Pollen Morphology in Sorbus L. (Rosaceae) and Its Taxonomic Implications
Abstract
:1. Introduction
2. Results
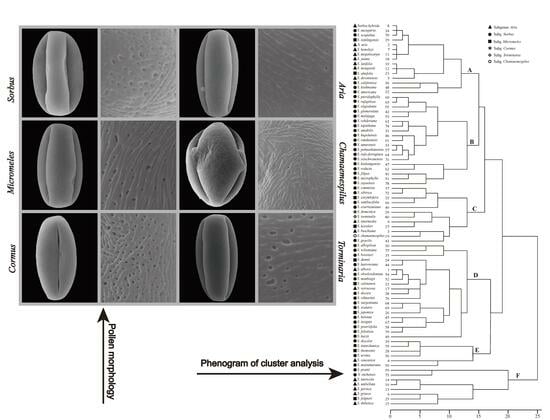
2.1. Pollen Size and Shape
2.2. Pollen Ornamentation
2.3. Cluster Analysis of Sorbus Based on Measured Data
3. Discussion
3.1. Pollen Size and Shape
3.2. Pollen Ornamentation
3.3. The Comparison of Pollen Morphology, Molecular Systematics, and Morphological Classification
3.4. Interspecific Clustering of Different Subgenera Based on Pollen Morphology
4. Materials and Methods
4.1. Sample Collection
4.2. Pollen Morphological Characteristics
4.3. Cluster Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phipps, J.B.; Robertson, K.R.; Smith, P.G.; Rohrer, J.R. A Checklist of the Subfamily Maloideae (Rosaceae). Can. J. Bot. 1990, 68, 2209–2269. [Google Scholar] [CrossRef]
- Sennikov, A.N.; Kurtto, A. A Phylogenetic Checklist of Sorbus s.l. (Rosaceae) in Europe. Memo. Soc. Fauna Flora Fenn. 2017, 93, 1–78. Available online: https://journal.fi/msff/article/view/64741 (accessed on 15 March 2022).
- Lu, L.D.; Spongberg, S.A. Sorbus. In Flora of China; Wu, Z.Y., Peter, H.R., Hong, D., Eds.; Science Press & Missouri Botanical Garden Press: Beijing, China; Saint Louis, MI, USA, 2003; Volume 9, pp. 144–170. [Google Scholar]
- Németh, C.; Papp, N.; Nosková, J.; Höhn, M. Speciation by Triparental Hybridization in Genus Sorbus (Rosaceae). Biol. Futura 2020, 71, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Linnaeus, C. Species Plantarum; Impensis Laurentii Salvii: Holmiae, Sweden, 1753; Volume 2, p. 1200. [Google Scholar]
- Kovanda, M.; Challice, J. The Genus Micromeles Revisited. Folia Geobot. 1981, 16, 181–193. [Google Scholar] [CrossRef]
- Hooker, J.D. Rosaceae. In The Flora of British India; L. Reeve: London, UK, 1879; Volume 2, pp. 307–388. [Google Scholar]
- Lindley, J.X. Observations on the Natural Group of Plants Called Pomaceae. Trans. Linn. Soc. Lond. 1821, 13, 88–106. [Google Scholar] [CrossRef]
- de Candolle, A.; de Candolle, A.P. Rosaceae. In Prodromus Systematis Naturalis Regni Vegetabilis, Sive, Enumeratio Contracta Ordinum Generum Specierumque Plantarum Huc Usque Cognitarium, Juxta Methodi Naturalis, Normas Digesta; Sumptibus Sociorum Treuttel & Würtz: Paris, France, 1825; Volume 2, pp. 525–639. [Google Scholar]
- Focke, W.O. Rosaceae. In Die Natürlichen Pflanzenfamilien Nebst Ihren Gattungen Und Wichtigeren Arten, Insbesondere Den Nutzpflanzen, Unter Mitwirkung Zahlreicher Hervorragender Fachgelehrten Begründet; Engler, A., Krause, K., Pilger, R., Prantl, K., et al., Eds.; Verlag von Wilhelm Engelman: Leipzig, Germany, 1894; Volume 3, pp. 1–61. [Google Scholar]
- Roemer, M.J. Familiarum Naturalium Regni Vegetabilis Synopses Monographicae; Landes-Industrie-Comptoir: Weimar, Germany, 1847; p. 314. [Google Scholar]
- Rehder, A.; Koehne, B.A.E. Rosaceae. In Plantae Wilsonianae: An Enumeration of the Woody Plants Collected in Western China for the Arnold Arboretum of Harvard University During the Years 1907, 1908, and 1910 by E. H. Wilson; Sargent, C.S., Wilson, E.H., Eds.; The University Press: Cambridge, UK, 1913; Volume 2, pp. 434–483. [Google Scholar]
- Wenzig, T. Die Pomaceen. Charaktere Der Gattungen Und Arten. Jahrb.; Notizblatt des Königl. Bot. Gart. Berlin 1883, 2, 287–307. [Google Scholar]
- Hedlund, T. Monographie Der Gattung Sorbus; Kessinger Publishing: Whitefish, MT, USA, 1901; p. 163. [Google Scholar]
- Wang, G.X.; Zhang, M.L. A Molecular Phylogeny of Sorbus (Rosaceae) Based on ITS Sequence. Yuan Yi Xue Bao 2011, 38, 2387–2394. [Google Scholar] [CrossRef]
- Yu, D.J.; Lu, L.D.; Gu, C.Z.; Guan, K.J.; Jiang, W.F. Rosaceae. In Flora of China; Editorial Committee of Flora of China; Chinese Academy of Sciences, Ed.; Science Press: Beijing, China, 1974; Volume 36, pp. 1–404. [Google Scholar]
- McAllister, H.; Mathew, B. The Genus Sorbus: Mountain Ash and Other Rowans; Royal Botanic Gardens: London, UK, 2005; p. 256. [Google Scholar]
- Li, Q.Y.; Guo, W.; Liao, W.B.; Macklin, J.A.; Li, J.H. Generic Limits of Pyrinae: Insights from Nuclear Ribosomal DNA Sequences. Bot. Stud. 2012, 53, 151–164. Available online: https://www.researchgate.net/publication/254607803 (accessed on 20 February 2022).
- Zheng, D.M.; Zhang, M.L. A Cladistic and Phenetic Analysis of the Infrageneric Relationships of Sorbus s. l. (Maloideae, Rosaceae) Based on the Morphological Characters. Yuan Yi Xue Bao 2007, 34, 723–728. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Donoghue, M.J. Expanded Phylogenetic and Dating Analyses of the Apples and Their Relatives (Pyreae, Rosaceae). Mol. Phylogenet Evol. 2012, 63, 230–243. [Google Scholar] [CrossRef]
- Zhang, S.D.; Jin, J.J.; Chen, S.Y.; Chase, M.W.; Soltis, D.E.; Li, H.T.; Yang, J.B.; Li, D.Z.; Yi, T.S. Diversification of Rosaceae since the Late Cretaceous Based on Plastid Phylogenomics. New Phytol. 2017, 214, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, C.H.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae Fruit Types Based on Nuclear Phylogeny in the Context of Geological Times and Genome Duplication. Mol. Biol. Evol. 2017, 34, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Shi, S.; Li, J.L.; Yu, J.; Wang, L.; Yang, X.Y.; Guo, L.; Zhou, S.L. Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription. BioMed. Res. Int. 2018, 2018, 7627191. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, E.T. Sorbus, L. In Western Asia and the Himalayas; Izdatel’stvo AN ArmSSR: Erevan, Armenia, 1978; p. 329. [Google Scholar]
- Rushforth, K. The Whitebeam Problem, and a Solution. Phytologia 2018, 100, 222–247. Available online: https://www.phytologia.org/uploads/2/3/4/2/23422706/100_4_222-247rushforthmalinae__rosaceae_12-5-18.pdf (accessed on 19 May 2022).
- Lechowicz, K.; Wrońska-Pilarek, D.; Bocianowski, J.; Maliński, T. Pollen Morphology of Polish Species from the Genus Rubus L. (Rosaceae) and Its Systematic Importance. PLoS ONE 2020, 15, e0237625. [Google Scholar] [CrossRef]
- Lu, L.; Fritsch, P.W.; Wang, H.; Li, H.T.; Li, D.Z.; Chen, J.Q. Pollen Morphology of Gaultheria L. And Related Genera of Subfamily Vaccinioideae: Taxonomic and Evolutionary Significance. Rev. Palaeobot. Palyno. 2009, 154, 106–123. [Google Scholar] [CrossRef]
- Wan, C.Y.; Liu, J.X. The Morphology and Systematics of the Pollen of Stellaria. Palynology 2017, 41, 533–546. [Google Scholar] [CrossRef]
- Xiong, X.H.; Zhou, X.M.; Li, M.; Xu, B.; Deng, H.N.; Yu, Q.; Gao, X.F. Pollen Morphology in Rubus (Rosaceae) and Its Taxonomic Implications. Plant Syst. Evol. 2019, 305, 705–716. [Google Scholar] [CrossRef]
- Song, J.H.; Moon, H.K.; Hong, S.P. Pollen Morphology of the Tribe Sorbarieae (Rosaceae). Plant Syst. Evol. 2016, 302, 853–869. [Google Scholar] [CrossRef]
- Sarwar, A.K.M.G.; Hoshino, Y.; Araki, H. Pollen Morphology and Its Taxonomic Significance in the Genus Bomarea Mirb. (Alstroemeriaceae)—I. Subgenera Baccata, Sphaerine, and Wichuraea. Acta Bot. Bras. 2015, 29, 425–432. [Google Scholar] [CrossRef]
- Banks, H.I.; Forest, F.; Lewis, G. Evolution and Diversity of Pollen Morphology in Tribe Cercideae (Leguminosae). Taxon 2014, 63, 299–314. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C.; Smith, B.M. Pollen Morphology of the Rosaceae of Western Canada. Grana 1988, 27, 95–113. [Google Scholar] [CrossRef]
- Liao, M.; Ullah, F.; Deng, H.N.; Zhang, J.Y.; Xu, B.; Gao, X.F. Pollen Morphology of the Genus Sophora (Fabaceae) and Its Taxonomic Implications. Microsc. Res. Techniq. 2022, 85, 1723–1741. [Google Scholar] [CrossRef]
- Yang, L.H.; Wu, Y.H.; Pei, X.; Guan, X.L.; Zheng, J. Pollen Morphological Characteristics and Cluster Analysis on Some Species in Sorbus Linn. J. Plant Resour. Eviron. 2019, 28, 84–90. [Google Scholar] [CrossRef]
- Bednorz, L.; Maciejewska, I.; Wronska-Pilarek, D.; Fujiki, T. Pollen Morphology of the Polish Species of the Genus Sorbus L. Acta Soc. Bot. Pol. 2005, 74, 315–322. [Google Scholar] [CrossRef]
- Bednorz, L.; Fujiki, T. Pollen Morphology of Some European Sorbus Species. Rocz. AR. Pozn. 2003, 6, 3–7. Available online: https://www.researchgate.net/publication/238084984 (accessed on 13 September 2023).
- Hebda, R.J.; Chinnappa, C.C. Studies on Pollen Morphology of Rosaceae. Acta Bot. Gallica 1994, 141, 183–193. [Google Scholar] [CrossRef]
- Jing, H.J. Taxonomic Revision of Sorbus L. (Rosaceae). Ph.D. Thesis, Sun Yat sen University, Guangzhou, China, 2015. [Google Scholar]
- Eide, F. Key for Northwest European Rosaceae Pollen. Grana 1981, 20, 101–118. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Sun, J.; Yu, J.; Zhou, S. Phylogeny and Classification of Prunus Sensu Lato (Rosaceae). J. Integr. Plant Biol. 2013, 55, 1069–1079. [Google Scholar] [CrossRef]
- Kovanda, M. Flower and Fruit Morphology of Sorbus in Correlation to the Taxonomy of the Genus. Preslia 1961, 33, 1–16. [Google Scholar]
- Li, M.; Toma, T.; Gao, Y.D.; Xu, B.; Zhu, Z.M.; Ju, W.B.; Gao, X.F. Molecular Phylogenetics and Historical Biogeography of Sorbus Sensu Stricto (Rosaceae). Mol. Phylogenet. Evol. 2017, 111, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.R. Leaf Characters of Sorbus Section Sorbus from China and Their Taxonomic Significance. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2019. [Google Scholar]
- Challice, J.; Kovanda, M. Flavonoids as Markers of Taxonomic Relationships in the Genus Sorbus in Europe. Preslia 1978, 50, 305–320. Available online: https://www.preslia.cz/archive/Preslia_50_1978_305-320.pdf (accessed on 22 February 2023).
- Persoon, C.H. Synopsis Plantarum, Seu Enchiridium Botanicum, Complectens Enumerationem Systematicam Specierum Hucusque Cognitarum; Parisiis Lutetiorum, C.F. Cramerum: Paris, France, 1807; Volume 2, p. 656. [Google Scholar]
- Host, N.T. Flora Austriaca; Arkose Press: Viennae, Austria, 1831; Volume 2, pp. 7–17. [Google Scholar]
- Robertson, K.R.; Phipps, J.B.; Rohrer, J.R.; Smith, P.G. A Synopsis of Genera in Maloideae (Rosaceae). Syst. Bot. 1991, 16, 376–394. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih Image to Imagej: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Erdtman, G. Handbook of Palynology-An Introduction to the Study of Pollen Grains and Spores; Munksgaard: Copenhagen, Denmark, 1969; p. 496. [Google Scholar]
- Wang, F.S.; Chien, N.S.; Zhang, Y.L.; Yang, H.Q. Pollen Flora of China; Science Press: Beijing, China, 1995; pp. 3–10. [Google Scholar]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]








| Characters | Subgenus (Number of Species) | ||||||
|---|---|---|---|---|---|---|---|
| Aira | Chamaemespilus | Cormus | Micromeles | Sorbus | Torminaria | Total | |
| Ornamentation Type I | 10 | 0 | 0 | 2 | 17 | 0 | 29 |
| Ornamentation Type II | 5 | 0 | 0 | 2 | 18 | 0 | 25 |
| Ornamentation Type III | 3 | 1 | 0 | 4 | 4 | 0 | 12 |
| Ornamentation Type IV | 0 | 0 | 0 | 1 | 6 | 0 | 7 |
| Ornamentation Type V | 0 | 0 | 1 | 0 | 5 | 1 | 7 |
| Suboblate | 3 | 0 | 0 | 1 | 3 | 0 | 7 |
| Spheroidal | 3 | 0 | 0 | 0 | 10 | 0 | 13 |
| Subprolate | 1 | 1 | 0 | 2 | 7 | 0 | 11 |
| Prolate | 8 | 0 | 1 | 6 | 25 | 1 | 41 |
| Perprolate | 3 | 0 | 0 | 0 | 5 | 0 | 8 |
| Hole density (0) | 5 | 0 | 0 | 3 | 24 | 0 | 32 |
| Hole density (0.18–2.81) | 12 | 0 | 0 | 5 | 25 | 1 | 43 |
| Hole density (3.74–5.70) | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| Characters | Type of Traits | Code |
|---|---|---|
| Length polar axis (P) | Quantitative | μm |
| Length of equatorial axis (E) | Quantitative | μm |
| Length of colpi (C) | Quantitative | μm |
| The ratio of colpus length to Polar axis length (C/P) | Quantitative | ratio |
| Pollen shape (P/E) | Qualitative | suboblate = 1; spheroidal = 2; subprolate = 3; prolate = 4; perprolate = 5 |
| Pollen ornamentation | Qualitative | striate-perforate = 1; striate = 2; cerebroid-perforate = 3; cerebroid = 4; foveolate = 5 |
| Hole density | Qualitative | absent = 0; 0.18–2.81/μm2 = 1; 3.74–5.70/μm2 = 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tian, C.-F.; Idrees, M.; Pathak, M.; Xiong, X.-H.; Gao, X.-F.; Wang, X.-R. Pollen Morphology in Sorbus L. (Rosaceae) and Its Taxonomic Implications. Plants 2023, 12, 3318. https://doi.org/10.3390/plants12183318
Li M, Tian C-F, Idrees M, Pathak M, Xiong X-H, Gao X-F, Wang X-R. Pollen Morphology in Sorbus L. (Rosaceae) and Its Taxonomic Implications. Plants. 2023; 12(18):3318. https://doi.org/10.3390/plants12183318
Chicago/Turabian StyleLi, Meng, Chang-Fen Tian, Muhammad Idrees, Mitra Pathak, Xian-Hua Xiong, Xin-Fen Gao, and Xian-Rong Wang. 2023. "Pollen Morphology in Sorbus L. (Rosaceae) and Its Taxonomic Implications" Plants 12, no. 18: 3318. https://doi.org/10.3390/plants12183318