Variability in Water Capacity of Small-Leaved Linden Associated with Both the Presence of Honeydew and Various Sources of Pollution
Abstract
:1. Introduction
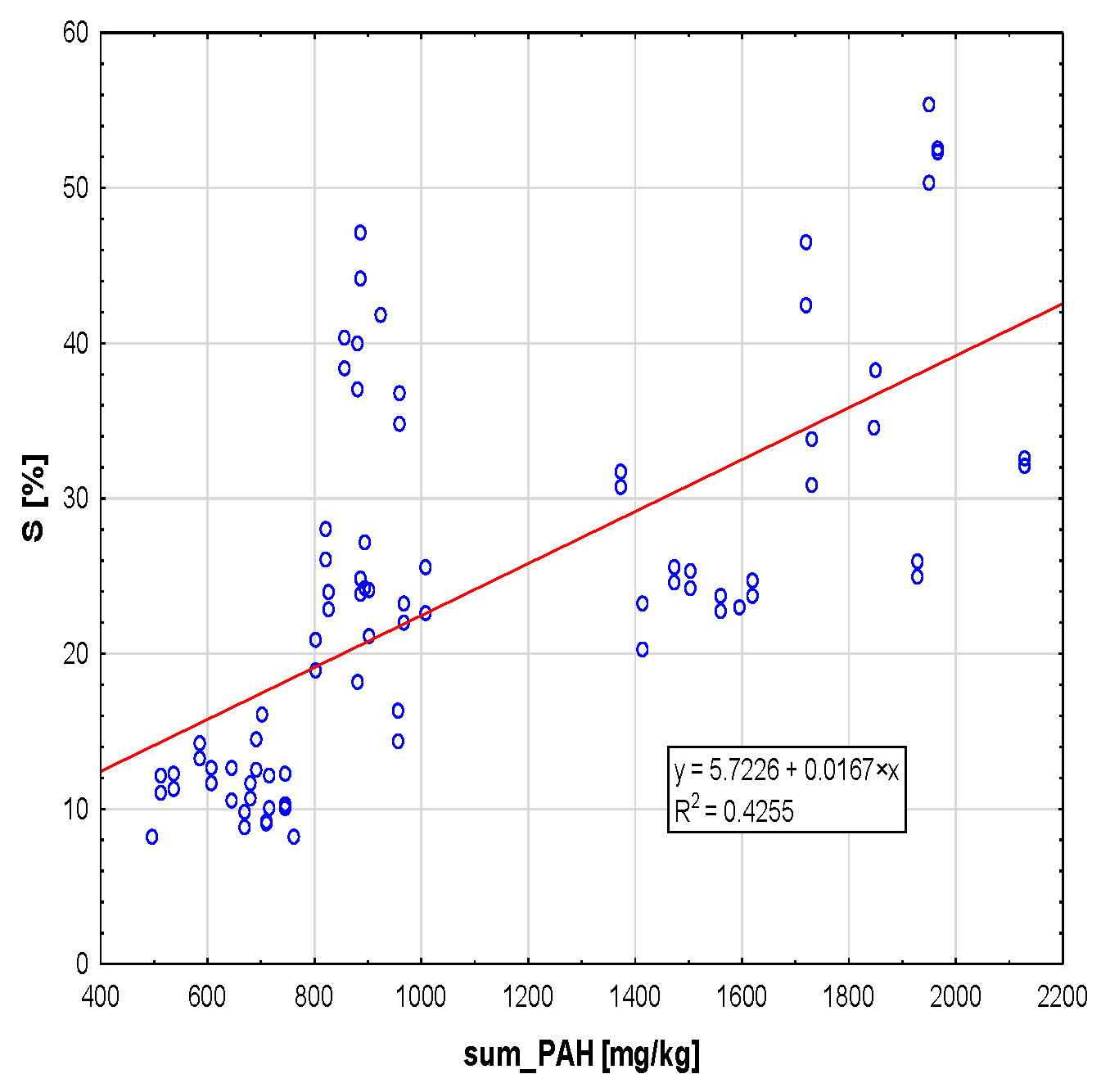
2. Results
2.1. General Dependence of S on SUM.PAH
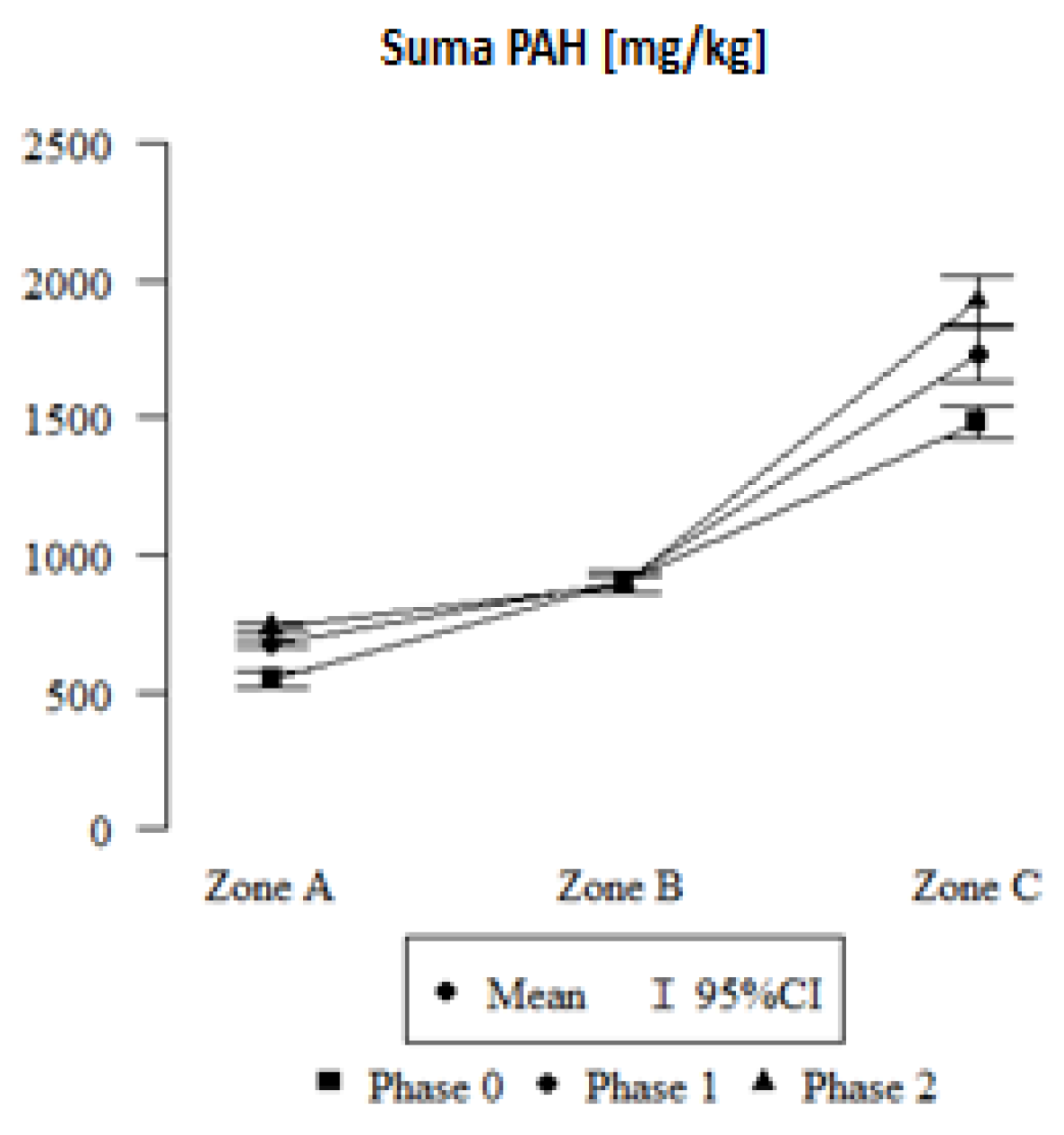
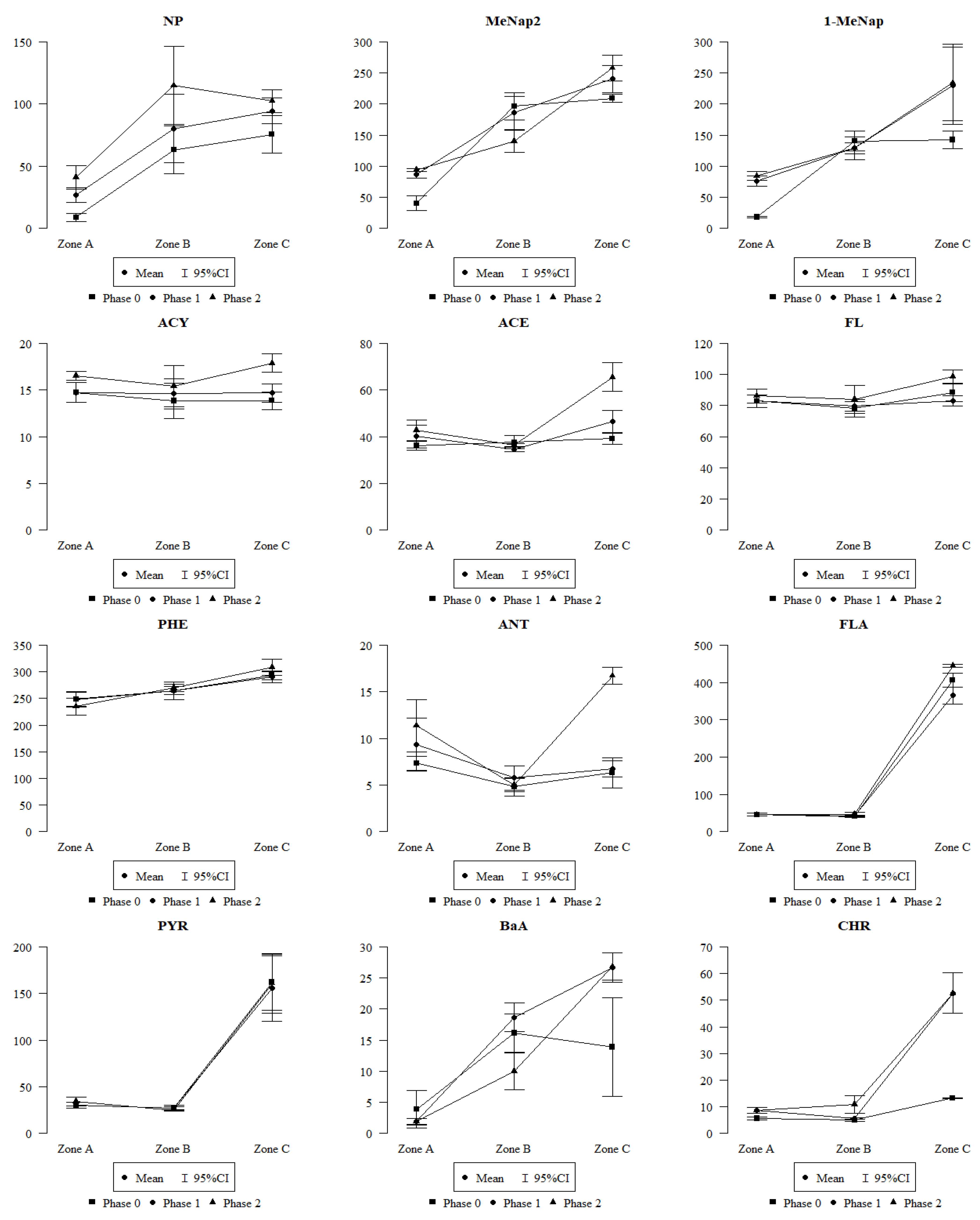
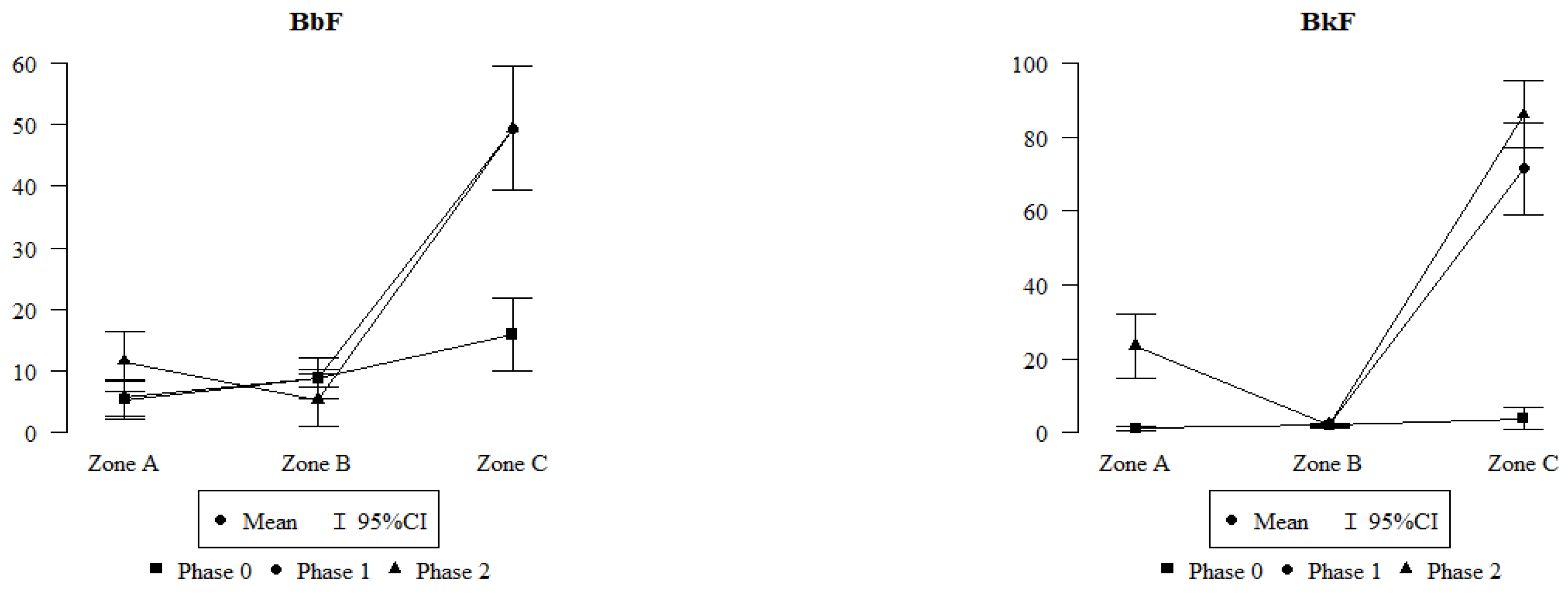
2.2. Comparison of Polycyclic Aromatic Hydrocarbons in Leaves Collected in Zones A, B, and C
3. Discussion
4. Materials and Methods
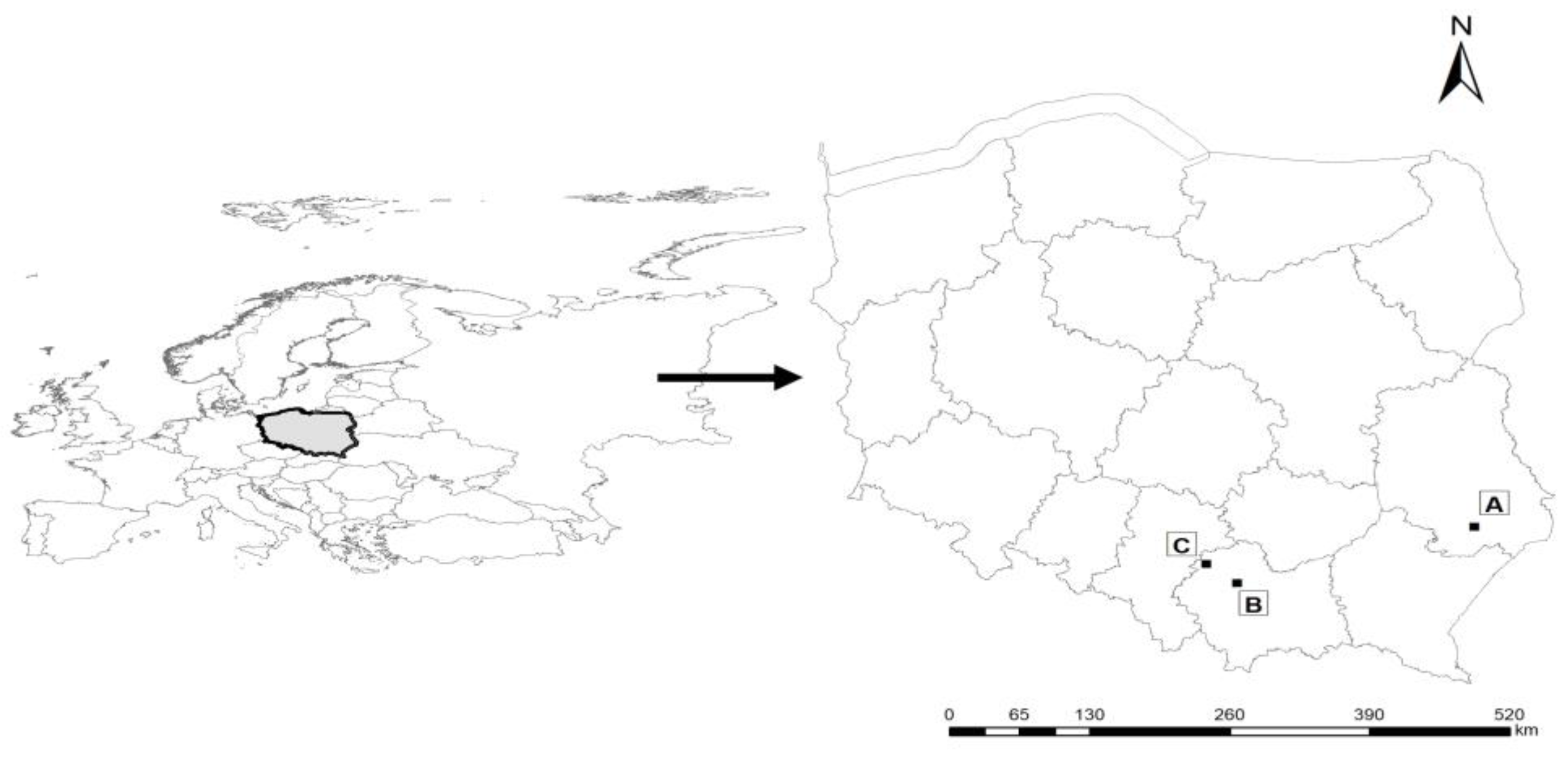
4.1. Sampling
4.2. Determination of the Size of Water Capacity
4.3. Determination of the Degree of Leaf Coverage by Honeydew
4.4. Measurement of the Content of Selected PAHs in Leaf Samples
4.5. Statistical Analysis
5. Conclusions
- Significant differences in the S of small-leaved linden leaves in each of the three locations were recorded, related to the quantity and quality of PAHs in the leaves;
- Differences in the amounts of PAHs in leaves were observed, depending on the emission sources (vehicle traffic and fire), as well as an increase in the amount of PAHs with an increase in the amount of honeydew on the leaves;
- Differences in the ability to retain water were observed with a large amount of honeydew on the surface of the assimilation apparatus.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi, S.M.M.; Attarod, P.; Van Stan, T.G.; Pypker, T.G. The importance of considering rainfall partitioning in afforestation intiatives in semiarid climates: A comparison of common planted tree spacies in Tehran, Iran. Sci. Total Environ. 2016, 568, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.T.; Aubrey, D.P.; Bader, M.Y.; Coenders-Gerrits, M.; Friesen, J.; Gutmann, E.D.; Guillemette, F.; Jiménez-Rodríguez, C.; Keim, R.F.; Klamerus-Iwan, A.; et al. Key questionson the evaporation and transport of intercepted precipitation. In Precipitation Partitioning Byvegetation; Van Stan, J.T., Gutmann, E., Friesen, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 269–280. [Google Scholar]
- Šraj, M.; Zabret, K.; Lebar, K. Temporal response of urban soil water content in relation to the rainfall and throughfall dynamics in the open and below the trees. J. Hydrol. Hydromech. 2022, 71, 210–220. [Google Scholar] [CrossRef]
- Papierowska, E.; Sikorska, D.; Szporak-Wasilewska, S.; Kleniewska, M.; Berezowski, T.; Chormański, J.; Debaene, G.; Szatyłowicz, J. Leaf wettability and plant surface water storage for common wetland species of the Biebrza peatlands (northeast Poland). J. Hydrol. Hydromech. 2023, 71, 169–176. [Google Scholar] [CrossRef]
- Sraj, M.; Brilly, M.; Mikoš, M. Rainfall interception by two deciduous Mediterranean forests of contrasting stature in Slovenia. Agric. For. Meteorol. 2008, 148, 121–134. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; Holder, C.D. The significance of leaf water repellency in ecohydrological research: A review. Ecohydrol 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Dunkerley, D. A Review of the Effects of Throughfall and Stemflow on Soil Properties and Soil Erosion. In Precipitation Partitioning by Vegetation: A Global Synthesis; Van Stan, J.T., Gutmann, E., Friesen, J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 183–213. [Google Scholar]
- Ma, Y.J.; Gao, S.Y.; Li, X.Y. Rainfall canopy partitioning and its influencing factors of riparian shrub in the alpine region. J. Desert Res. 2012, 32, 963–971. [Google Scholar]
- An, J.X.; Gao, G.Y.; Yuan, C.; Pinos, J.; Fu, B.J. Inter-and intra-event rainfall partitioning dynamics of two typical xerophytic shrubs in the Loess Plateau of China. Hydrol. Earth Syst. Sci. 2022, 26, 3885–3900. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1997, 99, 347–360. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytosta-bilisation in waxes, a 3 year Study. Int. J. Phytorem. 2012, 15, 245–256. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Błońska, E. Canopy storage capacity and wettability of leaves and needles: The effect of water temperature changes. J. Hydrol. 2018, 559, 534–540. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Witek, W. Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.). Water 2018, 10, 695. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- De Nicola, F.; Maisto, G.; Prati, M.V.; Alfani, A. Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environ. Pollut. 2008, 153, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Łyszczarz, S.; Lasota, J.; Szuszkiewicz, M.M.; Błońska, E. Soil texture as a key driver of polycyclic aromatic hydrocarbons (PAHs) distribution in forest topsoils. Sci. Rep. 2021, 11, 14708. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Importance of vegetation in removing polycyclic aromatic hydrocarbons from the atmosphere. Nature 1994, 370, 49–51. [Google Scholar] [CrossRef]
- Gałuszko, H.; Koteja, J.; Tworek, K. Bees on Honeydew; Sądecki Bartnik: Nowy Sącz, Poland, 1996; pp. 63–64. [Google Scholar]
- Gałuszka, H.; Tworek, K. Der Einfluss von einigen Witterungsfaktoren auf die Populationsdichte von Cinara pectinatae Nördl, Beekeeping Scientific Notebooks. Pszczel. Zesz. Nauk. 1993, 37, 103–111. [Google Scholar]
- Tyszkiewicz, S.; Obmiński, Z. Breeding and Cultivation of the Forest; PWRiL: Warsaw, Poland, 1963. [Google Scholar]
- Jaworski, A. Breeding Characteristics of Forest Trees; Gutenberg: Kraków, Poland, 1995. [Google Scholar]
- Boratyńska, K.; Dolatowski, J. Systematics and geographical distribution. In Our Forest Trees; Linden, T.M., Białobok, S., Eds.; Popular Science Monographs, PAN: Poznań, Poland, 1991; pp. 21–55. [Google Scholar]
- Nawrot, B.; Dzierżanowski, K.; Gawroński, S.W. Accumulation of particulate matter, PAHs and heavy metals in canopy of small-leaved lime. Environ. Prot. Nat. Resour. 2011, 49, 52–60. [Google Scholar]
- Holoubek, I.; Dušek, L.; Sáňka, M.; Hofman, J.; Čupr, P.; Jarkovský, J.; Zbíral, J.; Klánová, J. Soil burdens of persistent organic pollutants–Their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environ. Pollut. 2009, 157, 3207–3217. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Socha, J.; van Doorn, N.S. Linking heavy metal bioavailability (Cd, Cu, Zn and Pb) in Scots pine needles to soil properties in reclaimed mine areas. Sci. Total Environ. 2014, 470–471, 501–510. [Google Scholar] [CrossRef]
- Matyssek, R.; Wieser, G.; Calfapietra, C.; de Vries, W.; Dizengremel, P.; Ernst, D.; Jolivet, Y.; Mikkelsen, T.N.; Mohren, G.M.J.; Le Thiec, D.; et al. Forests under climate change and air pollution: Gaps in understanding and future directions for research. Environ. Pollut. 2012, 160, 57–65. [Google Scholar] [CrossRef]
- Kosiba, P. Variability of morphometric leaf traits in small-leaved linden (Tilia cordata Mill.) under the influence of air pollution. Acta Soc. Bot. Pol. 2008, 77, 125–137. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of partic-ulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytorem 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Juda-Rezler, K. Wastewater Discharge to the Environment; Publishing House of the Warsaw University of Technology: Warsaw, Poland, 2000. [Google Scholar]
- Schreuder, M.; Van Hove, L.W.A.; Brewer, C.A. Ozone Exposure Affects Leaf Wettability and Tree Water Balance. N. Phytol. 2001, 152, 443–454. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Błońska, E.; Lasota, J.; Waligórski, P.; Kalandyk, A. Seasonal variability of leaf water capacity and wettability under the influence of pollution in different city zones. Atmos. Pollut. Res. 2018, 9, 455–463. [Google Scholar] [CrossRef]
- Juda, J.; Chruściel, S.; Jaworski, W.; Jagusiewicz, A.; Nowicki, M.; Rutkowska, K.; Warchałowski, A.; Żak, W.; Bąkowski, K. Guidelines for the Calculation of Atmospheric Air Pollution; Ministry of Administration, Land Management and Environmental Protection; Department of Environmental Protection: Warsaw, Poland, 1977; Volume 59, pp. 1–19. [Google Scholar]
- WHO. Health Aspects of Air Pollution: Results from the WHO Projects, Systematic Review of Health Aspects of Air Pollution in Europe; WHO: Bonn, Germany, 2004; Volume 126. [Google Scholar]
- Laden, F.; Neas, L.M.; Dockery, D.W.; Schwatz, J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Persp. 2000, 108, 941–947. [Google Scholar] [CrossRef]
- Ghio, A.; Devlin, R. Inflammatory lung injury after bronchial instillation of air pollution particles. Am. J. Respir. Crit. Care Med. 2001, 164, 704–708. [Google Scholar] [CrossRef]
- Caricchia, A.M.; Chiavarini, S.; Pezza, M. Polycyclic aromatic hydrocarbons in the urban atmospheric particulate matter in the city of Naples (Italy). Atmos. Environ. 1999, 33, 3731–3738. [Google Scholar] [CrossRef]
- Goudarzi, G.; Baboli, Z.; Moslemnia, M.; Tobekhak, M.; Tahmasebi Birgani, Y.; Neisi, A.; Ghanemi, K.; Babaei, A.A.; Hashemzadeh, B.; Ahmadi Angali, K.; et al. Assessment of incremental lifetime cancer risks of ambient air PM10-bound PAHs in oil-rich cities of Iran. J. Environ. Health Sci. Eng. 2021, 19, 319–330. [Google Scholar] [CrossRef]
- Yu, L.; Mai, B.; Meng, X.; Bi, X.; Sheng, G.; Fu, J.; Peng, P. Particle-bound polychlorinated dibenzo-p-dioxins and dibenzofurans in the atmosphere of Guangzhou, China. Atmos. Environ. 2006, 40, 96–108. [Google Scholar] [CrossRef]
- Greszta, J.; Gruszka, A.; Kowalkowska, M. Impact of Immission on the Ecosystem; Silesia Publishing House: Katowice, Poland, 2002; pp. 25–28. [Google Scholar]
- Collins, C.D.; Bell, J.N.B. Effect of volatile organic compounds. In Air Pollution and Plant Life; Bell, J.N.B., Treshow, M., Eds.; Scientific and Technical Publishing House: Warsaw, Poland, 2004; pp. 193–205. [Google Scholar]
- Wysocki, C. The city as a specific habitat for vegetation. Sci. Nat. Technol. 2008, 2, 1–10. [Google Scholar]
- Klamerus-Iwan, A.; Gloor, E.; Sadowska-Rociek, A.; Błońska, E.; Lasota, J.; Łagan, S. Linking the contents of hydrophobic PAHs with the canopy water storage capacity of coniferous trees. Environ. Pollut. 2018, 242, 1176–1184. [Google Scholar]
- Kaupp, H.; Blumenstock, M.; McLachlan, M.S. Retention and mobility of atmospheric particle-accociated organic pollutant PCDD/Fs and PAHs on maize leaves. New Phytol. 2000, 148, 473–480. [Google Scholar]
- Jouraeva, V.A.; Johnson, D.L.; Hassett, J.P.; Nowak, D.J. Differences in accumulation of PAHs and metals on the leaves of Tilia × euchlora and Pyrus calleryana. Environ. Pollut. 2002, 120, 331–338. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Gawroński, S.W. Susceptibility of shrubs to accumulation of airborne microdust. Sci. Nat. Technol. 2011, 5, 124. [Google Scholar]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Muskała, P.; Sobik, M.; Błaś, M.; Polkowska, Ż.; Bokwa, A. Pollutant deposition via dew in urban and rural environment, Cracow, Poland. Atmos. Res. 2015, 151, 110–119. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef]
- Popek, R.; Łukowski, A.; Bates, C.; Oleksyn, J. Accumulation of particulate matter, heavy metals, and polycyclic aromatic hydrocarbons on the leaves of Tilia cordata Mill. in five Polish cities with different levels of air pollution. Int. J. Phytoremediation 2017, 19, 1134–1141. [Google Scholar] [CrossRef]
- Anna, S.-R.; Magdalena, S. Levels and risk assessment of polycyclic aromatic hydrocarbons, acrylamide and 5-hydroxymethylfurfural in yerba mate and its infusions. Food Control. 2023, 152, 109860. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 May 2023).


| Variable | Value | Parameter | 95%CI | p | |
|---|---|---|---|---|---|
| Zone | Zone A | ref. | |||
| Zone B | 8.569 | 4.141 | 12.997 | p < 0.001 * | |
| Zone C | 13.358 | 8.93 | 17.786 | p < 0.001 * | |
| Phase | Phase 0 | ref. | |||
| Phase 1 | 0.828 | −3.6 | 5.256 | p = 0.711 | |
| Phase 2 | −1.522 | −5.95 | 2.906 | p = 0.496 | |
| Interactions | Zone B: Phase 1 | 6.139 | −0.124 | 12.402 | p = 0.055 |
| Zone C: Phase 1 | 2.59 | −3.673 | 8.853 | p = 0.413 | |
| Zone B: Phase 2 | 19.231 | 12.968 | 25.494 | p < 0.001 * | |
| Zone C: Phase 2 | 20.749 | 14.486 | 27.012 | p < 0.001 * | |
| Variable | Value | Parameter | 95%CI | p | |
|---|---|---|---|---|---|
| Zone | Zone A | ref. | |||
| Zone B | 351.07 | 274.126 | 428.014 | p < 0.001 * | |
| Zone C | 935.566 | 858.621 | 1012.51 | p < 0.001 * | |
| Phase | Phase 0 | ref. | |||
| Phase 1 | 130.118 | 53.173 | 207.062 | p = 0.001 * | |
| Phase 2 | 187.866 | 110.921 | 264.81 | p < 0.001 * | |
| Interactions | Zone B: Phase 1 | −130.782 | −239.597 | −21.966 | p = 0.019 * |
| Zone C: Phase 1 | 112.588 | 3.773 | 221.404 | p = 0.043 * | |
| Zone B: Phase 2 | −192.443 | −301.259 | −83.628 | p = 0.001 * | |
| Zone C: Phase 2 | 252.02 | 143.205 | 360.836 | p < 0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwika, A.; Klamerus-Iwan, A.; Sadowska-Rociek, A. Variability in Water Capacity of Small-Leaved Linden Associated with Both the Presence of Honeydew and Various Sources of Pollution. Plants 2023, 12, 3443. https://doi.org/10.3390/plants12193443
Kwika A, Klamerus-Iwan A, Sadowska-Rociek A. Variability in Water Capacity of Small-Leaved Linden Associated with Both the Presence of Honeydew and Various Sources of Pollution. Plants. 2023; 12(19):3443. https://doi.org/10.3390/plants12193443
Chicago/Turabian StyleKwika, Agata, Anna Klamerus-Iwan, and Anna Sadowska-Rociek. 2023. "Variability in Water Capacity of Small-Leaved Linden Associated with Both the Presence of Honeydew and Various Sources of Pollution" Plants 12, no. 19: 3443. https://doi.org/10.3390/plants12193443





