Nutritional Value, Volatile Components, Functional Metabolites, and Antibacterial and Cytotoxic Activities of Different Parts of Millettia speciosa Champ., a Medicinal and Edible Plant with Potential for Development
Abstract
:1. Introduction
2. Results
2.1. Minerals and Trace Element Composition
2.2. Amino acid Composition
2.3. Fatty Acid Composition
2.4. Feasibility as Animal Feed
2.5. Chemical Composition of the Volatile Oil
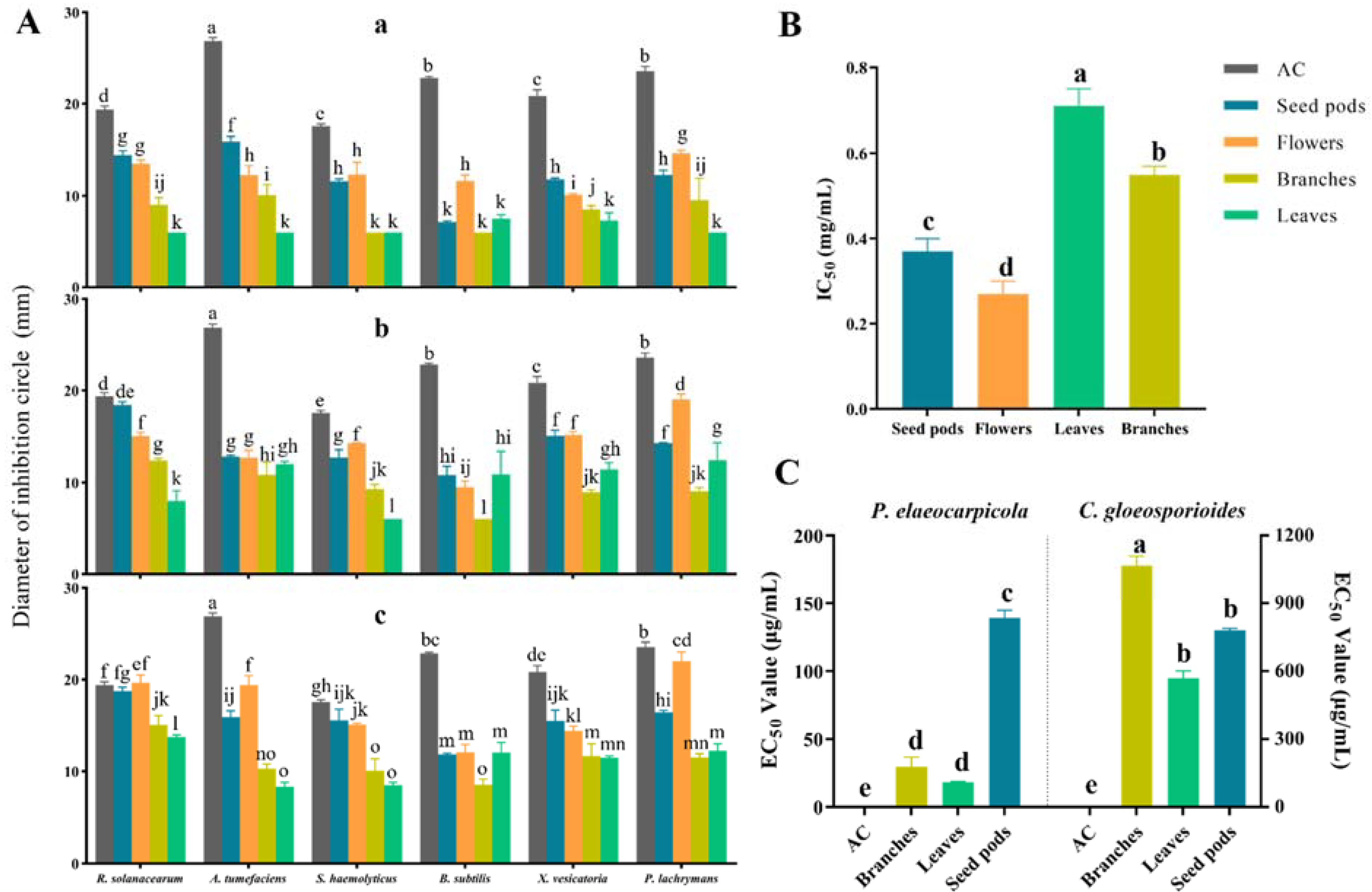
2.6. Antibacterial Activity
2.7. Cytotoxic Activity
2.8. Antifungal Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Trace and Mineral Elements
5.3. Amino Acids
5.4. Fatty Acids
5.5. Basic Components
5.6. Functional Metabolites
5.7. Preparation of Volatile Oil and GC-MS Analysis
5.8. Extraction of Nonvolatile Secondary Metabolites
5.9. Antibacterial Activity Evaluation
5.10. Antifungal Activity Evaluation
5.11. Cytotoxicity Evaluation Assay
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jiang, J. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zong, M.; Lou, W. Preparation, structural elucidation and immunomodulatory activity of a polysaccharide from Millettia Speciosa Champ. Ind. Crop. Prod. 2022, 182, 114889. [Google Scholar] [CrossRef]
- Yu, D.; Liang, X. Characterization and identification of isoflavonoids in the roots of Millettia speciosa Champ. by UPLC-Q-TOF-MS/MS. Curr. Pharm. Anal. 2019, 15, 580–591. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Xu, B.; Wu, E.; Cui, Y.; Hao, K.; Zhang, G.; Zhou, C.; Xu, Y.; Li, J.; et al. Polysaccharides from the roots of Millettia Speciosa Champ modulate gut health and ameliorate cyclophosphamide-induced intestinal injury and immunosuppression. Front. Immunol. 2021, 12, 766296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, J.; Chen, H.; Lian, Y.; Guo, H.; Su, Z.; Li, Y.; Zeng, H.; Zhang, X. Antifatigue and antioxidant activity of the polysaccharides isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, C.; Lin, Y.; Cai, J. Ameliorating effect on glycolipid metabolism and chemical profile of Millettia speciosa Champ. extract. J. Ethnopharmacol. 2021. 279, 114360. [CrossRef]
- Huang, Z.; Zeng, Y.; Chen, X.; Luo, S.; Pu, L.; Li, F.; Zong, M.; Lou, W. A novel polysaccharide from the roots of Millettia Speciosa Champ: Preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2020, 145, 547–557. [Google Scholar] [CrossRef]
- Luo, S.; Huang, Z.; Chen, X.; Zong, M.; Lou, W. Extraction and characterization of a functional protein from Millettia speciosa Champ. leaf. Nat. Prod. Res. 2023, 37, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Krstić, Đ.; Vukojević, V.; Mutić, J.; Fotirić Akšić, M.; Ličina, V.; Milojković-Opsenica, D.; Trifković, J. Distribution of elements in seeds of some wild and cultivated fruits. Nutrition and authenticity aspects. J. Sci. Food Agric. 2019, 99, 546–554. [Google Scholar]
- Pourhosseini, M.; Asgarpanah, J. Essential and fixed oil chemical profiles of Salvia aegyptiaca L. flowers and seeds. J. Chil. Chem. Soc. 2015, 60, 2747–2748. [Google Scholar] [CrossRef]
- Santos, A.P.; Lopes, M.C.; Limberger, R.P.; Apel, M.A.; Henriques, A.T.; Moreno, P.R.H. Analysis of the volatile oil from Pilocarpus pennatifolius Lemmaire (Rutaceae) leaves by GC-MS. Flavour Frag. J. 2004, 19, 325–326. [Google Scholar] [CrossRef]
- Rezaeih, K.A.P.; Gurbuz, B.; Uyanik, M.; Rahimi, A.; Arslan, N. Volatile constituents variability in Matricaria chamomilla L. from Ankara, Turkey. J. Essent. Oil Bear. Plants. 2015, 18, 255–260. [Google Scholar] [CrossRef]
- Javidnia, M.; Banani, A.; Miri, R.; Kamalinejad, M.; Javidnia, A. Constituents of the volatile oil of Inula oculus-christi L. from Iran. J. Essent. Oil Res. 2006, 18, 676–678. [Google Scholar] [CrossRef]
- Rashed, M.N. Bioenvironmental trace elements in warm climatic plant, pigeon pea (Cajanus cajan). Biol. Trace Elem. Res. 2021, 199, 1623–1632. [Google Scholar] [CrossRef]
- Hou, W.; Xue, X.; Li, X.; Khan, M.R.; Yan, J.; Ren, T.; Cong, R.; Lu, J. Interactive effects of nitrogen and potassium on: Grain yield, nitrogen uptake and nitrogen use efficiency of rice in low potassium fertility soil in China. Field Crop. Res. 2019, 236, 14–23. [Google Scholar] [CrossRef]
- ODS. Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/ (accessed on 5 March 2023).
- Yang, W.; Huang, G.; Chen, F.; Huang, H. Extraction/synthesis and biological activities of selenopolysaccharide. Trends Food Sci. Technol. 2021, 109, 211–218. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a bioactive micronutrient in the human diet and its cancer chemopreventive activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Hu, B.; Liu, L.; Huang, F.; Tian, J.; Hu, X.; Wang, Y. Dietary phosphorus requirement for juvenile bighead carp (Aristichthys nobilis). Aquac. Int. 2022, 30, 1675–1692. [Google Scholar] [CrossRef]
- Sobol, M.; Skiba, G.; Raj, S.; Kowalczyk, P.; Kramkowski, K.; Świątkiewicz, M.; Grela, E.R. Chemical body composition and bone growth of young pigs as affected by deficiency, adequate and excess of dietary phosphorus supply. Ann. Anim. Sci. 2022, 22, 1363–1372. [Google Scholar] [CrossRef]
- Uyan, O.; Koshio, S.; Ishikawa, M.; Uyan, S.; Ren, T.; Yokoyama, S.; Komilus, C.F.; Michael, F.R. Effects of dietary phosphorus and phospholipid level on growth, and phosphorus deficiency signs in juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2007, 267, 44–54. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef] [PubMed]
- Pęksa, A.; Miedzianka, J.; Nemś, A. Amino acid composition of flesh-coloured potatoes as affected by storage conditions. Food Chem. 2018, 266, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Rahmoune, B.; Zerrouk, L.Z.; Bouzaa, S.; Morsli, A.; Khelifi-Slaoui, M.; Ludwig-Müller, J.; Khelifi, L. Amino acids profiling in Datura stramonium and study of their variations after inoculation with plant growth promoting Rhizobacteria. Biologia 2019, 74, 1373–1383. [Google Scholar] [CrossRef]
- Grosshagauer, S.; Pirkwieser, P.; Kraemer, K.; Somoza, V. The future of moringa foods: A food chemistry perspective. Front. Nutr. 2021, 8, 751076. [Google Scholar] [CrossRef] [PubMed]
- Yushipitsina, G.G.; Chuprova, N.A.; Repyakh, S.M. Fractionation and amino acid composition of proteins of the woody verdure of sea buckthorn. Chem. Nat. Compd. 1988, 24, 348–350. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- Zhou, J.; Han, D. Proximate, amino acid and mineral composition of pupae of the silkworm Antheraea pernyi in China. J. Food Compos. Anal. 2007, 19, 850–853. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G. In vitro anti-diabetic activity of free amino acid and protein amino acid extracts from four Iranian medicinal plants. Iran. J. Sci. Technol. 2021, 45, 443–454. [Google Scholar] [CrossRef]
- Mattaini, K.R.; Sullivan, M.R.; Vander Heiden, M.G. The importance of serine metabolism in cancer. J. Cell Biol. 2016, 214, 249–257. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Chinarak, K.; Panpipat, W.; Summpunn, P.; Panya, A.; Phonsatta, N.; Cheong, L.Z.; Chaijan, M. Insights into the effects of dietary supplements on the nutritional composition and growth performance of sago palm weevil (Rhynchophorus ferrugineus) larvae. Food Chem. 2021, 363, 130279. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Herrera, C.C.; Acosta-Estrada, B.; Chuck-Hernández, C.; Serrano-Sandova, S.N.; Guardado-Félix, D.; Pérez-Carrillo, E. Nutritional content of edible grasshopper (Sphenarium purpurascens) fed on alfalfa (Medicago sativa) and maize (Zea mays). CyTA-J. Food 2020, 18, 257–263. [Google Scholar] [CrossRef]
- Tian, J.; Tian, L.; Chen, M.; Chen, Y.; Wei, A. Low temperature affects fatty acids profiling and key synthesis genes expression patterns in Zanthoxylum bungeanum maxim. Int. J. Mol. Sci. 2022, 23, 2319. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The potential of fatty acids and their derivatives as antifungal agents: A review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Kalac, P.; Samkova, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar]
- Jafari, E.; Ghanbarian, G.; Bahmanzadegan, A. Essential oil composition of aerial parts of Micromeria persica Boiss. from Western of Shiraz, Iran. Nat. Prod. Res. 2018, 32, 991–996. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Han, D.; Lu, J.; Yin, S.; Hu, H.; Zhao, C. Combination of palmitic acid and methylseleninic acid induces mitochondria-dependent apoptosis via attenuation of the IRE1α arm and enhancement of CHOP in hepatoma. ACS Omega 2021, 6, 15708–15715. [Google Scholar] [CrossRef]
- KARA, K. The investigation of fatty acids compositions of Jerusalem artichoke (Helianthus tuberosus) herbage harvested at different phenological stages. Ank. Univ. Vet. Fak. Derg. 2021, 68, 259–267. [Google Scholar] [CrossRef]
- Kmínková, M.; Winterová, R.; Kučera, J. Fatty acids in lipids of carp (Cyprinus carpio) tissues. Czech. J. Food Sci. 2001, 19, 177–181. [Google Scholar] [CrossRef]
- Fernandes, C.E.; Da Silva Vasconcelos, M.A.; De Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; De Melo Filho, A.B. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Nascimento, K.S.; Edvan, R.L.; Santos, C.O.; Sousa, J.M.S.; Nascimento, R.R.; Miranda, R.S.; Bezerra, L.R.; Biagiotti, D.; Lima Neto, A.F.; Araújo, M.J. Production aspects of hay of tropical pasture of Urochloa brizantha, Megathyrsus maximus and Andropogon gayanus: Forage mass yield characteristics, evaluation of losses, dehydration and nutritional value of hays. Crop Pasture Sci. 2022, 73, 1425–1437. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Edouard, N.; Lebas, F.; Maize Green Forage. Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/358 (accessed on 30 July 2023).
- Goossen, C.P.; Bosworth, S.C.; Darby, H.M.; Kraft, J. Microwave pretreatment allows accurate fatty acid analysis of small fresh weight (100 g) dried alfalfa, ryegrass, and winter rye samples. Anim. Feed Sci. Technol. 2018, 239, 74–84. [Google Scholar] [CrossRef]
- Yari, M.; Valizadeh, R.; Ali Nnaserian, A.; Jonker, A.; Yu, P. Carbohydrate and lipid spectroscopic molecular structures of different alfalfa hay and their relationship with nutrient availability in ruminants. Asian Australas. J. Anim. Sci. 2017, 30, 1575–1589. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Lv, J.; Sun, X.; Kong, F.; Wang, S.; Wang, Y.; Yang, H.; Cao, Z.; Li, S.; et al. Comparison of ruminal degradability, indigestible neutral detergent fiber, and total-tract digestibility of three main crop straws with alfalfa hay and corn silage. Animals 2021, 11, 3218. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Mowery, R.A.; Scarlata, C.J.; Chambliss, C.K. Compositional analysis of water-soluble materials in corn stover. J. Agric. Food Chem. 2007, 55, 5912–5918. [Google Scholar] [CrossRef]
- Holík, M.; Kunzová, E.; Ludvíková, V.; Hakl, J.. Impact of long-term manure and mineral fertilization on accumulation of non-structural carbohydrates in lucerne forage. Agronomy 2022, 12, 639. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Milla, P.G.; Peñalver, R.; Nieto, G. Health benefits of uses and applications of Moringa oleifera in bakery products. Plants 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Maibody, S.A.M.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M. An investigation on chemical/mineral compositions, ruminal microbial fermentation, and feeding value of some leaves as alternative forages for finishing goats during the dry season. AMB Express 2021, 11, 76. [Google Scholar] [CrossRef]
- Levickienė, D.; Jarienė, E.; Gajewski, M.; Danilčenko, H.; Vaitkevičienė, N.; Przybył, J.L.; Sitarek, M. Influence of harvest time on biologically active compounds and the antioxidant activity in leaves of mulberry grown in Lithuania. Not. Bot. Horti Agrobot. Cluj-Na. 2017, 45, 431–436. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; He, Y.; He, H.; Chen, X.; Liu, T.; Zhu, B. Wild vs. cultivated Zingiber striolatum diels: Nutritional and biological activity differences. Plants 2023, 12, 2180. [Google Scholar]
- Ganesan, T.; Subban, M.; Leslee, D.B.C.; Kuppannan, S.B.; Seedevi, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Geethangili, M.; Ding, S.T. A review of the phytochemistry and pharmacology of Phyllanthus urinaria L. Front. Pharmacol. 2018, 9, 1109. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Z.; Gerendás, J. Interactive effects of phosphorus supply and light intensity on glucosinolates in pakchoi (Brassica campestris L. ssp. chinensis var. communis). Plant Soil 2009, 323, 323–333. [Google Scholar] [CrossRef]
- Han, J.; Pan, X.; Chen, Q. Distribution and safety assessment of heavy metals in fresh meat from Zhejiang, China. Sci Rep 2022, 12, 3241. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ru, Y.; Feng, L.; Wang, Z.; He, X.; Zhang, X. A comparative study of nutrient composition, bioactive properties and phytochemical characteristics of Stauntonia obovatifoliola flesh and pericarp. Front. Nutr. 2022, 9, 1013971. [Google Scholar] [CrossRef]
- FAO. Amino-Acid Content of Foods and Biological Data on Proteins. Available online: http://www.fao.org/3/AC854T/AC854T00.htm#TOC (accessed on 5 March 2023).
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.; Dias, J.; Marques, A. Nutritional value and sensory properties of common carp (Cyprinus carpio L.) fillets enriched with sustainable and natural feed ingredients. Food Chem. Toxicol. 2021, 152, 112197. [Google Scholar] [CrossRef]
- Wu, H.; Ge, M.; Chen, H.; Jiang, S.; Lin, L.; Lu, L. Comparison between the nutritional qualities of wild-caught and rice-field male Chinese mitten crabs (Eriocheir sinensis). LWT-Food Sci. Technol. 2020, 117, 108663. [Google Scholar] [CrossRef]
- Han, T.; Wang, L.; Zhang, Y.; Zhang, J.; Han, D.; Lv, N.; Han, X.; Zhao, G.; Wang, M. The changes of nutrient composition of piled laying hen manure and anaerobic fermentation for recycling as a dietary ingredient for ruminants. J. Environ. Manage. 2018, 206, 768–773. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Chen, X.; Zhu, B.; Liu, T.; Yang, J. Nutritional composition and antioxidant activity of Gonostegia hirta: An underexploited, potentially wdible, wild plant. Plants 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mao, Z.; Wu, C.; Shahid, H.; Wang, S.; Shan, T. Diversity and antibacterial and antioxidant activities of fungal endophytes from the roots of Eucalyptus deglupta. Sains Malays. 2022, 51, 1687–1696. [Google Scholar] [CrossRef]
- Köller, W.; Wilcox, W.F.; Parker, D.M. Sensitivity of Venturia inaequalis populations to anilinopyrimi-dine fungicides and their contribution to scab management in New York. Plant Dis. 2005, 89, 357–365. [Google Scholar] [CrossRef]
- Lei, Z.; Yao, J.; Liu, H.; Ma, C.; Yang, W. Synthesis and bioactivity of novel sulfonate scaffold-containing pyrazolecarbamide derivatives as antifungal and antiviral agents. Front. Chem. 2022, 10, 928842. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, Z.; Yang, C.; Guo, B.; Miao, J.; Chen, Y.; Tang, L.; Li, Y. Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi. Mol. Divers. 2022, 26, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Mao, Z.; Guo, H.; Xie, Y.; Cui, Z.; Sun, J.; Wu, H.; Wen, X.; Wang, J.; Shan, T. Mollicellins O-R, four new depsidones isolated from the endophytic fungus Chaetomium sp. Eef-10. Molecules 2018, 23, 3218. [Google Scholar] [CrossRef]

| Element | Content (mg/kg) | Element | Content (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Flower | Leaves | Seeds | Flower | Leaves | Seeds | ||
| Li | <0.1 | <0.1 | <0.1 | Ni | 2.2 | 0.91 | 3.0 |
| B | 8.3 | 16 | 7.8 | Cu | 5.3 | 5.7 | 7.1 |
| Na | 22 | 31 | <3 | Zn | 19 | 26 | 38 |
| Mg | 1101 | 1417 | 2074 | As | <0.1 | <0.1 | <0.1 |
| Al | 31 | 54 | <2 | Se | <0.1 | 0.24 | 0.59 |
| K | 10,642 | 9547 | 7919 | Rb | 24 | 20 | 18 |
| Ca | 1436 | 3598 | 925 | Sr | 2.2 | 6.2 | 0.24 |
| Ti | 0.44 | 0.44 | <0.1 | Ag | <0.1 | <0.1 | <0.1 |
| V | <0.1 | <0.1 | <0.1 | Cd | <0.05 | <0.05 | <0.05 |
| Cr | 0.42 | 0.52 | <0.1 | Sn | <0.1 | <0.1 | <0.1 |
| Mn | 86 | 137 | 37 | Sb | <0.1 | <0.1 | <0.1 |
| Fe | 78 | 106 | 34 | Ba | 4.3 | 8.4 | 0.42 |
| Co | <0.1 | 0.10 | <0.1 | Hg | <0.05 | <0.05 | <0.05 |
| Mo | 0.37 | <0.1 | 0.26 | Pb | 0.48 | 0.47 | <0.1 |
| Fatty Acid | Percentage (%) | Fatty Acid | Percentage (%) |
|---|---|---|---|
| Lauric acid (C12:0) | 0.0156 | Erucic acid (C22:1) | 0.0811 |
| Myristic acid (C14:0) | 0.130 | Tricosoic acid (C23:0) | 0.0998 |
| Pentadecanoic acid (C15:0) | 0.0121 | Docosadienoic acid (C22:2) | 0.0160 |
| Palmitic acid (C16:0) | 21.3 | Timnodonic acid (C20:5) | 0.0180 |
| Palmitoleic acid (C16:1) | 0.338 | Tetracosanoic acid (C24:0) | 0.798 |
| Heptadecanoic acid (C17:0) | 0.127 | Docosahexaenoic acid (C22:6) | 0.0309 |
| Stearic acid (C18:0) | 4.99 | Total SFAs | 29.27 |
| Oleic acid (C18:1) | 27.3 | Total MUFAs | 28.02 |
| Linoleic acid (C18:2) | 41.9 | Total PUFAs | 42.79 |
| Linolenic acid (C18:3) | 0.807 | UFAs: SFAs | 2.42 |
| Arachidic acid (C20:0) | 0.580 | n-6/n-3 | 48.95 |
| Eicosanoenoic acid (C20:1) | 0.305 | n-3/n-6 | 0.02 |
| Heneicosanoic acid (C21:0) | 0.0559 | AI | 0.31 |
| Eicosadienoic acid (C20:2) | 0.0230 | TI | 0.70 |
| Docosanoic acid (C22:0) | 1.16 |
| Measured Item | Detected Value | Measured Item | Detected Value |
|---|---|---|---|
| Basic Components (DM%) | Functional Metabolites (DM%) | ||
| Crude protein | 14.2 | Total phenols | 6.09 |
| WSC | 5.82 | Simple phenols | 1.93 |
| NDF | 60.48 | Hydrolyzed tannins | 4.17 |
| ADF | 49.29 | Condensed tannins | 0.31 |
| Moisture | 4.2 | Total flavonoids | 4.81 |
| Crude fat | 1.4 | Mineral elements (DM%) | |
| Crude ash | 4.4 | P (Total) | 0.14 |
| Acid detergent lignin | 29.02 |
| No. | RT | Compound | Molecular Formula | SI | RI a | RI b | Molecular Mass | Relative Percentage (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 14.128 | Linalool | C10H18O | 96 | 1080 | 1080 | 154 | 0.52 |
| 2 | 16.977 | α-Terpineol | C10H18O | 87 | 1178 | 1192 | 154 | 1.49 |
| 3 | 18.234 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | C10H18O | 96 | 1217 | 1217 | 154 | 0.27 |
| 4 | 18.991 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (E)- | C10H18O | 97 | 1238 | 1235 | 154 | 1.09 |
| 5 | 19.441 | Nonanoic acid | C9H18O2 | 95 | 1244 | 1248 | 158 | 0.36 |
| 6 | 22.768 | Tetradecane | C14H30 | 97 | 1426 | 1395 [11] | 198 | 0.08 |
| 7 | 24.345 | 2,6,10-Trimethyltridecane | C16H34 | 95 | 1476 | 1462 | 226 | 0.07 |
| 8 | 25.273 | Pentadecane | C15H32 | 96 | 1526 | 1496 [12] | 212 | 0.08 |
| 9 | 26.262 | 2-Bromo dodecane | C12H25Br | 80 | 1513 | 1505 | 248 | 0.43 |
| 10 | 26.847 | Dodecanoic acid | C12H24O2 | 93 | 1542 | 1558 | 200 | 0.91 |
| 11 | 27.64 | Hexadecane | C16H34 | 94 | 1621 | 1593 [11] | 226 | 0.31 |
| 12 | 28.993 | Bisabolol oxide B | C15H26O2 | 83 | 1669 | 1651 [13] | 238 | 0.16 |
| 13 | 29.876 | Heptadecane | C17H36 | 95 | 1710 | 1698 [14] | 240 | 0.18 |
| 14 | 30.616 | Cryptomerione | C15H22O | 90 | 1725 | 1725 | 218 | 0.15 |
| 15 | 31.356 | Tetradecanoic acid | C14H28O2 | 99 | 1747 | 1748 | 228 | 2.53 |
| 16 | 32.014 | Octadecane | C18H38 | 95 | 1795 | 1797 [11] | 254 | 0.15 |
| 17 | 32.964 | Hexahydrofarnesyl acetone | C18H36O | 96 | 1835 | 1843 | 268 | 1.11 |
| 18 | 33.339 | Pentadecanoic acid | C15H30O2 | 95 | 1840 | 1833 | 242 | 0.71 |
| 19 | 33.535 | 1-Nonadecene | C19H38 | 93 | 1854 | 1892 | 266 | 0.37 |
| 20 | 34.571 | Hexadecanoic acid, methyl ester | C17H34O2 | 98 | 1913 | 1915 | 270 | 0.37 |
| 21 | 35.308 | Dibutyl phthalate | C16H22O4 | 93 | 1915 | 1922 | 278 | 0.59 |
| 22 | 35.858 | n-Hexadecanoic acid | C16H32O2 | 99 | 1950 | 1946 | 256 | 21.73 |
| 23 | 38.506 | Phytol | C20H40O | 94 | 2086 | 2096 | 296 | 2.51 |
| 24 | 39.183 | Linolenic acid | C18H30O2 | 95 | 2111 | 2116 | 278 | 0.39 |
| 25 | 42.778 | Pentacosane | C25H52 | 94 | 2501 | 2501 [14] | 352.407 | 5.86 |
| 26 | 45.133 | Tetracosane | C24H50 | 98 | 2393 | 2397 [14] | 338.391 | 19.96 |
| 27 | 48.638 | Nonacosane | C29H60 | 99 | 2845 | 2895 [11] | 408.47 | 5.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, Y.; Li, Y.; Huang, Y.; Zhang, Y.; Yang, L.; Xu, X.; Wu, F.; Du, B.; Mao, Z.; et al. Nutritional Value, Volatile Components, Functional Metabolites, and Antibacterial and Cytotoxic Activities of Different Parts of Millettia speciosa Champ., a Medicinal and Edible Plant with Potential for Development. Plants 2023, 12, 3900. https://doi.org/10.3390/plants12223900
Wang W, Yan Y, Li Y, Huang Y, Zhang Y, Yang L, Xu X, Wu F, Du B, Mao Z, et al. Nutritional Value, Volatile Components, Functional Metabolites, and Antibacterial and Cytotoxic Activities of Different Parts of Millettia speciosa Champ., a Medicinal and Edible Plant with Potential for Development. Plants. 2023; 12(22):3900. https://doi.org/10.3390/plants12223900
Chicago/Turabian StyleWang, Wei, Yigang Yan, Yitong Li, Yinyin Huang, Yirong Zhang, Lan Yang, Xiaoli Xu, Fengqi Wu, Bing Du, Ziling Mao, and et al. 2023. "Nutritional Value, Volatile Components, Functional Metabolites, and Antibacterial and Cytotoxic Activities of Different Parts of Millettia speciosa Champ., a Medicinal and Edible Plant with Potential for Development" Plants 12, no. 22: 3900. https://doi.org/10.3390/plants12223900





