Synergism in Two-Component Insecticides with Dillapiole against Fall Armyworm
Abstract
:1. Introduction
2. Results
2.1. Acute Toxicity
2.2. Acute Effects of Binary Mixtures
3. Discussion
3.1. Acute Toxicity
3.2. Acute Effects of Binary Mixtures
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Distillation and Rectification
4.3. Chemicals
4.4. Analyses of the Essential Oil and Distilled Fractions
4.5. Acute Toxicity of Compounds
4.6. Acute Effects of Binary Mixtures with Dillapiole
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 9235:2021 Aromatic Natural Raw Materials—Vocabulary, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2021. [Google Scholar]
- Nollett, L.M.L.; Hathore, H.S. Green Pesticides Handbook: Essential Oils for Pest Control, 1st ed.; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1498759380. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco Cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Gillij, Y.G.; Gleiser, R.M.; Zygadlo, J.A. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour. Technol. 2008, 99, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Appl. Clay Sci. 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Pavela, R. Acute and Synergistic Effects of Some Monoterpenoid Essential Oil Compounds on the House Fly (Musca domestica L.). J. Essent. Oil Bear. Plants 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Fazolin, M.; Monteiro, A.F.M.; Malavazi, F.W.; Estrela, J.L.V. Óleos essenciais como sinergistas de formulações inseticidas. In Inseticidas Botânicos No Brasil: Aplicações, Potencialidades e Perspectivas; Ribeiro, R.P., Vendramim, J.D., Baldin, E.L.L., Eds.; FEALQ: Piracicaba, Brazil, 2023; pp. 557–615. ISBN 978-65-89722-11-3. [Google Scholar]
- Womack, E.D.; Williams, W.P.; Smith, J.S.; Warburton, M.L.; Bhattramakki, D. Mapping Quantitative Trait Loci for Resistance to Fall Armyworm (Lepidoptera: Noctuidae) Leaf-Feeding Damage in Maize Inbred Mp705. J. Econ. Entomol. 2020, 113, 956–963. [Google Scholar] [CrossRef]
- Omuut, G.; Mollel, H.G.; Kanyesigye, D.; Akohoue, F.; Adumo Aropet, S.; Wagaba, H.; Otim, M.H. Genetic analyses and detection of point mutations in the acetylcholinesterase-1 gene associated with organophosphate insecticide resistance in fall armyworm (Spodoptera frugiperda) populations from Uganda. BMC Genom. 2023, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, R.; Martins, E.; Macedo, C.; Queiroz, P.; Praça, L.; Soares, C.M.; Moreira, H.; Grisi, I.; Silva, J.; Soberon, M.; et al. Evidence of Field-Evolved Resistance of Spodoptera frugiperda to Bt Corn Expressing Cry1F in Brazil That Is Still Sensitive to Modified Bt Toxins. PLoS ONE 2015, 10, e0119544. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, A.R.B.; Rodrigues, J.G.; Kanno, R.H.; de Amaral, F.S.A.E.; Malaquias, J.B.; Silva-Brandão, K.L.; Cônsoli, F.L.; Omoto, C. Susceptibility monitoring and comparative gene expression of susceptible and resistant strains of Spodoptera frugiperda to lambda-cyhalothrin and chlorpyrifos. Pest Manag. Sci. 2023, 79, 2206–2219. [Google Scholar] [CrossRef] [PubMed]
- POWO Piper, L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:326982-2 (accessed on 18 June 2023).
- Guimarães, E.F.; Medeiros, E.V.S.S.; Queiroz, G.A.; Piper, L. Available online: https://floradobrasil2020.jbrj.gov.br/FB12738 (accessed on 18 June 2023).
- Andrade, E.H.A.A.; Guimarães, E.F.; Maia, J.G.S. Variabilidade Química em Óleos Essenciais de Espécies de Piper da Amazônia; FEQ-UFPA: Belém, Brazil, 2009; ISBN 9788563005007. [Google Scholar]
- Liu, S.Q. The Activity of Analogs of the Natural Product Dillapiol and Sesamol as Detoxification Enzyme Inhibitors and Insecticide Synergists. Ph.D. Thesis, University of Ottawa, Ottawa, ON, USA, 2015. [Google Scholar]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper aduncum essential oil: A promising insecticide, acaricide and antiparasitic. A review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef]
- Fazolin, M.; Monteiro, A.F.M.; Bizzo, H.R.; Gama, P.E.; Viana, L.D.O.; de Lima, M.É.C. Insecticidal activity of Piper aduncum oil: Variation in dillapiole content and chemical and toxicological stability during storage. Acta Amaz. 2022, 52, 179–188. [Google Scholar] [CrossRef]
- Fazolin, M.; Bizzo, H.R.; Monteiro, A.F.M. Potential use of terpenoids for control of insect pests. In Terpenoids: Recent Advances in Extraction, Biochemistry and Biotechnology; Oliveira, M.S., Souza-Filho, A.P., Eds.; Bentham Books: Sharjah, United Arab Emirates, 2022; pp. 246–278. ISBN 978-1-68108-964-5. [Google Scholar]
- Usseglio, V.L.; Dambolena, J.S.; Zunino, M.P. Can Essential Oils Be a Natural Alternative for the Control of Spodoptera frugiperda? A Review of Toxicity Methods and Their Modes of Action. Plants 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests. Agric. Food Sci. 2001, 10, 243–259. [Google Scholar] [CrossRef]
- Mao, W.; Zangerl, A.R.; Berenbaum, M.R.; Schuler, M.A. Metabolism of myristicin by Depressaria pastinacella CYP6AB3v2 and inhibition by its metabolite. Insect Biochem. Mol. Biol. 2008, 38, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Scotti, M.; Silva, V.; Santos, S.; Cavalcanti, S.; Junior, F. Chemometric Studies on Potential Larvicidal Compounds Against Aedes Aegypti. Med. Chem. 2014, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira, A.C.; Ribeiro-Pinto, L.F.; Paumgartten, J.R. In vitro inhibition of CYP2B1 monooxygenase by β-myrcene and other monoterpenoid compounds. Toxicol. Lett. 1997, 92, 39–46. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lee, B.-H.; Choi, W.-S.; Park, B.-S.; Kim, J.-G.; Campbell, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L.). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.-J.; Kang, J.S.; Kim, G.-H.; Park, I.-K. Insecticidal and Acetylcholine Esterase Inhibition Activity of Apiaceae Plant Essential Oils and Their Constituents against Adults of German Cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef]
- Heshmati Afshar, F.; Maggi, F.; Iannarelli, R.; Cianfaglione, K.; Isman, M.B. Comparative toxicity of Helosciadium nodiflorum essential oils and combinations of their main constituents against the cabbage looper, Trichoplusia ni (Lepidoptera). Ind. Crops Prod. 2017, 98, 46–52. [Google Scholar] [CrossRef]
- Moraes, M.M.; Camara, C.A.G.; Silva, M.M.C. Comparative toxicity of essential oil and blends of selected terpenes of Ocotea species from Pernambuco, Brazil, against Tetranychus urticae Koch. An. Acad. Bras. Cienc. 2017, 89, 1417–1429. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Keane, S.; Ryan, M. Purification, characterisation, and inhibition by monoterpenes of acetylcholinesterase from the waxmoth, Galleria mellonella (L.). Insect Biochem. Mol. Biol. 1999, 29, 1097–1104. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; Pluempanupat, W.; Aungsirisawat, C.; Boonyarit, P.; Goff, G.L.; Bullangpoti, V. Effect of Plant Essential Oils and Their Major Constituents on Cypermethrin Tolerance Associated Detoxification Enzyme Activities in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Waliwitiya, R.; Nicholson, R.A.; Kennedy, C.J.; Lowenberger, C.A. The Synergistic Effects of Insecticidal Essential Oils and Piperonyl Butoxide on Biotransformational Enzyme Activities in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Hematpoor, A.; Liew, S.Y.; Chong, W.L.; Azirun, M.S.; Lee, V.S.; Awang, K. Inhibition and Larvicidal Activity of Phenylpropanoids from Piper sarmentosum on Acetylcholinesterase against Mosquito Vectors and Their Binding Mode of Interaction. PLoS ONE 2016, 11, e0155265. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.V.; Monnerat, R.G.; Borges, M.; Carvalho, R.S. Metodologia de Criação Para Avaliação de Agentes Entomopatogênicos; Embrapa: Brasilia, Brazil, 2001. [Google Scholar]
- Estrela, J.L.V.; Fazolin, M.; Catani, V.; Alécio, M.R.; Lima, M.S. de Toxicidade de óleos essenciais de Piper aduncum e Piper hispidinervum em Sitophilus zeamais. Pesqui. Agropecuária Bras. 2006, 41, 217–222. [Google Scholar] [CrossRef]
- Robertson, L.J.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315373775. [Google Scholar]
- Al-Sarar, A.; Hall, F.R.; Downer, R.A. Impact of spray application methodology on the development of resistance to cypermethrin and spinosad by fall armywormSpodoptera frugiperda (JE Smith). Pest Manag. Sci. 2006, 62, 1023–1031. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: New York, NY, USA, 1971; ISBN 9780521135900. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Enthomology 1925, 18, 265–267. [Google Scholar] [CrossRef]
- B-Bernard, C.; Philogène, B.J.R. Insecticide synergists: Role, importance, and perspectives. J. Toxicol. Environ. Health 1993, 38, 199–223. [Google Scholar] [CrossRef]
- Guedes, R.N.; Picanço, M.C.; Guedes, N.M.P.; Madeira, N.R. Sinergismo do óleo mineral sobre a toxicicidade de inseticidas para Scrobipalpula absoluta (Lepidoptera: Gelechidae). Pesqui. Agropecuária Bras. 1995, 30, 313–318. [Google Scholar]
- Frankenhuyzen, K. van Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Abdelgaleil, S.A.M.; Rabie, R.Y.A. Insecticidal and synergistic effects of Majorana hortensis essential oil and some of its major constituents. Entomol. Exp. Appl. 2009, 131, 225–232. [Google Scholar] [CrossRef]
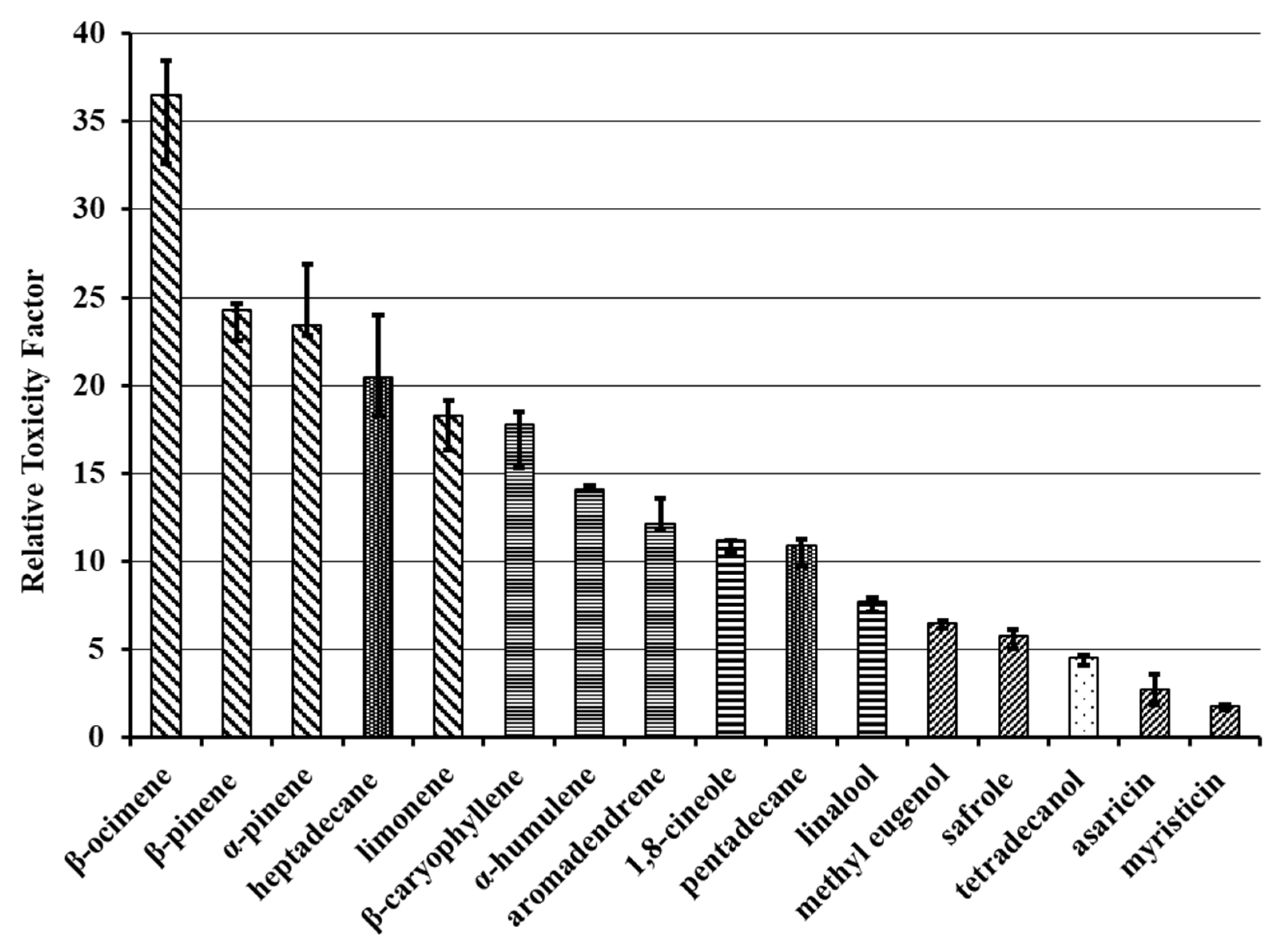
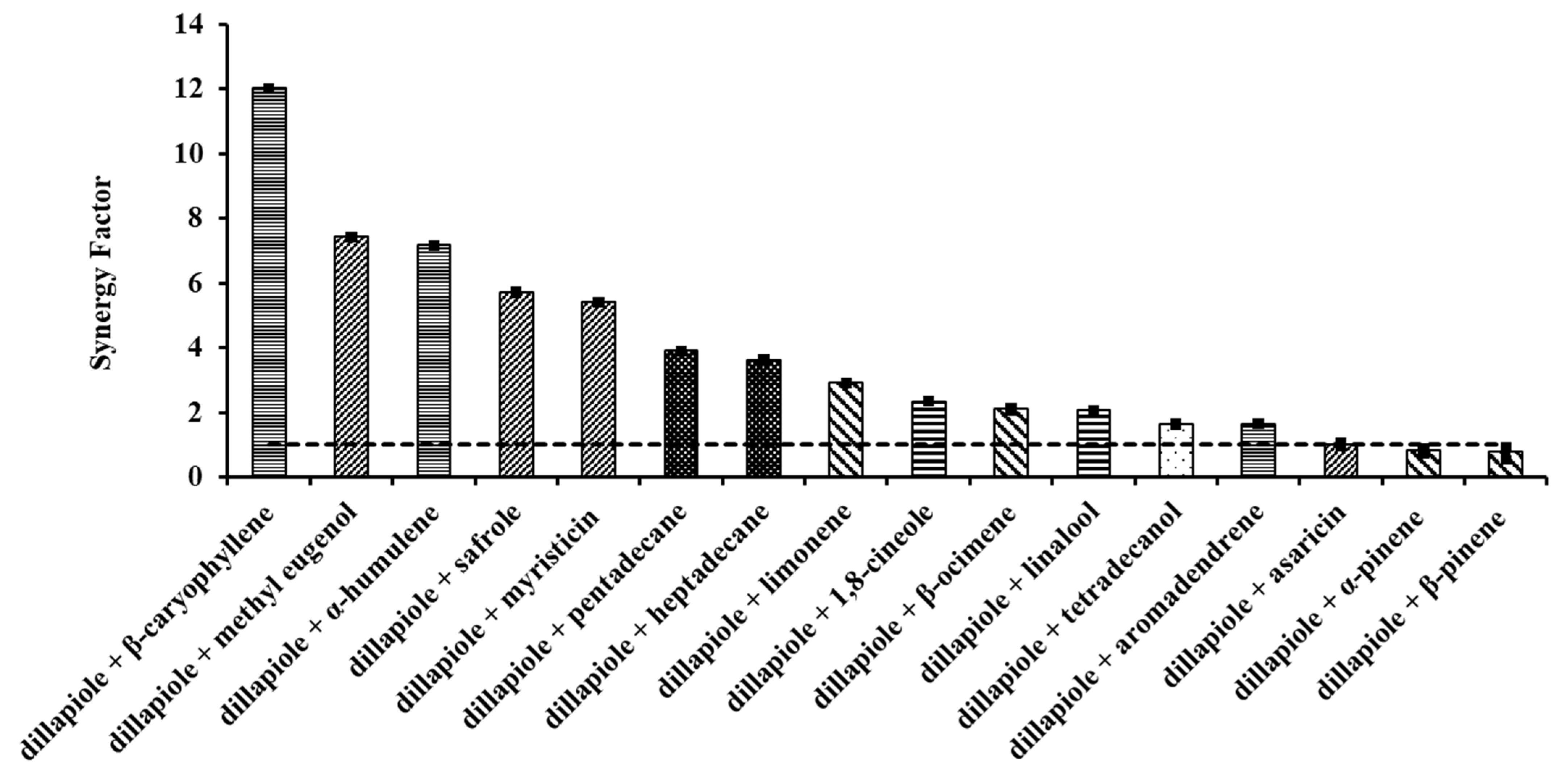
| Compounds | LD50 (ppm) | DF | Prob. χ2 | Pearson’s χ2 | R2 | Angular Coefficient ± SEM |
|---|---|---|---|---|---|---|
| dillapiole | 0.35 | 22 | 0.7040 | 18.0332 | 0.72 | 1.17 ± 0.15 |
| myristicin | 0.62 | 18 | 0.8764 | 11.4053 | 0.88 | 1.84 ± 0.16 |
| asaricin | 0.95 | 26 | 0.3946 | 24.4778 | 0.66 | 0.33 ± 0.05 |
| tetradecanol | 1.59 | 22 | 0.4554 | 22.0761 | 0.79 | 0.93 ± 0.10 |
| safrole | 2.02 | 26 | 0.5895 | 23.7626 | 0.81 | 2.05 ± 0.20 |
| methyl eugenol | 2.28 | 22 | 0.3867 | 23.2679 | 0.82 | 1.03 ± 0.10 |
| linalool | 2.71 | 38 | 0.1225 | 48.1414 | 0.85 | 0.90 ± 0.06 |
| pentadecane | 3.81 | 18 | 0.7978 | 12.8948 | 0.89 | 1.85 ± 0.15 |
| 1,8-cineole | 3.91 | 34 | 0.9993 | 1.6763 | 0.88 | 0.94 ± 0.06 |
| aromadendrene | 4.24 | 18 | 0.2576 | 21.4431 | 0.75 | 0.64 ± 0.09 |
| α-humulene | 4.94 | 20 | 0.1034 | 28.2604 | 0.75 | 0.83 ± 0.11 |
| β-caryophyllene | 6.24 | 16 | 0.6323 | 13.5492 | 0.80 | 0.49 ± 0.06 |
| limonene | 6.41 | 46 | 0.9988 | 16.6017 | 0.86 | 1.33 ± 0.08 |
| heptadecane | 7.17 | 13 | 0.7230 | 9.6409 | 0.73 | 0.29 ± 0.05 |
| α-pinene | 8.21 | 17 | 0.4060 | 17.7305 | 0.90 | 0.68 ± 0.05 |
| β-pinene | 8.52 | 18 | 0.0661 | 27.7428 | 0.66 | 0.90 ± 0.15 |
| β-ocimene | 12.81 | 22 | 0.9044 | 13.9184 | 0.85 | 1.61 ± 0.14 |
| Binary Mixtures | LD50 (ppm) | DF | Prob. χ2 | Pearson’s χ2 | R2 | Angular Coefficient ± SEM |
|---|---|---|---|---|---|---|
| dillapiole + β-caryophyllene | 0.03 | 28 | 0.8542 | 20.2750 | 0.85 | 0.26 ± 0.02 |
| dillapiole + methyl eugenol | 0.05 | 22 | 1.6690 | 28.2693 | 0.85 | 0.62 ± 0.05 |
| dillapiole + α-humulene | 0.05 | 22 | 0.8690 | 14.8408 | 0.89 | 0.52 ± 0.04 |
| dillapiole + safrole | 0.06 | 26 | 0.9123 | 16.8910 | 0.74 | 0.27 ± 0.03 |
| dillapiole + myristicin | 0.05 | 22 | 0.9887 | 9.7158 | 0.87 | 0.50 ± 0.04 |
| dillapiole + pentadecane | 0.09 | 26 | 0.1261 | 34.3629 | 0.72 | 0.58 ± 0.07 |
| dillapiole + heptadecane | 0.10 | 22 | 0.6245 | 19.3366 | 0.86 | 1.08 ± 0.09 |
| dillapiole + limonene | 0.12 | 22 | 0.8088 | 16.1412 | 0.83 | 0.90 ± 0.09 |
| dillapiole + 1,8 cineole | 0.15 | 22 | 0.9257 | 13.2597 | 0.83 | 0.95 ± 0.09 |
| dillapiole + β-ocimene | 0.17 | 22 | 0.9066 | 13.8542 | 0.84 | 0.66 ± 0.06 |
| dillapiole + linalool | 0.17 | 30 | 0.1910 | 36.5375 | 0.71 | 0.83 ± 0.10 |
| dillapiole + tetradecanol | 0.21 | 14 | 0.1258 | 20.1389 | 0.71 | 1.17 ± 0.20 |
| dillapiole + aromadendrene | 0.21 | 14 | 0.6326 | 11.6721 | 0.81 | 2.14 ± 0.28 |
| dillapiole + asaricin | 0.34 | 17 | 0.7140 | 13.3269 | 0.88 | 0.69 ± 0.06 |
| pure dillapiole | 0.35 | 22 | 0.7040 | 18.0332 | 0.72 | 1.17 ± 0.1 |
| dillapiole + α-pinene | 0.42 | 20 | 0.3115 | 22.5492 | 0.80 | 0.58 ± 0.06 |
| dillapiole + β-pinene | 0.44 | 17 | 0.1183 | 24.0415 | 0.69 | 0.36 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazolin, M.; Bizzo, H.R.; Monteiro, A.F.M.; Lima, M.E.C.; Maisforte, N.S.; Gama, P.E. Synergism in Two-Component Insecticides with Dillapiole against Fall Armyworm. Plants 2023, 12, 3042. https://doi.org/10.3390/plants12173042
Fazolin M, Bizzo HR, Monteiro AFM, Lima MEC, Maisforte NS, Gama PE. Synergism in Two-Component Insecticides with Dillapiole against Fall Armyworm. Plants. 2023; 12(17):3042. https://doi.org/10.3390/plants12173042
Chicago/Turabian StyleFazolin, Murilo, Humberto R. Bizzo, André F. M. Monteiro, Maria E. C. Lima, Natália S. Maisforte, and Paola E. Gama. 2023. "Synergism in Two-Component Insecticides with Dillapiole against Fall Armyworm" Plants 12, no. 17: 3042. https://doi.org/10.3390/plants12173042







