Plant Serpins: Potential Inhibitors of Serine and Cysteine Proteases with Multiple Functions
Abstract
:1. Introduction
2. Results
2.1. Developed Countries Invest in Studies of Plant Serpins
2.2. Identification and Characterization of Serpins in Different Plant Species
2.3. Biological Pathways of Plant Serpins
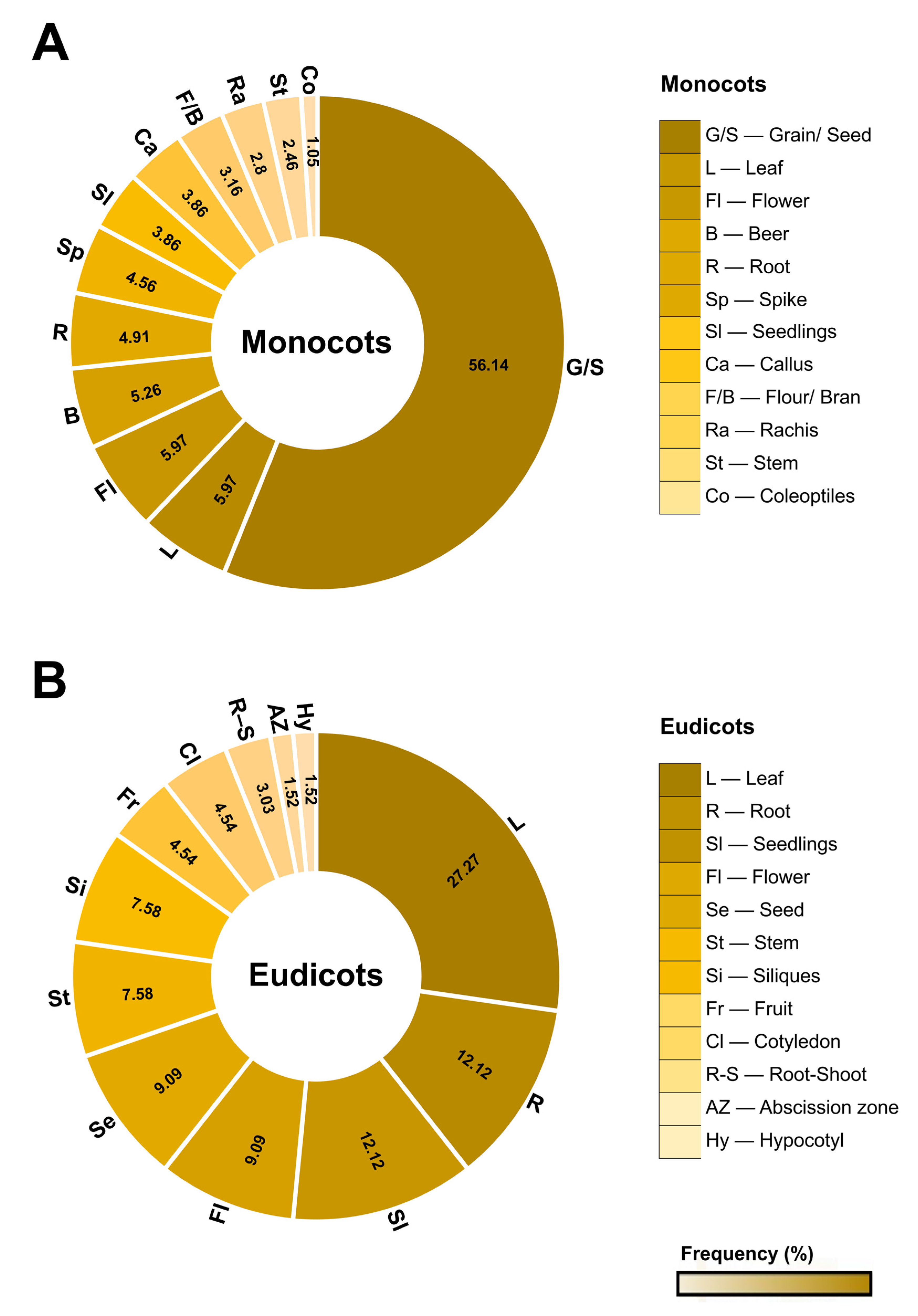
2.4. Serpins Identified in Different Organs or Tissues of Plants
2.5. Inhibitory Potential and the Various Targets of Plant Serpins
2.6. Additional Functions of Plant Serpins
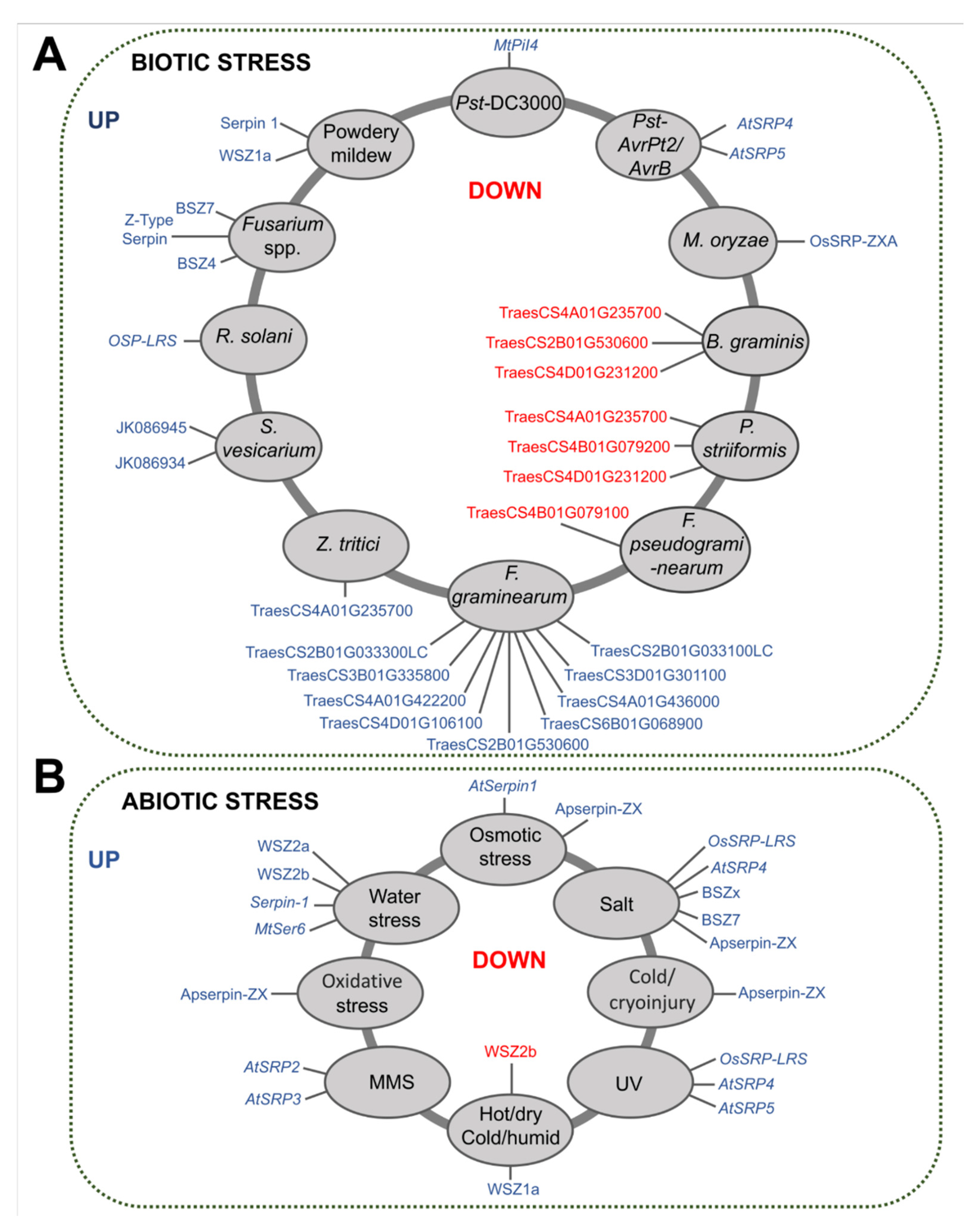
2.7. Action of Serpins in Plant Defense and Stress Events
3. Discussion
3.1. Serpins Are Ubiquitous in Different Plant Species
3.2. Serpins Are Promiscuous Inhibitors and Have Inhibitory Activity against Different Serine and Cysteine Proteases
3.3. Serpins Are Multifunctional
3.4. Serpins Are Defense Proteins Related to the Control of Stress-Induced Cell Death
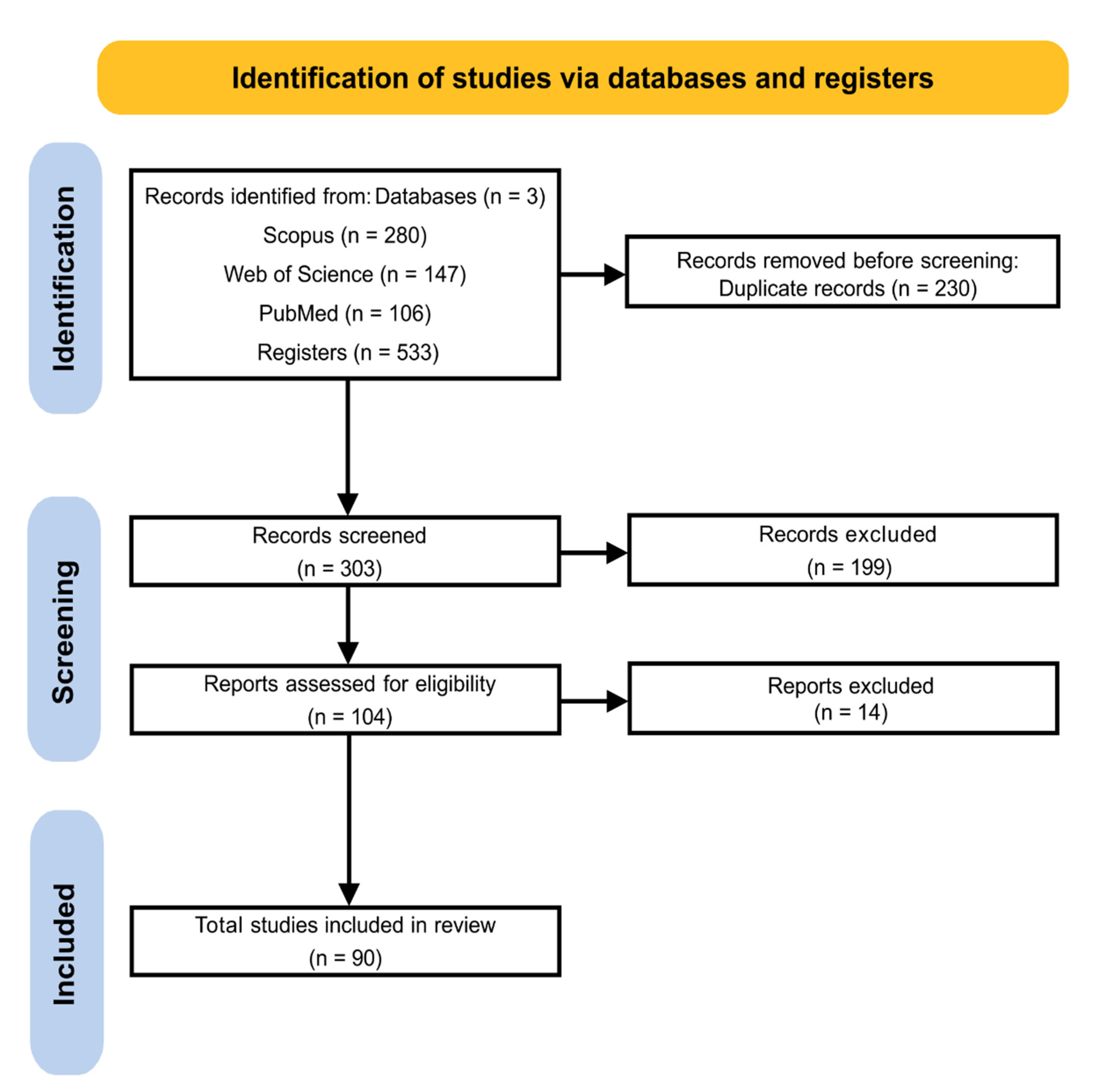
4. Materials and Methods
4.1. Planning
4.2. Execution
4.3. Summarization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly-Robinson, G.A.; Reihill, J.A.; Lundy, F.T.; McGarvey, L.P.; Lockhart, J.C.; Litherland, G.J.; Thornbury, K.D.; Martin, S.L. The Serpin Superfamily and Their Role in the Regulation and Dysfunction of Serine Protease Activity in COPD and Other Chronic Lung Diseases. Int. J. Mol. Sci. 2021, 22, 6351. [Google Scholar] [CrossRef]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.W.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The Serpins Are an Expanding Superfamily of Structurally Similar but Functionally Diverse Proteins. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef]
- Irving, J.A.; Steenbakkers, P.J.M.; Lesk, A.M.; Op den Camp, H.J.M.; Pike, R.N.; Whisstock, J.C. Serpins in Prokaryotes. Mol. Biol. Evol. 2002, 19, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Gettins, P.G.W. Serpin Structure, Mechanism, and Function. Chem. Rev. 2002, 102, 4751–4803. [Google Scholar] [CrossRef] [PubMed]
- Sanrattana, W.; Maas, C.; de Maat, S. SERPINs—From Trap to Treatment. Front. Med. 2019, 6, 25. [Google Scholar] [CrossRef]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a Serpin–Protease Complex Shows Inhibition by Deformation. Nature 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Mkaouar, H.; Akermi, N.; Kriaa, A.; Abraham, A.-L.; Jablaoui, A.; Soussou, S.; Mokdad-Gargouri, R.; Maguin, E.; Rhimi, M. Serine Protease Inhibitors and Human Wellbeing Interplay: New Insights for Old Friends. PeerJ 2019, 7, e7224. [Google Scholar] [CrossRef]
- Bouton, M.; Geiger, M.; Sheffield, W.P.; Irving, J.A.; Lomas, D.A.; Song, S.; Satyanarayanan, R.S.; Zhang, L.; McFadden, G.; Lucas, A.R. The Under-appreciated World of the Serpin Family of Serine Proteinase Inhibitors. EMBO Mol. Med. 2023, 15, e17144. [Google Scholar] [CrossRef]
- Köhnlein, T.; Welte, T. Alpha-1 Antitrypsin Deficiency: Pathogenesis, Clinical Presentation, Diagnosis, and Treatment. Am. J. Med. 2008, 121, 3–9. [Google Scholar] [CrossRef]
- Zou, Z.; Anisowicz, A.; Hendrix, M.J.; Thor, A.; Neveu, M.; Sheng, S.; Rafidi, K.; Seftor, E.; Sager, R. Maspin, a Serpin with Tumor-Suppressing Activity in Human Mammary Epithelial Cells. Science 1994, 263, 526–529. [Google Scholar] [CrossRef]
- Miao, R.Q.; Agata, J.; Chao, L.; Chao, J. Kallistatin Is a New Inhibitor of Angiogenesis and Tumor Growth. Blood 2002, 100, 3245–3252. [Google Scholar] [CrossRef]
- Chao, J.; Chai, K.X.; Chen, L.M.; Xiong, W.; Chao, S.; Woodley-Miller, C.; Wang, L.X.; Lu, H.S.; Chao, L. Tissue Kallikrein-Binding Protein Is a Serpin. I. Purification, Characterization, and Distribution in Normotensive and Spontaneously Hypertensive Rats. J. Biol. Chem. 1990, 265, 16394–16401. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Chen, D.; Zhao, W.; Wang, H.; Yang, M.; Xiang, Z.; Yuan, H. SerpinA3N Attenuates Ischemic Stroke Injury by Reducing Apoptosis and Neuroinflammation. CNS Neurosci. Ther. 2022, 28, 566–579. [Google Scholar] [CrossRef]
- Dahl, S.W.; Rasmussen, S.K.; Hejgaard, J. Heterologous Expression of Three Plant Serpins with Distinct Inhibitory Specificities. J. Biol. Chem. 1996, 271, 25083–25088. [Google Scholar] [CrossRef]
- Dahl, S.W.; Rasmussen, S.K.; Petersen, L.C.; Hejgaard, J. Inhibition of Coagulation Factors by Recombinant Barley Serpin BSZx. FEBS Lett. 1996, 394, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, H.; Rasmussen, S.K.; Roberts, T.H.; Hejgaard, J. Inhibitory Serpins from Wheat Grain with Reactive Centers Resembling Glutamine-Rich Repeats of Prolamin Storage Proteins. Cloning and Characterization of Five Major Molecular Forms. J. Biol. Chem. 2000, 275, 33272–33279. [Google Scholar] [CrossRef]
- Lampl, N.; Alkan, N.; Davydov, O.; Fluhr, R. Set-Point Control of RD21 Protease Activity by AtSerpin1 Controls Cell Death in Arabidopsis. Plant J. 2013, 74, 498–510. [Google Scholar] [CrossRef]
- Lema Asqui, S.; Vercammen, D.; Serrano, I.; Valls, M.; Rivas, S.; Van Breusegem, F.; Conlon, F.L.; Dangl, J.L.; Coll, N.S. AtSERPIN1 Is an Inhibitor of the Metacaspase AtMC1-Mediated Cell Death and Autocatalytic Processing in Planta. New Phytol. 2018, 218, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, L.; Singh, P.K.; Singh, S.; Nandi, A.K. Down-Regulation of Rice Serpin Gene OsSRP-LRS Exaggerates Stress-Induced Cell Death. J. Plant Biol. 2015, 58, 327–332. [Google Scholar] [CrossRef]
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-Induced Senescence of Medicago truncatula Nodules Involves Serpin and Ferritin to Control Proteolytic Activity and Iron Levels. New Phytol. 2018, 220, 196–208. [Google Scholar] [CrossRef]
- Cohen, M.; Davydov, O.; Fluhr, R. Plant Serpin Protease Inhibitors: Specificity and Duality of Function. J. Exp. Bot. 2019, 70, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Fluhr, R. Noncanonical Interactions between Serpin and β-Amylase in Barley Grain Improve β-Amylase Activity in Vitro. Plant Direct 2018, 2, e00054. [Google Scholar] [CrossRef]
- Tolstyko, E.A.; Lezzhov, A.A.; Pankratenko, A.V.; Serebryakova, M.V.; Solovyev, A.G.; Morozov, S.Y. Detection and in Vitro Studies of Cucurbita Maxima Phloem Serpin-1 RNA-Binding Properties. Biochimie 2020, 170, 118–127. [Google Scholar] [CrossRef] [PubMed]
- de Serres, F.; Blanco, I. Role of Alpha-1 Antitrypsin in Human Health and Disease. J. Intern. Med. 2014, 276, 311–335. [Google Scholar] [CrossRef]
- Kryvalap, Y.; Czyzyk, J. The Role of Proteases and Serpin Protease Inhibitors in β-Cell Biology and Diabetes. Biomolecules 2022, 12, 67. [Google Scholar] [CrossRef]
- Scott, B.M.; Sheffield, W.P. Engineering the Serpin α1-antitrypsin: A Diversity of Goals and Techniques. Protein Sci. 2020, 29, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Tilvawala, R.; Nemmara, V.V.; Reyes, A.C.; Sorvillo, N.; Salinger, A.J.; Cherpokova, D.; Fukui, S.; Gutch, S.; Wagner, D.; Thompson, P.R. The Role of SERPIN Citrullination in Thrombosis. Cell Chem. Biol. 2021, 28, 1728–1739.e5. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Dong, J.; Jin, R.; Yang, L.; Huo, L.; Chen, L.; Zhao, W.; Gao, X. Proteomics Unravels New Candidate Genes of Dasypyrum Villosum for Improving Wheat Quality. J. Proteom. 2021, 245, 104292. [Google Scholar] [CrossRef]
- Alvarez-Alfageme, F.; Maharramov, J.; Carrillo, L.; Vandenabeele, S.; Vercammen, D.; Van Breusegem, F.; Smagghe, G. Potential Use of a Serpin from Arabidopsis for Pest Control. PLoS ONE 2011, 6, e20278. [Google Scholar] [CrossRef]
- Jin, Z.; Li, X.-M.; Gao, F.; Sun, J.-Y.; Mu, Y.-W.; Lu, J. Proteomic Analysis of Differences in Barley (Hordeum vulgare) Malts with Distinct Filterability by DIGE. J. Proteom. 2013, 93, 93–106. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Gao, F.; Lu, J.; Cai, G.; Dong, J.; Yu, J.; Yang, M. Characterization of Barley Serpin Z7 That Plays Multiple Roles in Malt and Beer. J. Agric. Food Chem. 2014, 62, 5643–5650. [Google Scholar] [CrossRef]
- Johnson, E.T.; Skory, C.D.; Naumann, T.A.; Jairajpuri, M.A.; Dowd, P.F. Three Sorghum Serpin Recombinant Proteins Inhibit Midgut Trypsin Activity and Growth of Corn Earworm. Agri Gene 2016, 2, 11–16. [Google Scholar] [CrossRef]
- Luengo-Gil, G.; Calvo, M.I.; Martín-Villar, E.; Águila, S.; Bohdan, N.; Antón, A.I.; Espín, S.; Ayala de la Peña, F.; Vicente, V.; Corral, J.; et al. Antithrombin Controls Tumor Migration, Invasion and Angiogenesis by Inhibition of Enteropeptidase. Sci. Rep. 2016, 6, 27544. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, D.; Ambadapadi, S.; Davids, J.; Ryden, S.; Samy, H.; Bartee, M.; Sobel, E.; Dai, E.; Liu, L.; et al. Serpin Treatment Suppresses Inflammatory Vascular Lesions in Temporal Artery Implants (TAI) from Patients with Giant Cell Arteritis. PLoS ONE 2015, 10, e0115482. [Google Scholar] [CrossRef] [PubMed]
- Azouz, N.P.; Klingler, A.M.; Callahan, V.; Akhrymuk, I.V.; Elez, K.; Raich, L.; Henry, B.M.; Benoit, J.L.; Benoit, S.W.; Noé, F.; et al. Alpha 1 Antitrypsin Is an Inhibitor of the SARS-CoV-2–Priming Protease TMPRSS2. Pathog. Immun. 2021, 6, 55–74. [Google Scholar] [CrossRef]
- Wettstein, L.; Weil, T.; Conzelmann, C.; Müller, J.A.; Groß, R.; Hirschenberger, M.; Seidel, A.; Klute, S.; Zech, F.; Prelli Bozzo, C.; et al. Alpha-1 Antitrypsin Inhibits TMPRSS2 Protease Activity and SARS-CoV-2 Infection. Nat. Commun. 2021, 12, 1726. [Google Scholar] [CrossRef]
- Bhattacharjee, L.; Singh, D.; Gautam, J.K.; Nandi, A.K. Arabidopsis thaliana Serpins AtSRP4 and AtSRP5 Negatively Regulate Stress-Induced Cell Death and Effector-Triggered Immunity Induced by Bacterial Effector AvrRpt2. Physiol. Plant. 2017, 159, 329–339. [Google Scholar] [CrossRef]
- Iimure, T.; Kihara, M.; Ichikawa, S.; Ito, K.; Takeda, K.; Sato, K. Development of DNA Markers Associated with Beer Foam Stability for Barley Breeding. Theor. Appl. Genet. 2011, 122, 199–210. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, X.; Zhang, Y.; Xu, J.; Li, X. Construction of a Comprehensive Beer Proteome Map Using Sequential Filter-Aided Sample Preparation Coupled with Liquid Chromatography Tandem Mass Spectrometry. J. Sep. Sci. 2019, 42, 2835–2841. [Google Scholar] [CrossRef]
- Grosse-Holz, F.M.; van der Hoorn, R.A.L. Juggling Jobs: Roles and Mechanisms of Multifunctional Protease Inhibitors in Plants. New Phytol. 2016, 210, 794–807. [Google Scholar] [CrossRef]
- Law, R.H.P.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An Overview of the Serpin Superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA 2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, D.; Belenghi, B.; van de Cotte, B.; Beunens, T.; Gavigan, J.-A.; De Rycke, R.; Brackenier, A.; Inzé, D.; Harris, J.L.; Van Breusegem, F. Serpin1 of Arabidopsis thaliana Is a Suicide Inhibitor for Metacaspase 9. J. Mol. Biol. 2006, 364, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lampl, N.; Budai-Hadrian, O.; Davydov, O.; Joss, T.V.; Harrop, S.J.; Curmi, P.M.G.; Roberts, T.H.; Fluhr, R. Arabidopsis AtSerpin1, Crystal Structure and in Vivo Interaction with Its Target Protease RESPONSIVE TO DESICCATION-21 (RD21). J. Biol. Chem. 2010, 285, 13550–13560. [Google Scholar] [CrossRef]
- Koh, E.; Carmieli, R.; Mor, A.; Fluhr, R. Singlet Oxygen-Induced Membrane Disruption and Serpin-Protease Balance in Vacuolar-Driven Cell Death. Plant Physiol. 2016, 171, 1616–1625. [Google Scholar] [CrossRef]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. Serpin1 and WSCP Differentially Regulate the Activity of the Cysteine Protease RD21 during Plant Development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 2212–2217. [Google Scholar] [CrossRef]
- van Midden, K.P.; Peric, T.; Klemenčič, M. Plant Type I Metacaspases Are Proteolytically Active Proteases despite Their Hydrophobic Nature. FEBS Lett. 2021, 595, 2237–2247. [Google Scholar] [CrossRef]
- Lundgard, R.; Svensson, B. A 39 Kd Barley Seed Protein of the Serpin Superfamily Inhibits α-Chymotrypsin. Carlsberg Res. Commun. 1989, 54, 173–180. [Google Scholar] [CrossRef]
- Hejgaard, J. Inhibitory Serpins from Rye Grain with Glutamine as P1 and P2 Residues in the Reactive Center. FEBS Lett. 2001, 488, 149–153. [Google Scholar] [CrossRef]
- Hejgaard, J.; Hauge, S. Serpins of Oat (Avena sativa) Grain with Distinct Reactive Centres and Inhibitory Specificity. Physiol. Plant. 2002, 116, 155–163. [Google Scholar] [CrossRef]
- Yoo, B.-C.; Aoki, K.; Xiang, Y.; Campbell, L.R.; Hull, R.J.; Xoconostle-Cázares, B.; Monzer, J.; Lee, J.-Y.; Ullman, D.E.; Lucas, W.J. Characterization of Cucurbita Maxima Phloem Serpin-1 (CmPS-1). A Developmentally Regulated Elastase Inhibitor. J. Biol. Chem. 2000, 275, 35122–35128. [Google Scholar] [CrossRef] [PubMed]
- Hejgaard, J.; Laing, W.A.; Marttila, S.; Gleave, A.P.; Roberts, T.H. Serpins in Fruit and Vegetative Tissues of Apple (Malus domestica): Expression of Four Serpins with Distinct Reactive Centres and Characterisation of a Major Inhibitory Seed Form, MdZ1b. Funct. Plant Biol. 2005, 32, 517–527. [Google Scholar] [CrossRef]
- Chen, G.; Li, R.; Shen, X. ApSerpin-ZX from Agapanthus Praecox, Is a Potential Cryoprotective Agent to Plant Cryopreservation. Cryobiology 2021, 98, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Handoyo, T.; Ishii, T.; Kumazawa, S.; Morita, N.; Suyama, K. Proteomic Analysis of Wheat Flour Allergens. J. Agric. Food Chem. 2007, 55, 6863–6870. [Google Scholar] [CrossRef]
- Šotkovský, P.; Hubálek, M.; Hernychová, L.; Novák, P.; Havranová, M.; Šetinová, I.; Kitanovičová, A.; Fuchs, M.; Stulík, J.; Tučková, L. Proteomic Analysis of Wheat Proteins Recognized by IgE Antibodies of Allergic Patients. Proteomics 2008, 8, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Sander, I.; Rozynek, P.; Rihs, H.-P.; Van Kampen, V.; Chew, F.T.; Lee, W.S.; Kotschy-Lang, N.; Merget, R.; Brüning, T.; Raulf-Heimsoth, M. Multiple Wheat Flour Allergens and Cross-Reactive Carbohydrate Determinants Bind IgE in Baker’s Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Huebener, S.; Tanaka, C.K.; Uhde, M.; Zone, J.J.; Vensel, W.H.; Kasarda, D.D.; Beams, L.; Briani, C.; Green, P.H.R.; Altenbach, S.B.; et al. Specific Nongluten Proteins of Wheat Are Novel Target Antigens in Celiac Disease Humoral Response. J. Proteome Res. 2015, 14, 503–511. [Google Scholar] [CrossRef]
- Tolstyko, E.A.; Chergintsev, D.A.; Tolicheva, O.A.; Vinogradova, D.S.; Konevega, A.L.; Morozov, S.Y.; Solovyev, A.G. RNA Binding by Plant Serpins in Vitro. Biochemistry 2021, 86, 1214–1224. [Google Scholar] [CrossRef]
- Bezdi, M.S.; Toorchi, M.; Pourabad, R.F.; Zarghami, N.; Nouri, M.-Z.; Komatsu, S. Proteome Analysis of Gut and Salivary Gland Proteins of Fifth-Instar Nymph and Adults of the Sunn Pest, Eurygaster Integriceps. Arch. Insect Biochem. Physiol. 2012, 81, 105–119. [Google Scholar] [CrossRef]
- Irving, J.A.; Pike, R.N.; Lesk, A.M.; Whisstock, J.C. Phylogeny of the Serpin Superfamily: Implications of Patterns of Amino Acid Conservation for Structure and Function. Genome Res. 2000, 10, 1845–1864. [Google Scholar] [CrossRef]
- Roberts, T.H.; Marttila, S.; Rasmussen, S.K.; Hejgaard, J. Differential Gene Expression for Suicide-Substrate Serine Proteinase Inhibitors (Serpins) in Vegetative and Grain Tissues of Barley. J. Exp. Bot. 2003, 54, 2251–2263. [Google Scholar] [CrossRef]
- Izuhara, K.; Ohta, S.; Kanaji, S.; Shiraishi, H.; Arima, K. Recent Progress in Understanding the Diversity of the Human Ov-Serpin/Clade B Serpin Family. Cell. Mol. Life Sci. 2008, 65, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; Ginsburg, D.; Day, D.E.; Berkenpas, M.B.; Verhamme, I.M.; Kvassman, J.-O.; Shore, J.D. Serpin-Protease Complexes Are Trapped as Stable Acyl-Enzyme Intermediates. J. Biol. Chem. 1995, 270, 25309–25312. [Google Scholar] [CrossRef]
- Stratikos, E.; Gettins, P.G.W. Formation of the Covalent Serpin-Proteinase Complex Involves Translocation of the Proteinase by More than 70 Å and Full Insertion of the Reactive Center Loop into β-Sheet A. Proc. Natl. Acad. Sci. USA 1999, 96, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, J.; Zhou, Z.S.; Zhu, C.C.; Hu, L.B.; Wang, L.; Yang, L.; Yang, Z.M. Ectopic Expression of a Proteinase Inhibitor I4 (MtPiI4) Gene from Medicago truncatula Confers Plant Resistance to Pseudomonas Syringae Pv. Tomato DC3000. Plant Mol. Biol. Rep. 2015, 33, 1686–1696. [Google Scholar] [CrossRef]
- Silverman, G.A.; Whisstock, J.C.; Askew, D.J.; Pak, S.C.; Luke, C.J.; Cataltepe, S.; Irving, J.A.; Bird, P.I. Human Clade B Serpins (Ov-Serpins) Belong to a Cohort of Evolutionarily Dispersed Intracellular Proteinase Inhibitor Clades That Protect Cells from Promiscuous Proteolysis. Cell. Mol. Life Sci. 2004, 61, 301–325. [Google Scholar] [CrossRef]
- Silverman, G.A.; Whisstock, J.C.; Bottomley, S.P.; Huntington, J.A.; Kaiserman, D.; Luke, C.J.; Pak, S.C.; Reichhart, J.-M.; Bird, P.I. Serpins Flex Their Muscle: I. Putting the Clamps on Proteolysis in Diverse Biological Systems. J. Biol. Chem. 2010, 285, 24299–24305. [Google Scholar] [CrossRef]
- Fluhr, R.; Lampl, N.; Roberts, T.H. Serpin Protease Inhibitors in Plant Biology. Physiol. Plant. 2012, 145, 95–102. [Google Scholar] [CrossRef]
- Levashina, E.A.; Langley, E.; Green, C.; Gubb, D.; Ashburner, M.; Hoffmann, J.A.; Reichhart, J.-M. Constitutive Activation of Toll-Mediated Antifungal Defense in Serpin-Deficient Drosophila. Science 1999, 285, 1917–1919. [Google Scholar] [CrossRef]
- Dong, C.; Huang, T.-C.; Roberts, T.H. Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress. Int. J. Mol. Sci. 2023, 24, 2707. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat.-Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Svendsen, I.; Hejgaard, J. A Plant Serpin Gene. Structure, Organization and Expression of the Gene Encoding Barley Protein Z4. Eur. J. Biochem. 1990, 194, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Curioni, A.; Pressi, G.; Furegon, L.; Peruffo, A.D.B. Major Proteins of Beer and Their Precursors in Barley: Electrophoretic and Immunological Studies. J. Agric. Food Chem. 1995, 43, 2620–2626. [Google Scholar] [CrossRef]
- Johnson, R.R.; Dyer, W.E. Degradation of Endosperm MRNAs during Dry Afterripening of Cereal Grains. Seed Sci. Res. 2000, 10, 233–241. [Google Scholar] [CrossRef]
- Keates, S.E.; Kostman, T.A.; Anderson, J.D.; Bailey, B.A. Altered Gene Expression in Three Plant Species in Response to Treatment with Nep1, a Fungal Protein That Causes Necrosis. Plant Physiol. 2003, 132, 1610–1622. [Google Scholar] [CrossRef]
- Borén, M.; Larsson, H.; Falk, A.; Jansson, C. The Barley Starch Granule Proteome—Internalized Granule Polypeptides of the Mature Endosperm. Plant Sci. 2004, 166, 617–626. [Google Scholar] [CrossRef]
- Shatters, R.G., Jr.; Bausher, M.G.; Hunter, W.B.; Chaparro, J.X.; Dang, P.M.; Niedz, R.P.; Mayer, R.T.; McCollum, T.G.; Sinisterra, X. Putative Protease Inhibitor Gene Discovery and Transcript Profiling during Fruit Development and Leaf Damage in Grapefruit (Citrus paradisi Macf.). Gene 2004, 326, 77–86. [Google Scholar] [CrossRef]
- Østergaard, O.; Finnie, C.; Laugesen, S.; Roepstorff, P.; Svennson, B. Proteome Analysis of Barley Seeds: Identification of Major Proteins from Two-Dimensional Gels (Pl 4–7). Proteomics 2004, 4, 2437–2447. [Google Scholar] [CrossRef]
- Salt, L.J.; Robertson, J.A.; Jenkins, J.A.; Mulholland, F.; Mills, E.N.C. The Identification of Foam-Forming Soluble Proteins from Wheat (Triticum aestivum) Dough. Proteomics 2005, 5, 1612–1623. [Google Scholar] [CrossRef]
- Perrocheau, L.; Rogniaux, H.; Boivin, P.; Marion, D. Probing Heat-Stable Water-Soluble Proteins from Barley to Malt and Beer. Proteomics 2005, 5, 2849–2858. [Google Scholar] [CrossRef]
- Petersen, M.L.C.; Hejgaard, J.; Thompson, G.A.; Schulz, A. Cucurbit Phloem Serpins Are Graft-Transmissible and Appear to Be Resistant to Turnover in the Sieve Element-Companion Cell Complex. J. Exp. Bot. 2005, 56, 3111–3120. [Google Scholar] [CrossRef]
- Macewicz, J.; Orzechowski, S.; Dobrzyńska, U.; Haebel, S. Is Quantity of Protein in Barley Forms Determined by Proteins Localized in the Subaleurone Layer? Acta Physiol. Plant. 2006, 28, 409–416. [Google Scholar] [CrossRef]
- Schoonheim, P.J.; Veiga, H.; da Costa Pereira, D.; Friso, G.; van Wijk, K.J.; de Boer, A.H. A Comprehensive Analysis of the 14-3-3 Interactome in Barley Leaves Using a Complementary Proteomics and Two-Hybrid Approach. Plant Physiol. 2007, 143, 670–683. [Google Scholar] [CrossRef]
- Sancho, A.I.; Gillabert, M.; Tapp, H.; Shewry, P.R.; Skeggs, P.K.; Mills, E.N.C. Effect of Environmental Stress during Grain Filling on the Soluble Proteome of Wheat (Triticum aestivum) Dough Liquor. J. Agric. Food Chem. 2008, 56, 5386–5393. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Atwell, B.J.; Roberts, T.H. Serpin Genes AtSRP2 and AtSRP3 Are Required for Normal Growth Sensitivity to a DNA Alkylating Agent in Arabidopsis. BMC Plant Biol. 2009, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Finnie, C.; Bagge, M.; Steenholdt, T.; Østergaard, O.; Bak-Jensen, K.S.; Backes, G.; Jensen, A.; Giese, H.; Larsen, J.; Roepstorff, P.; et al. Integration of the Barley Genetic and Seed Proteome Maps for Chromosome 1H, 2H, 3H, 5H and 7H. Funct. Integr. Genom. 2009, 9, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Petti, C.; Khan, M.; Doohan, F. Lipid Transfer Proteins and Protease Inhibitors as Key Factors in the Priming of Barley Responses to Fusarium Head Blight Disease by a Biocontrol Strain of Pseudomonas Fluorescens. Funct. Integr. Genom. 2010, 10, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, E.; Aldini, G.; Regazzoni, L.; Kravchuk, A.V.; Citterio, A.; Righetti, P.G. Les Maîtres de LOrge: The Proteome Content of Your Beer Mug. J. Proteome Res. 2010, 9, 5262–5269. [Google Scholar] [CrossRef] [PubMed]
- Eggert, K.; Pawelzik, E. Proteome Analysis of Fusarium Head Blight in Grains of Naked Barley (Hordeum vulgare subsp. nudum). Proteomics 2011, 11, 972–985. [Google Scholar] [CrossRef]
- Witzel, K.; Pietsch, C.; Strickert, M.; Matros, A.; Röder, M.S.; Weschke, W.; Wobus, U.; Mock, H.-P. Mapping of Quantitative Trait Loci Associated with Protein Expression Variation in Barley Grains. Mol. Breed. 2011, 27, 301–314. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Tanaka, C.K.; Hurkman, W.J.; Whitehand, L.C.; Vensel, W.H.; Dupont, F.M. Differential Effects of a Post-Anthesis Fertilizer Regimen on the Wheat Flour Proteome Determined by Quantitative 2-DE. Proteome Sci. 2011, 9, 46. [Google Scholar] [CrossRef]
- Eremia, M.C.; Lupescu, I.; Tcacenco, L. Bioactive Compounds Obtained by Immobilisation of Serine Protease Inhibitors. Int. J. Nano Biomater. 2011, 3, 335–343. [Google Scholar] [CrossRef]
- Debiton, C.; Merlino, M.; Chambon, C.; Bancel, E.; Decourteix, M.; Planchot, V.; Branlard, G. Analyses of Albumins, Globulins and Amphiphilic Proteins by Proteomic Approach Give New Insights on Waxy Wheat Starch Metabolism. J. Cereal Sci. 2011, 53, 160–169. [Google Scholar] [CrossRef]
- Francis, S.E.; Ersoy, R.A.; Ahn, J.-W.; Atwell, B.J.; Roberts, T.H. Serpins in Rice: Protein Sequence Analysis, Phylogeny and Gene Expression during Development. BMC Genom. 2012, 13, 449. [Google Scholar] [CrossRef]
- Bielskienė, K.; Labeikytė, D.; Sjakste, N.; Bagdonienė, L.; Juodka, B. Phosphatase Activity in Barley Proteins Tightly Bound to DNA and Its Development-Dependent Changes. Biochemistry 2012, 77, 679–688. [Google Scholar] [CrossRef]
- Mameri, H.; Denery-Papini, S.; Pietri, M.; Tranquet, O.; Larré, C.; Drouet, M.; Paty, E.; Jonathan, A.-M.; Beaudouin, E.; Moneret-Vautrin, D.-A.; et al. Molecular and Immunological Characterization of Wheat Serpin (Tri a 33). Mol. Nutr. Food Res. 2012, 56, 1874–1883. [Google Scholar] [CrossRef]
- Singh, A.M.; Singh, S.K.; Ahlawat, A.K.; Jain, N.; Singh, G.P.; Ravi, I.; Yadav, M.; Misra, P.C. Distribution of Alleles of Grain Quality Genes in Indian Bread Wheat Varieties. Indian J. Genet. Plant Breed. 2012, 72, 208–216. [Google Scholar]
- Sun, H.; Cao, F.; Wang, N.; Zhang, M.; Ahmed, I.M.; Zhang, G.; Wu, F. Differences in Grain Ultrastructure, Phytochemical and Proteomic Profiles between the Two Contrasting Grain Cd-Accumulation Barley Genotypes. PLoS ONE 2013, 8, e79158. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, J.; Chen, G.; Bian, Y.; Lv, D.; Li, X.; Wang, Z.; Yan, Y. ITRAQ-Based Quantitative Proteome and Phosphoprotein Characterization Reveals the Central Metabolism Changes Involved in Wheat Grain Development. BMC Genom. 2014, 15, 1029. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Bronsoms, S.; Quarles Van Ufford, H.C.; Halkes, S.B.A.; Virkola, R.; Liskamp, R.M.J.; Beukelman, C.J.; Pieters, R.J.; Pérez, J.F.; Martín-Orúe, S.M. A Proteinaceous Fraction of Wheat Bran May Interfere in the Attachment of Enterotoxigenic E. Coli K88 (F4+) to Porcine Epithelial Cells. PLoS ONE 2014, 9, e104258. [Google Scholar] [CrossRef] [PubMed]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.; Fitzgerald, G.; Khan, A.; Seneweera, S. Rising CO2 Concentration Altered Wheat Grain Proteome and Flour Rheological Characteristics. Food Chem. 2015, 170, 448–454. [Google Scholar] [CrossRef]
- Picariello, G.; Mamone, G.; Cutignano, A.; Fontana, A.; Zurlo, L.; Addeo, F.; Ferranti, P. Proteomics, Peptidomics, and Immunogenic Potential of Wheat Beer (Weissbier). J. Agric. Food Chem. 2015, 63, 3579–3586. [Google Scholar] [CrossRef]
- Dong, K.; Zhen, S.; Cheng, Z.; Cao, H.; Ge, P.; Yan, Y. Proteomic Analysis Reveals Key Proteins and Phosphoproteins upon Seed Germination of Wheat (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 1017. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.T.; Sousa, L.; de Sousa, A.T.; Pais, M.S. Mechanisms of Resistance/Tolerance of Pyrus Communis to Stemphylium Vesicarium. A Transcriptome Analysis. Agrofor. Syst. 2015, 89, 991–1017. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Zehirov, G.; Kirova, E.; Simova-Stoilova, L. Transcript Profiling of Serine- and Cysteine Protease Inhibitors in Triticum aestivum Varieties with Different Drought Tolerance. Cereal Res. Commun. 2016, 44, 79–88. [Google Scholar] [CrossRef]
- Ghorbani, S.; Hoogewijs, K.; Pečenková, T.; Fernandez, A.; Inzé, A.; Eeckhout, D.; Kawa, D.; De Jaeger, G.; Beeckman, T.; Madder, A.; et al. The SBT6.1 Subtilase Processes the GOLVEN1 Peptide Controlling Cell Elongation. J. Exp. Bot. 2016, 67, 4877–4887. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, C.; Zhen, S.; Cao, M.; Zeller, F.J.; Hsam, S.L.K.; Yan, Y. Identification of Drought Stress Related Proteins from 1Sl(1B) Chromosome Substitution Line of Wheat Variety Chinese Spring. Bot. Stud. 2016, 57, 20. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Tanaka, C.K.; Whitehand, L.C.; Vensel, W.H. Effects of Post-Anthesis Fertilizer on the Protein Composition of the Gluten Polymer in a US Bread Wheat. J. Cereal Sci. 2016, 68, 66–73. [Google Scholar] [CrossRef]
- Cao, H.; He, M.; Zhu, C.; Yuan, L.; Dong, L.; Bian, Y.; Zhang, W.; Yan, Y. Distinct Metabolic Changes between Wheat Embryo and Endosperm during Grain Development Revealed by 2D-DIGE-Based Integrative Proteome Analysis. Proteomics 2016, 16, 1515–1536. [Google Scholar] [CrossRef]
- Guo, B.; Luan, H.; Lin, S.; Lv, C.; Zhang, X.; Xu, R. Comparative Proteomic Analysis of Two Barley Cultivars (Hordeum vulgare L.) with Contrasting Grain Protein Content. Front. Plant Sci. 2016, 7, 542. [Google Scholar] [CrossRef] [PubMed]
- Saadati, M.; Toorchi, M. The Study of Plant Protein Accumulation in Gut of Insect Using Proteomics Technique: Wheat–Sunn Pest Interaction. J. Saudi Soc. Agric. Sci. 2017, 16, 205–209. [Google Scholar] [CrossRef]
- Kosová, K.; Chrpová, J.; Šantrůček, J.; Hynek, R.; Štěrbová, L.; Vítámvás, P.; Bradová, J.; Prášil, I.T. The Effect of Fusarium Culmorum Infection and Deoxynivalenol (DON) Application on Proteome Response in Barley Cultivars Chevron and Pedant. J. Proteom. 2017, 169, 112–124. [Google Scholar] [CrossRef] [PubMed]
- García-Molina, M.D.; Muccilli, V.; Saletti, R.; Foti, S.; Masci, S.; Barro, F. Comparative Proteomic Analysis of Two Transgenic Low-Gliadin Wheat Lines and Non-Transgenic Wheat Control. J. Proteom. 2017, 165, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Philosoph-Hadas, S.; Ma, C.; Jiang, C.-Z.; Riov, J.; Mugasimangalam, R.; Kochanek, B.; Salim, S.; Reid, M.S.; Meir, S. The Tomato Hybrid Proline-Rich Protein Regulates the Abscission Zone Competence to Respond to Ethylene Signals. Hortic. Res. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fluhr, R. Singlet Oxygen Plays an Essential Role in the Root’s Response to Osmotic Stress. Plant Physiol. 2018, 177, 1717–1727. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Yang, X.; Li, Y.; Wang, C.; He, D. Proteomic Analysis of the Impacts of Powdery Mildew on Wheat Grain. Food Chem. 2018, 261, 30–35. [Google Scholar] [CrossRef]
- Hoernstein, S.N.W.; Fode, B.; Wiedemann, G.; Lang, D.; Niederkrüger, H.; Berg, B.; Schaaf, A.; Frischmuth, T.; Schlosser, A.; Decker, E.L.; et al. Host Cell Proteome of Physcomitrella Patens Harbors Proteases and Protease Inhibitors under Bioproduction Conditions. J. Proteome Res. 2018, 17, 3749–3760. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Li, M.; Ma, X.; Tian, E.; Sun, A.; Yin, Y. Analysis of the Natural Dehydration Mechanism during Middle and Late Stages of Wheat Seeds Development by Some Physiological Traits and ITRAQ-Based Proteomic. J. Cereal Sci. 2018, 80, 102–110. [Google Scholar] [CrossRef]
- Benbow, H.R.; Jermiin, L.S.; Doohan, F.M. Serpins: Genome-Wide Characterisation and Expression Analysis of the Serine Protease Inhibitor Family in Triticum aestivum. G3 Genes Genomes Genet. 2019, 9, 2709–2722. [Google Scholar] [CrossRef]
- Tanner, G.J.; Colgrave, M.L.; Blundell, M.J.; Howitt, C.A.; Bacic, A. Hordein Accumulation in Developing Barley Grains. Front. Plant Sci. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Riahi, J.; Amri, B.; Chibani, F.; Azri, W.; Mejri, S.; Bennani, L.; Zoghlami, N.; Matros, A.; Mock, H.P.; Ghorbel, A.; et al. Comparative Analyses of Albumin/Globulin Grain Proteome Fraction in Differentially Salt-Tolerant Tunisian Barley Landraces Reveals Genotype-Specific and Defined Abundant Proteins. Plant Biol. 2019, 21, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Jørgensen, B.; Aziz, E.; Batool, R.; Naseer, S.; Rasmussen, S.K. Genome Wide Identification and Comparative Analysis of the Serpin Gene Family in Brachypodium and Barley. Plants 2020, 9, 1439. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Shen, Y.; Zhang, C.; Kong, X.; Li, X.; Hua, Y. Endopeptidases, Exopeptidases, and Glutamate Decarboxylase in Soybean Water Extract and Their in Vitro Activity. Food Chem. 2021, 360, 130026. [Google Scholar] [CrossRef]
- le Roux, M.-S.L.; Burger, N.F.V.; Vlok, M.; Kunert, K.J.; Cullis, C.A.; Botha, A.-M. EMS Derived Wheat Mutant BIG8-1 (Triticum aestivum L.)—A New Drought Tolerant Mutant Wheat Line. Int. J. Mol. Sci. 2021, 22, 5314. [Google Scholar] [CrossRef]
- Távora, F.T.P.K.; Bevitori, R.; Mello, R.N.; Cintra, M.M.D.F.; Oliveira-Neto, O.B.; Fontes, W.; Castro, M.S.; Sousa, M.V.; Franco, O.L.; Mehta, A. Shotgun Proteomics Coupled to Transient-Inducible Gene Silencing Reveal Rice Susceptibility Genes as New Sources for Blast Disease Resistance. J. Proteom. 2021, 241, 104223. [Google Scholar] [CrossRef]




| Species | Serpin * | |
|---|---|---|
| Monocots | Hordeum vulgare | BSZ7 (Q43492.2) |
| BSZx (Q40066.1) | ||
| BSZ4 (P06293.2) | ||
| Triticum aestivum | WSZ1a (Q41593) | |
| WSZ1b (P93693) | ||
| WSZ1c (Q9ST58) | ||
| WSZ2a (Q9ST57) | ||
| WSZ2b (P93692) | ||
| Secale cereale | RSZb, RSZc1, RSZc2, RSZd, RSZe, RSZf | |
| Avena sativa | OSZa, OSZb, OSZc, OSZd | |
| Oryza sativa | OsSRP-LRS (Os03g41419) | |
| Sorghum bicolor | Sbser1, Sbser2, Sbser3 | |
| Eudicots | Arabidopsis thaliana | AtSerpin1 (Q9S7T8) |
| AtSRP2 (At2g14540) | ||
| AtSRP3 (At1g64030) | ||
| AtSRP4 (At2g26390) | ||
| AtSRP5 (At2g25240) | ||
| Curcubita maxima | CmPS-1 (AAG02411.1) | |
| Cucumis sativus | CsPS-1 | |
| Malus domestica | MdZ1b | |
| Medicago truncatula | MtPiI4, MtSer6 | |
| Agapanthus praecox | ApSerpin-ZX | |
| Algae | Chlamydomonas reinhardtii | CrSERPIN |
| Classification | Inhibitor | Protease a | Authors |
|---|---|---|---|
| Plant | AtSerpin1 | Metacaspase (AtMC9) | [43] |
| AtSerpin1 | Papain (RD21) | [44] | |
| [17] | |||
| [45] | |||
| [46] | |||
| AtSerpin1 | Metacaspase (AtMC1) | [18] | |
| Green alga | CrSERPIN | Metacaspase (CrMCA-I) | [47] |
| Animal (vertebrate) | BSZ7 | Chymotrypsin, trypsin b | [48] |
| BSZx | Trypsin, chymotrypsin, cathepsin G | [14] | |
| BSZx | Plasm kallikrein, thrombin, coagulation factor VIIa/sTF, coagulation factor Xa, protein C b, leukocyte elastase b, coagulation factor XIIa b, urokinase-type plasminogen activator (u-PA) b | [15] | |
| BSZ4 | Leukocyte cathepsin G | [14] | |
| BSZ4 | Chymotrypsin, leukocyte cathepsin G | [22] | |
| WSZ1a | Chymotrypsin, cathepsin G | [14] | |
| WSZ1a | Chymotrypsin, cathepsin G, leukocyte elastase b, pancreas elastase b, coagulation factor Xa b | [16] | |
| WSZ1b, WSZ1c | Chymotrypsin, cathepsin G, leukocyte elastase b, pancreas elastase b | [16] | |
| WSZ2a | Chymotrypsin, cathepsin G, pancreas elastase | [16] | |
| WSZ2b | Chymotrypsin, cathepsin G, trypsin b, plasmin, plasm kallikrein, coagulation factor VIIa/sTF b | [16] | |
| RSZb, RSZc1, RSZc2, RSZe | Chymotrypsin | [49] | |
| RSZf | Chymotrypsin, cathepsin G | [49] | |
| OSZa | Pancreas elastase | [50] | |
| OSZb | Pancreas elastase, chymotrypsin | [50] | |
| OSZc | Trypsin, chymotrypsin b | [50] | |
| CmPS-1 | Pancreas elastase | [51] | |
| MdZ1b | Trypsin, plasmin b, chymotrypsin b, urokinase-type plasminogen activator (u-PA) b | [52] | |
| ApSerpin ZX | Trypsin | [53] | |
| Animal (invertebrate) | AtSerpin1 | Trypsin, chymotrypsin, cathepsin B and L | [29] |
| Sbser1, Sbser2, Sbser3 | Trypsin | [32] | |
| Bacterium | BSZx | Subtilisin Carlsberg and Novo b | [15] |
| Other Conditions | ||
|---|---|---|
| Serpins | Description | Authors |
| WSZ2b, WSZ1c | Serpins were identified as wheat allergens because they interacted with IgE antibodies from the sera of patients with allergies to wheat, identified by electrophoresis and IgE-immunoblotting analysis. | [54] |
| WSZ1b | [54,55] | |
| WSZ2a | WSZ2A was identified among the IgE-binding proteins of German bakers with work-related wheat flour allergies (asthma/rhinitis). | [56] |
| Serpin 3 | The serpins reacted with the IgG and IgA antibodies of patients with celiac disease or dermatitis herpetiformis. The antibody reactivity to non-gluten antigens was further confirmed with a recombinant serpin, Serpin 3 (gi: 224589270), which showed reactivity with IgG and/or IgA antibodies from the patients. | [57] |
| BSZ7 | In particular, when the serpin Z7 was added to the wort, changes in its filterability were observed, such as a slight increase in viscosity and a reduction in the separation rate and turbidity, which may affect the final quality of the beer. | [30] |
| BSZ4 | The presence of serpin Z4 changed β-amylase enzymatic properties by increasing the maximal catalytic velocity and stabilizing β-amylase activity during rising temperature and oxidative stress. | [22] |
| CmPS-1 | C. maxima phloem serpin 1 (CmPS1) is able to bind RNA in vitro, and among the different RNAs tested, it has the highest affinity for tRNA in plants. | [23,58] |
| AtSerpin1 | Similar to CmPS-1, AtSerpin1 formed complexes with tRNA from yeast and A. thaliana leaves. | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.M.; Santos, A.S.; Santos, A.S.; Zugaib, M.; Pirovani, C.P. Plant Serpins: Potential Inhibitors of Serine and Cysteine Proteases with Multiple Functions. Plants 2023, 12, 3619. https://doi.org/10.3390/plants12203619
Ferreira MM, Santos AS, Santos AS, Zugaib M, Pirovani CP. Plant Serpins: Potential Inhibitors of Serine and Cysteine Proteases with Multiple Functions. Plants. 2023; 12(20):3619. https://doi.org/10.3390/plants12203619
Chicago/Turabian StyleFerreira, Monaliza Macêdo, Ariana Silva Santos, Adriadna Souza Santos, Maria Zugaib, and Carlos Priminho Pirovani. 2023. "Plant Serpins: Potential Inhibitors of Serine and Cysteine Proteases with Multiple Functions" Plants 12, no. 20: 3619. https://doi.org/10.3390/plants12203619






