1308 | 3.652 | 0.0151 * | |
| Residual | 3.582 | 100 | 0.03582 |
| Attribute | ANOVA Table | SS | DF | MS | F | p Value |
|---|---|---|---|---|---|---|
| Synthesis–Dopant (Control) | Synthesis:dopant | 0.3042 | 4 | 0.07605 | 4.092 | 0.0040 ** |
| Synthesis | 0.2926 | 1 | 0.2926 | 15.75 | 0.0001 *** | |
| Dopant | 1.422 | 4 | 0.3555 | 19.13 | <0.0001 **** | |
| Residual | 1.970 | 106 | 0.01858 | |||
| Attribute | ANOVA table | SS | DF | MS | F | pvalue |
| Synthesis–Dopant (No control) | Synthesis:dopant | 0.2642 | 3 | 0.08805 | 4.539 | 0.0050 ** |
| Synthesis | 0.5076 | 1 | 0.5076 | 26.17 | 0.0001 *** | |
| Dopant | 0.3641 | 3 | 0.1214 | 6.256 | 0.0006 *** | |
| Residual | 1.921 | 99 | 0.01940 |
| Attribute | ANOVA Table | SS | DF | MS | F | p Value |
|---|---|---|---|---|---|---|
| Synthesis–Dopant (Control) | Synthesis:dopant | 1.023 | 4 | 0.2558 | 5.729 | 0.0002 * |
| Synthesis | 0.1865 | 1 | 0.1865 | 4.177 | 0.0416 * | |
| Dopant | 1.521 | 4 | 0.3801 | 8.514 | <0.0001 * | |
| Residual | 17.90 | 401 | 0.04465 | |||
| Attribute | ANOVA table | SS | DF | MS | F | pvalue |
| Synthesis–Dopant (No control) | Synthesis:dopant | 0.9994 | 3 | 0.3331 | 7.326 | <0.0001 * |
| Synthesis | 0.3385 | 1 | 0.3385 | 7.445 | 0.0067 * | |
| Dopant | 0.6999 | 3 | 0.2333 | 5.131 | 0.0017 * | |
| Residual | 16.96 | 373 | 0.04547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páramo, L.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Esquivel, K. Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2: Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification. Plants 2023, 12, 659. https://doi.org/10.3390/plants12030659
Páramo L, Feregrino-Pérez AA, Vega-González M, Escobar-Alarcón L, Esquivel K. Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2: Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification. Plants. 2023; 12(3):659. https://doi.org/10.3390/plants12030659
Chicago/Turabian StylePáramo, Luis, Ana Angélica Feregrino-Pérez, Marina Vega-González, Luis Escobar-Alarcón, and Karen Esquivel. 2023. "Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2: Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification" Plants 12, no. 3: 659. https://doi.org/10.3390/plants12030659