Genome-Wide Identification and Expression Analysis of MTP (Metal Ion Transport Proteins) Genes in the Common Bean
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of MTP Genes in the Common Bean
2.2. Phylogenetic Analysis of PvMTP Gene Families in the Common Bean
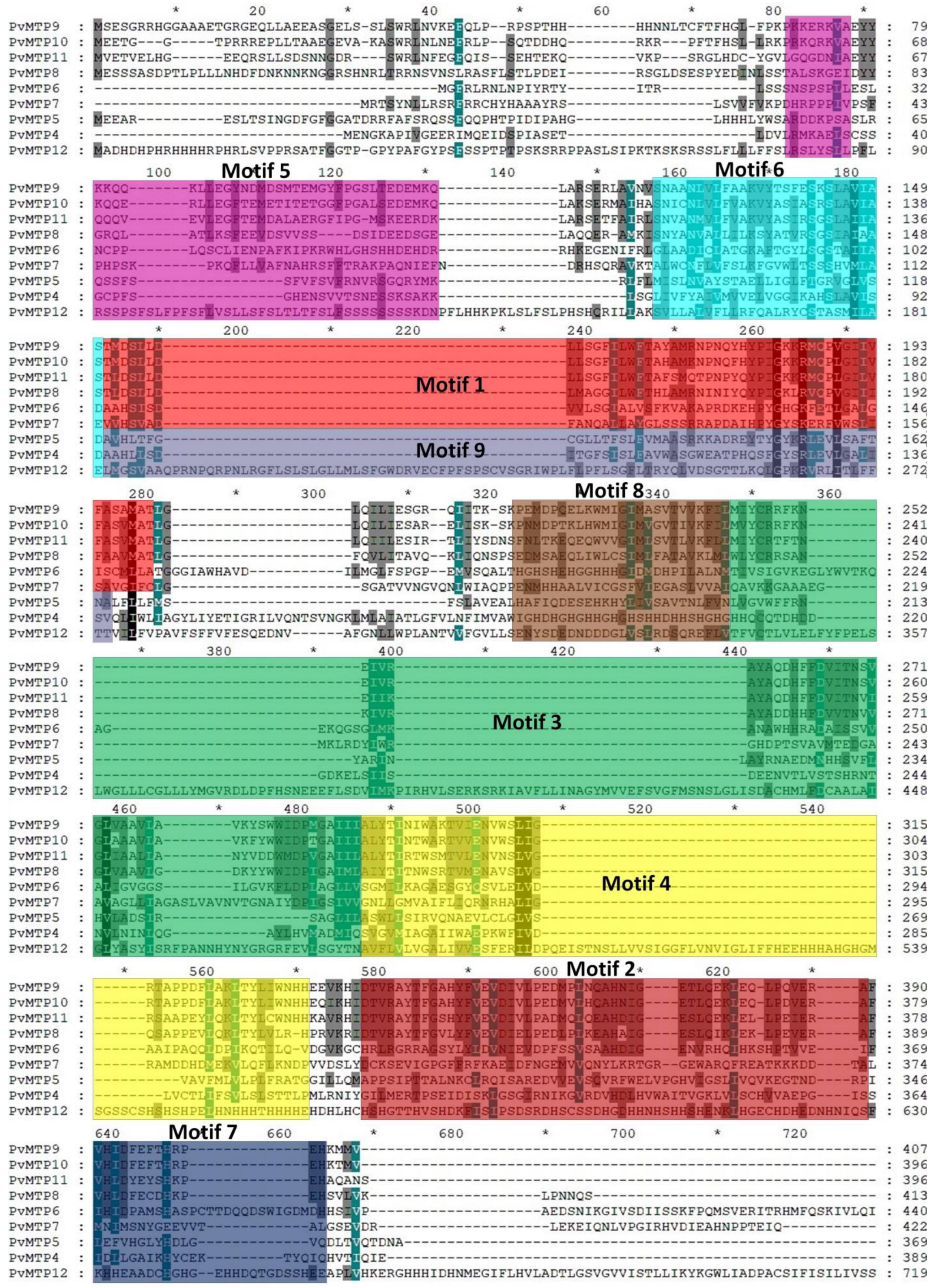
2.3. Motifs and Exon/Intron Organization
2.4. Promoter Region Cis Motif Analysis of PvMTP Genes
2.5. Protein–Protein Interaction Analysis among the PvMTP Family Members
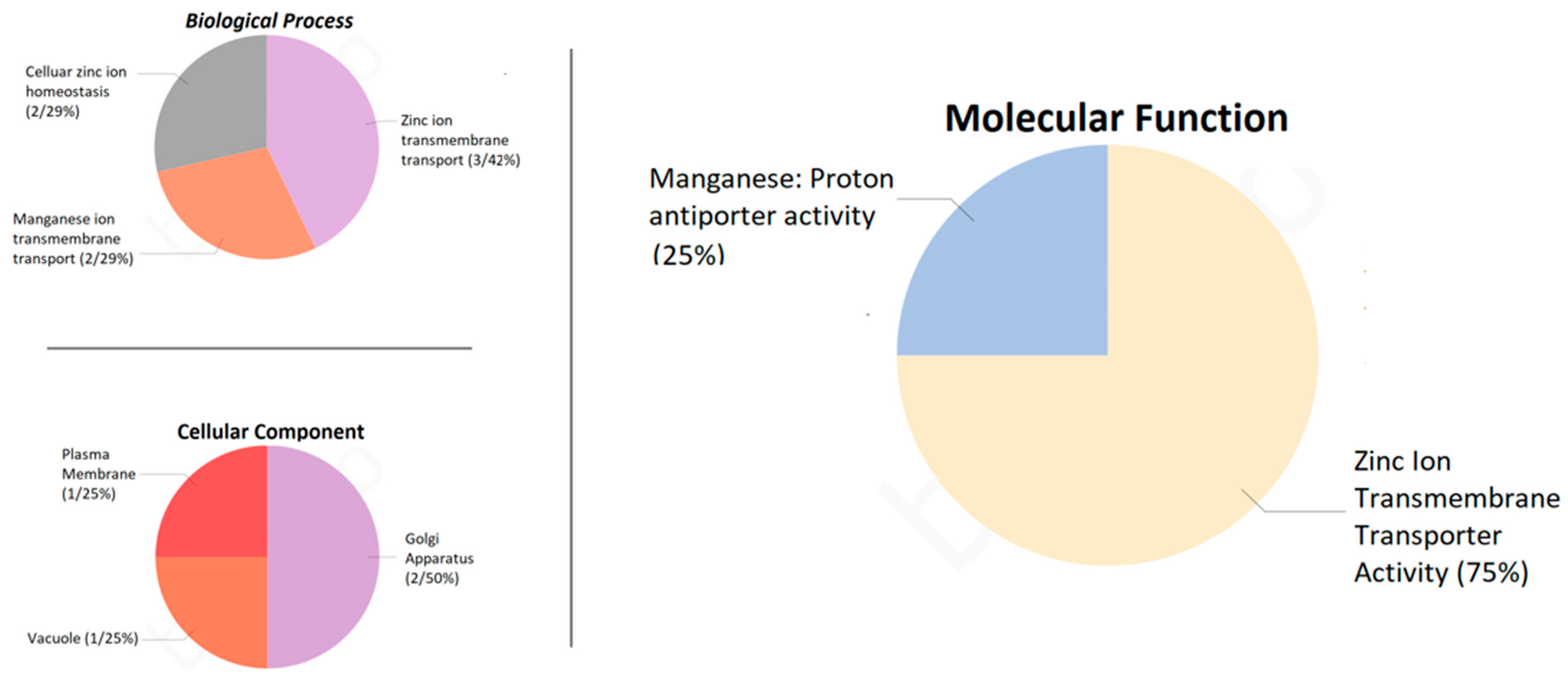
2.6. Subcellular Localizations and Gene Ontology of the PvMTP Gene Family
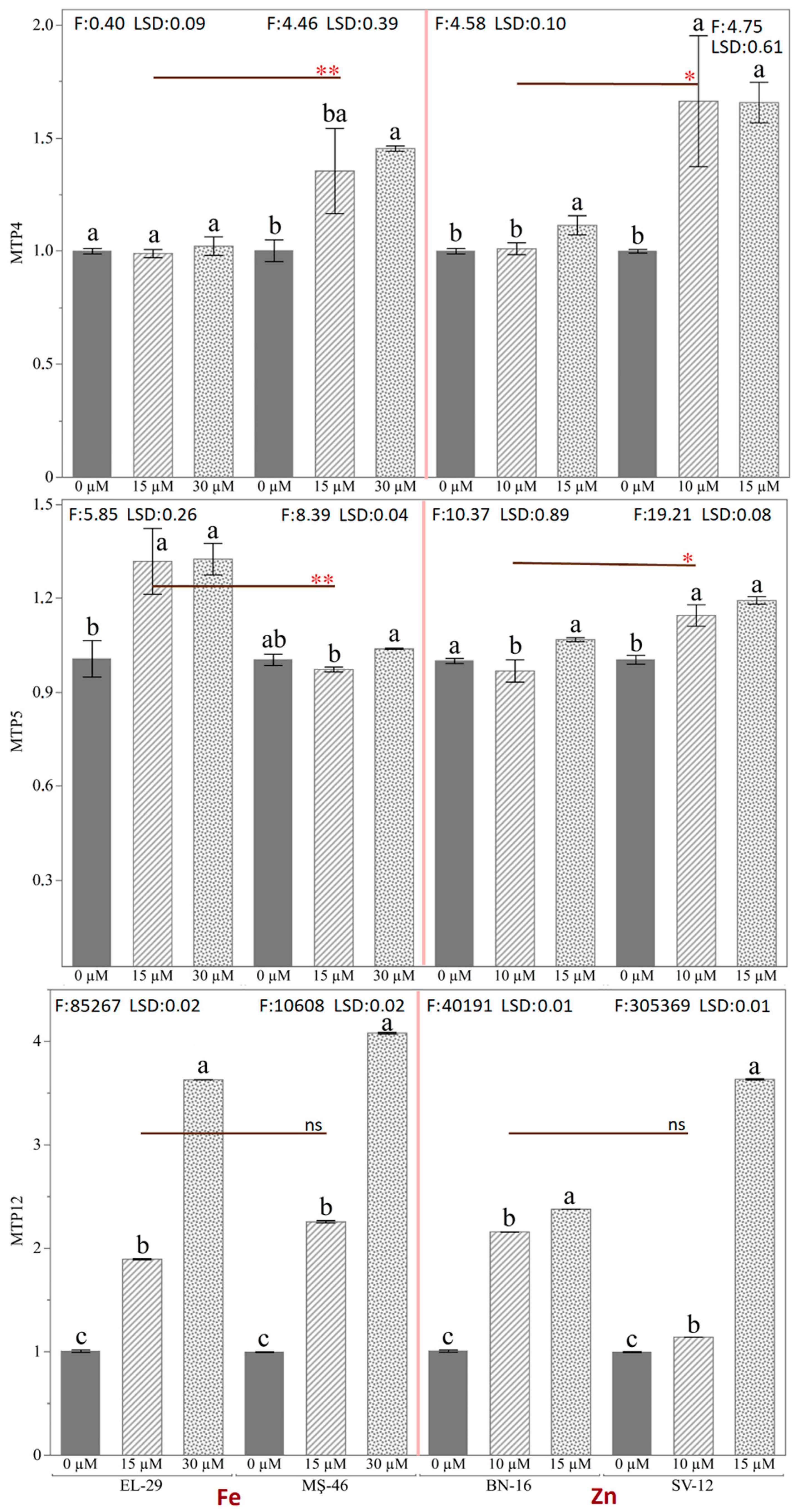
2.7. Effect of Zn and Fe Application on PvMTP4, PvMTP5, and PvMTP12 Genes in Common Bean Genotypes
2.8. Effect of Fe and Zn Application on Fe and Zn Accumulation in Common Bean Genotypes
3. Materials and Methods
3.1. Plant Material
3.2. Identification of MTP Genes in Common Beans
3.3. Chromosome Localization and Phylogenetic Analysis
3.4. Identification of Exon–Intron Structures and Protein Motifs
3.5. Promoter Region Cis Motif Analysis, Subcellular Localization, and Protein–Protein Interactions
3.6. Primer Design and qRT PCR Analysis
3.7. RNA Extraction, DNAse Treatment, and cDNA Synthesis
3.8. Plant Growth, Fe and Zn Applications
3.9. Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, M.; Imran, M.Z.H.; Alam, M.; Rahman, M. Effect of boiling and roasting on physicochemical and antioxidant properties of dark red kidney bean (Phaseolus vulgaris). Food Res. 2021, 5, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef]
- Kulaz, H.; Najafi, S.; Tuncturk, M.; Tuncturk, R.; Yilmaz, H. Chromosome analysis of some Phaseolus vulgaris L. genotypes in Turkey. Bangladesh J. Bot. 2022, 51, 281–288. [Google Scholar] [CrossRef]
- Ha, J.; Lee, S.H. Updates on Legume Genome Sequencing. In Legume Genomics: Methods and Protocols; Humana: Louisville, KY, USA, 2020; pp. 1–18. [Google Scholar]
- Nadeem, M.A.; Yeken, M.Z.; Shahid, M.Q.; Habyarimana, E.; Yılmaz, H.; Alsaleh, A.; Hatipoğlu, R.; Çilesiz, Y.; Khawar, K.M.; Baloch, F.S.; et al. Common bean as a potential crop for future food security: An overview of past, current and future contributions in genomics, transcriptomics, transgenics and proteomics. Biotechnol. Biotechnol. Equip. 2021, 35, 759–787. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low-and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Izquierdo, P.; Astudillo, C.; Grusak, M.A. A legume biofortification quandary: Variability and genetic control of seed coat micronutrient accumulation in common beans. Front. Plant Sci. 2013, 4, 275. [Google Scholar] [CrossRef]
- FAO. 2022. Available online: https://www.fao.org/faostat/en/. (accessed on 18 May 2023).
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA A Cancer J. Clin. 2012, 62, 30–67. [Google Scholar]
- Ikerd, J. The Economic Pamphleteer: Can we afford good food? J. Agric. Food Syst. Community Dev. 2022, 11, 5–8. [Google Scholar] [CrossRef]
- Burchi, F.; Fanzo, J.; Frison, E. The role of food and nutrition system approaches in tackling hidden hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron deficiency without anaemia: A diagnosis that matters. Clin. Med. 2021, 21, 107. [Google Scholar] [CrossRef]
- Belay, A.; Gashu, D.; Joy, E.J.; Lark, R.M.; Chagumaira, C.; Likoswe, B.H.; Zerfu, D.; Ander, E.L.; Young, S.D.; Broadley, M.R.; et al. Zinc deficiency is highly prevalent and spatially dependent over short distances in Ethiopia. Sci. Rep. 2021, 11, 6510. [Google Scholar] [CrossRef] [PubMed]
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A.; Williams, L.E.; Fett, J.P. Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front. Plant Sci. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Ricachenevsky, F.K.; de Abreu Waldow, V.; Fett, J.P. Iron biofortification in rice: It’s a long way to the top. Plant Sci. 2012, 190, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Sahrawat, K.L.; Rai, K.N.; Blair, M.W.; Andersson, M.S.; Pfeiffer, W. 3 Nutritionally Enhanced Staple Food Crops. Plant Breed. Rev. 2012, 36, 169. [Google Scholar]
- Menguer, P.K.; Farthing, E.; Peaston, K.A.; Ricachenevsky, F.K.; Fett, J.P.; Williams, L.E. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 2013, 64, 2871–2883. [Google Scholar] [CrossRef] [PubMed]
- Gustin, J.L.; Loureiro, M.E.; Kim, D.; Na, G.; Tikhonova, M.; Salt, D.E. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 2009, 57, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 2007, 8, 107. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, B. Identification of a rice metal tolerance protein OsMTP11 as a manganese transporter. PLoS ONE 2017, 12, e0174987. [Google Scholar] [CrossRef]
- Haque, A.M.; Rahman, M.A.; Das, U.; Rahman, M.M.; Elseehy, M.M.; El-Shehawi, A.M.; Parvez, M.S.; Kabir, A.H. Changes in physiological responses and MTP (metal tolerance protein) transcripts in soybean (Glycine max) exposed to differential iron availability. Plant Physiol. Biochem. 2022, 179, 1–9. [Google Scholar] [CrossRef]
- Kobae, Y.; Uemura, T.; Sato, M.H.; Ohnishi, M.; Mimura, T.; Nakagawa, T.; Maeshima, M. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 2004, 45, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Arrivault, S.; Senger, T.; Krämer, U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 2006, 46, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Gruber, B.D.; Pittman, J.K.; White, R.G.; Leung, H.; Miao, Y.; Jiang, L.; Ryan, P.R.; Richardson, A.E. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007, 51, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, S.; Liu, B.; Zhang, M.; Wu, K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 2012, 31, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, R.; Filiz, E.; Eroglu, S. Genome-wide exploration of metal tolerance protein (MTP) genes in common wheat (Triticum aestivum): Insights into metal homeostasis and biofortification. BioMetals 2017, 30, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. MTP1 mops up excess zinc in Arabidopsis cells. Trends Plant Sci. 2005, 10, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Dräger, D.B.; Desbrosses-Fonrouge, A.G.; Krach, C.; Chardonnens, A.N.; Meyer, R.C.; Saumitou-Laprade, P.; Krämer, U. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J. 2004, 39, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, X.; Li, D.; Ma, L.; Dong, J.; Wang, T. Identification and characterization of MtMTP1, a Zn transporter of CDF family, in the Medicago truncatula. Plant Physiol. Biochem. 2009, 47, 1089–1094. [Google Scholar] [CrossRef]
- Li, J.; Dong, R.; Jia, Y.; Huang, J.; Zou, X.; An, N.; Song, J.; Chen, Z. Characterization of metal tolerance proteins and functional analysis of GmMTP8. 1 involved in manganese tolerance in soybean. Front. Plant Sci. 2021, 12, 683813. [Google Scholar] [CrossRef]
- Papierniak-Wygladala, A.; Kozak, K.; Barabasz, A.; Palusińska, M.; Całka, M.; Maślińska, K.; Antosiewicz, D.M. Identification and characterization of a tobacco metal tolerance protein, NtMTP2. Metallomics 2020, 12, 2049–2064. [Google Scholar] [CrossRef]
- Gustin, J.L.; Zanis, M.J.; Salt, D.E. Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol. Biol. 2011, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Elbaiomy, R.G.; Elrys, A.S.; Wang, Y.; Zhu, Y.; Huang, Q.; Yan, K.; Zhao, X.; Abbas, M.; Li, J.; et al. Genome-wide identification and expression analysis of metal tolerance protein gene family in Medicago truncatula under a broad range of heavy metal stress. Front. Genet. 2021, 12, 713224. [Google Scholar] [CrossRef] [PubMed]
- Ram, H.; Kaur, A.; Gandass, N.; Singh, S.; Deshmukh, R.; Sonah, H.; Sharma, T.R. Molecular characterization and expression dynamics of MTP genes under various spatio-temporal stages and metal stress conditions in rice. PLoS ONE 2019, 14, e0217360. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Z.; Tong, Y.H.; Zhou, X.; Ling, L.L.; Chun, C.P.; Cao, L.; Zeng, M.; Peng, L.Z. Genome-wide identification of sweet orange (Citrus sinensis) metal tolerance proteins and analysis of their expression patterns under zinc, manganese, copper, and cadmium toxicity. Gene 2017, 629, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, L.; Chen, J.; Lu, Y.; Qin, Y.; Yan, Y.; Liu, F.; Tan, J. Genome-wide investigation on metal tolerance protein (MTP) genes in leguminous plants: Glycine max, Medicago truncatula, and Lotus japonicus. Acta Physiol. Plant. 2023, 45, 18. [Google Scholar] [CrossRef]
- Ravel, C.; Fiquet, S.; Boudet, J.; Dardevet, M.; Vincent, J.; Merlino, M.; Michard, R.; Martre, P. Conserved cis-regulatory modules in promoters of genes encoding wheat high-molecular-weight glutenin subunits. Front. Plant Sci. 2014, 5, 621. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, Z.; Abedi, A.; Kordrostami, M.; Burritt, D.J.; Hossain, M.A. Genome-wide identification and characterization of the metal tolerance protein (MTP) family in grape (Vitis vinifera L.). 3 Biotech. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Tang, Y.; Wang, D.; Chen, X.; Yao, Y.; Guo, Y. Genome-wide identification, comprehensive gene feature, evolution, and expression analysis of plant metal tolerance proteins in tobacco under heavy metal toxicity. Front. Genet. 2019, 10, 345. [Google Scholar] [CrossRef]
- Struk, S.; Jacobs, A.; Sánchez Martín-Fontecha, E.; Gevaert, K.; Cubas, P.; Goormachtig, S. Exploring the protein–protein interaction landscape in plants. Plant Cell Environ. 2019, 42, 387–409. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genom. 2008, 2008, 1–12. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Meier, B.; von Wirén, N.; Peiter, E. The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Peiter, E.; Montanini, B.; Gobert, A.; Pedas, P.; Husted, S.; Maathuis, F.J.; Blaudez, D.; Chalot, M.; Sanders, D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Craddock, G.R.; Hammar, H.E. Factors Affecting Plant Growth in High-Zinc Medium: I. Influence of Iron on Growth of Flax at Various Zinc Levels 1. Agron. J. 1969, 61, 562–565. [Google Scholar] [CrossRef]
- Brar, M.S.; Sekhon, G.S. Interaction of zinc with other micronutrient cations, 2: Effect of iron on zinc65 absorption by rice seedlings and its translocation within the plants. Plant Soil 1976, 45, 145–150. [Google Scholar] [CrossRef]
- Briat, J.F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of phosphate starvation response 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Stefan, H.; Hell, R.; Krämer, U. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell. 2012, 24, 724–737. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Grusak, M.A.; Graham, R.; Beebe, S.E. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- Yeken, M.Z.; Akpolat, H.; Karaköy, T.; Çiftçi, V. Assessment of mineral content variations for biofortification of the bean seed. Int. J. Agric. Wildl. Sci. 2018, 4, 261–269. [Google Scholar]
- Hoppler, M.; Egli, I.; Petry, N.; Gille, D.; Zeder, C.; Walczyk, T.; Blair, M.W.; Hurrell, R.F. Iron speciation in beans (Phaseolus vulgaris) biofortified by common breeding. J. Food Sci. 2014, 79, C1629–C1634. [Google Scholar] [CrossRef]
- Pinheiro, C.; Baeta, J.P.; Pereira, A.M.; Domingues, H.; Ricardo, C.P. Diversity of seed mineral composition of Phaseolus vulgaris L. germplasm. J. Food Compos. Anal. 2010, 23, 319–325. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rojas, M.; Yan, X.; Blair, M.W.; Pedraza, F.; Mun˜oz, F.; Tohme, J.; Lynch, J.P. Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Sci. 2006, 46, 413–423. [Google Scholar] [CrossRef]
- Islam, F.M.A.; Basford, K.E.; Redden, R.J.; González, A.V.; Kroonenberg, P.M.; Beebe, S. Genetic variability in cultivated common bean beyond the two major gene pools. Genet. Resour. Crop Evol. 2002, 49, 271–283. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Higgins, D.G.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Nicholas, K.B. Genedoc: A Tool for Editing and Annoting Multiple Sequence Alignments. 1997. Available online: http://wwwpscedu/biomed/genedoc (accessed on 11 December 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35 (Suppl. 2), W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Yeken, M.Z.; Özer, G.; Çiftçi, V. Genome-Wide Identification and Expression Analysis of DGK (Diacylglycerol Kinase) Genes in Common Bean. J. Plant Growth Regul. 2023, 42, 2558–2569. [Google Scholar] [CrossRef]
- De Figueiredo, M.A.; Boldrin, P.F.; Hart, J.J.; de Andrade, M.J.; Guilherme, L.R.; Glahn, R.P.; Li, L. Zinc and selenium accumulation and their effect on iron bioavailability in common bean seeds. Plant Physiol. Biochem. 2017, 111, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Kacar, B. Bitki ve Toprağın Kimyasal Analizleri; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı: Istanbul, Turkey, 1994. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Allaire, J.J.B. R Studio: Integrated Development Environment for R; RStudio. Inc.: Boston, MA, USA, 2012; Volume 770, pp. 165–171. [Google Scholar]






| Gen ID | Phytozome ID | Chr: Location | Protein | ||||
|---|---|---|---|---|---|---|---|
| Domain Family | Length (aa) | kDa | pI | Instability Index | |||
| PvMTP4 | Phvul.011G061200.1 | Chr11: 5461254..5462423 | PF01545 | 389 | 42.47 | 5.94 | 25.85 |
| PvMTP5 | Phvul.001G142750.1 | Chr1: 38584559..38590051 | PF01545 | 369 | 41.35 | 7.85 | 37.63 |
| PvMTP6 | Phvul.007G165166.1 | Chr7: 27868992..27875439 | PF01545-PF16916 | 490 | 53.21 | 6.55 | 45.77 |
| PvMTP7 | Phvul.008G254900.1 | Chr8: 60275955..60280336 | PF01545 | 422 | 46.77 | 8.97 | 35.47 |
| PvMTP8 | Phvul.006G080700.1 | Chr6: 19280258..19283725 | PF01545-PF16916 | 413 | 46.16 | 5.44 | 50.45 |
| PvMTP9 | Phvul.011G178800.1 | Chr11: 48990796..48998055 | PF01545-PF16916 | 407 | 46.47 | 6.77 | 46.31 |
| PvMTP10 | Phvul.005G049300.1 | Chr5: 5742399..5745782 | PF01545-PF16916 | 396 | 45.42 | 8.36 | 41.51 |
| PvMTP11 | Phvul.008G015500.1 | Chr8: 1278329..1281961 | PF01545-PF16916 | 396 | 44.98 | 5.08 | 39.58 |
| PvMTP12 | Phvul.002G176300.1 | Chr2: 33458940..33461381 | PF01545 | 813 | 91.05 | 6.67 | 45.33 |
| Motif | Motif Sequence |
|---|---|
| Motif 1 | TLDSLLDLLSGFILWFTAYAMRNPNQYQYPIGKKRMQPLGIIVFASVMAT |
| Motif 2 | DTVRAYTFGAHYFVEVDIVLPEDMPLQZAHNIGESLQEKLEQLPEVERAF |
| Motif 3 | MIYCRRFKNEIVRAYAQDHFFDVITNVVGLVAAVLAVKYYWWIDPIGAII |
| Motif 4 | ALYTINTWARTVIENVWSLIGRSAPPDFLQKLTYLIWNHHE |
| Motif 5 | RKVAEYYKQQZKLLEGFTEMDSJTETGYFPGALSEDEMKQ |
| Motif 6 | SNAANJVLFVAKVYASIRSGSLAIIAS |
| Motif 7 | VHJDFEFTHKPEH |
| Motif 8 | KPNMDPEQLQWMIGIMLSVTIVKFIL |
| Motif 9 | SDAAHLLFDCAALSISLFAVWASRWPABRHYSYGYGRLEVLSAFTN |
| Motif | Putative Function | PvMTP4 | PvMTP5 | PvMTP6 | PvMTP7 | PvMTP8 | PvMTP9 | PvMTP10 | PvMTP11 | PvMTP12 |
|---|---|---|---|---|---|---|---|---|---|---|
| TATA-box | core promoter element | 15 | 25 | 21 | 14 | 36 | 71 | 10 | 12 | 7 |
| Box III | protein binding site | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| CCAAT-box | MYBHv1 binding site | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| CAAT-box | promoter and enhancer regions | 12 | 16 | 8 | 13 | 6 | 6 | 4 | 7 | 4 |
| AT-rich element | AT-rich (ATBP-1) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| HD-Zip 3 | protein binding site | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| G-box | light responsiveness | 0 | 0 | 1 | 3 | 0 | 1 | 1 | 0 | 0 |
| GT1-motif | light responsiveness | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 3 |
| 3-AF1 binding site | light responsiveness | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| TCCC-motif | light responsiveness | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 6 |
| GATA-motif | light responsiveness | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sp1 | light responsiveness | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| AE-box | light responsiveness | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| I-box | light responsiveness | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| LAMP-element | light responsiveness | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MRE | light responsiveness | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TCT-motif | light responsiveness | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| chs-CMA2a | light responsiveness | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Box 4 | light responsiveness | 0 | 2 | 0 | 1 | 0 | 7 | 0 | 0 | 0 |
| ATCT-motif | light responsiveness | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| chs-CMA1a | light responsiveness | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| ATC-motif | light responsiveness | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| AT1-motif | light responsiveness | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| LTR | low-temperature responsiveness | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| MBS | drought inducibility | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| O2-site | zein metabolism regulation | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| circadian | circadian control | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| ARE | anaerobic induction | 4 | 0 | 3 | 3 | 0 | 2 | 8 | 2 | 1 |
| CAT-box | meristem expression | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 0 |
| TC-rich repeats | defense and stress responsiveness | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 1 |
| TGA-element | auxin-responsiveness | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 |
| AuxRR-core | auxin responsiveness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| CGTCA-motif | MeJA-responsiveness | 0 | 2 | 4 | 1 | 0 | 0 | 3 | 0 | 1 |
| G-Box | MeJA-responsiveness | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| TGACG-motif | MeJA-responsiveness | 0 | 2 | 4 | 1 | 0 | 0 | 3 | 0 | 1 |
| ABRE | abscisic acid responsiveness | 0 | 2 | 2 | 2 | 0 | 0 | 4 | 0 | 0 |
| TCA-element | salicylic acid responsiveness | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| GARE-motif | gibberellin-responsiveness | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Primer | Phytozome ID | Size | Sequence Information |
|---|---|---|---|
| PvMTP4 F. PvMTP4 R. | Phvul.011G061200.1 | 73 bp | 5′-AATCTTCAGGGGGCGTATTTGC-3′ 5′-TGGCTCCAGCAATCATCACTCC-3′ |
| PvMTP5 F. PvMTP5 R. | Phvul.001G142750.1 | 151 bp | 5′- AGGGCAACTGGTGGTATCTTG-3′ 5′-CGATCACATGACCTGGCACTA-3′ |
| PvMTP12 F. PvMTP12 R. | Phvul.002G176300.1 | 113 bp | 5′-TGATCCCGCCTGCTCAATTT-3′ 5′-CATGTTCTTGCACCCTTGGC-3′ |
| Beta Aktin F. Beta Aktin R. | - | - | 5′-TGAGCAAGGAGATTACAGCATTGG-3′ 5′- CATACTCTGCCTTCGCAATCCAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, H.; Özer, G.; Baloch, F.S.; Çiftçi, V.; Chung, Y.S.; Sun, H.-J. Genome-Wide Identification and Expression Analysis of MTP (Metal Ion Transport Proteins) Genes in the Common Bean. Plants 2023, 12, 3218. https://doi.org/10.3390/plants12183218
Yilmaz H, Özer G, Baloch FS, Çiftçi V, Chung YS, Sun H-J. Genome-Wide Identification and Expression Analysis of MTP (Metal Ion Transport Proteins) Genes in the Common Bean. Plants. 2023; 12(18):3218. https://doi.org/10.3390/plants12183218
Chicago/Turabian StyleYilmaz, Hilal, Göksel Özer, Faheem Shehzad Baloch, Vahdettin Çiftçi, Yong Suk Chung, and Hyeon-Jin Sun. 2023. "Genome-Wide Identification and Expression Analysis of MTP (Metal Ion Transport Proteins) Genes in the Common Bean" Plants 12, no. 18: 3218. https://doi.org/10.3390/plants12183218






