Arbuscular Mycorrhizae Alter Photosynthetic Responses to Drought in Seedlings of Artemisia tridentata
Abstract
:1. Introduction
2. Results
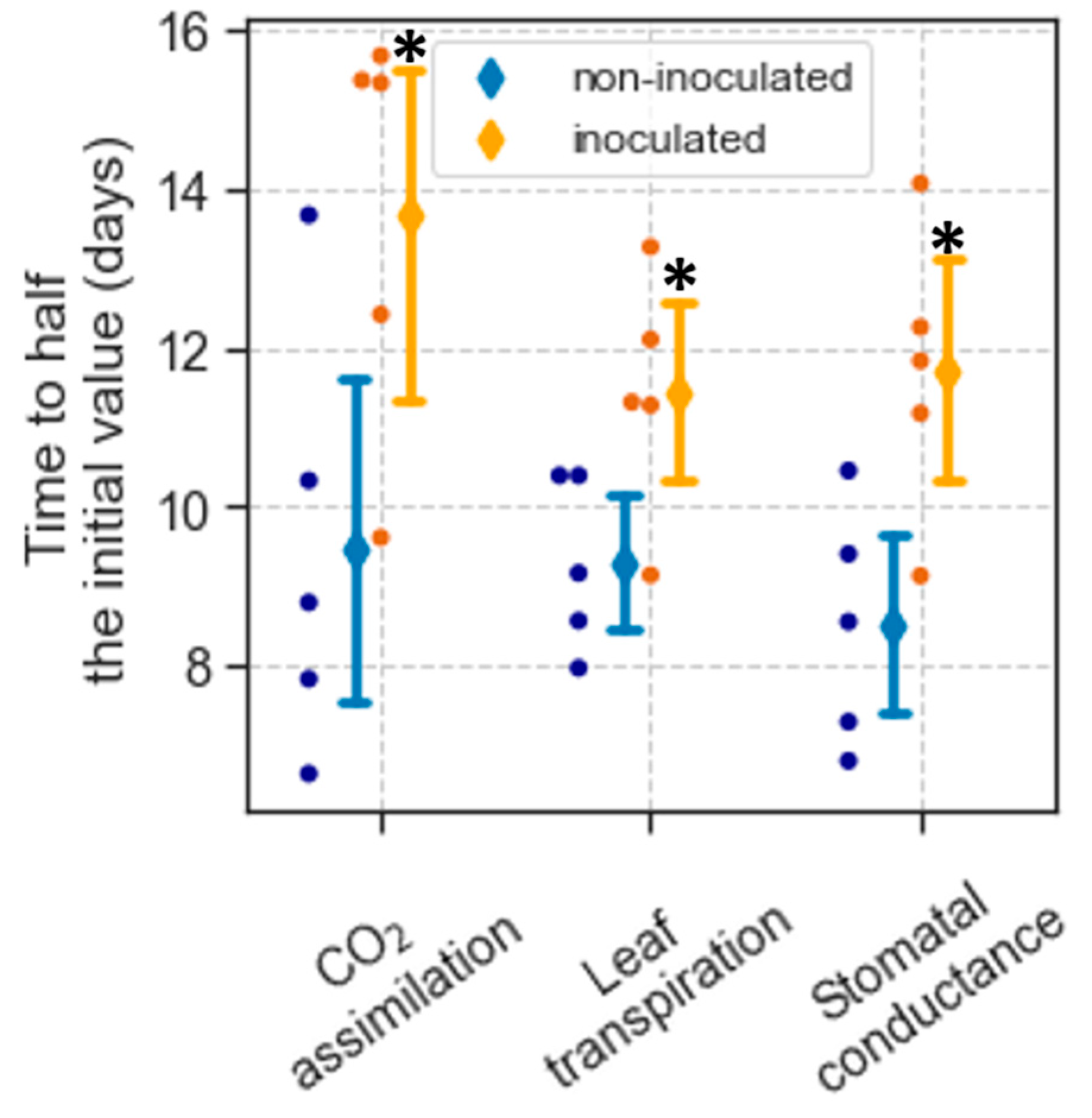
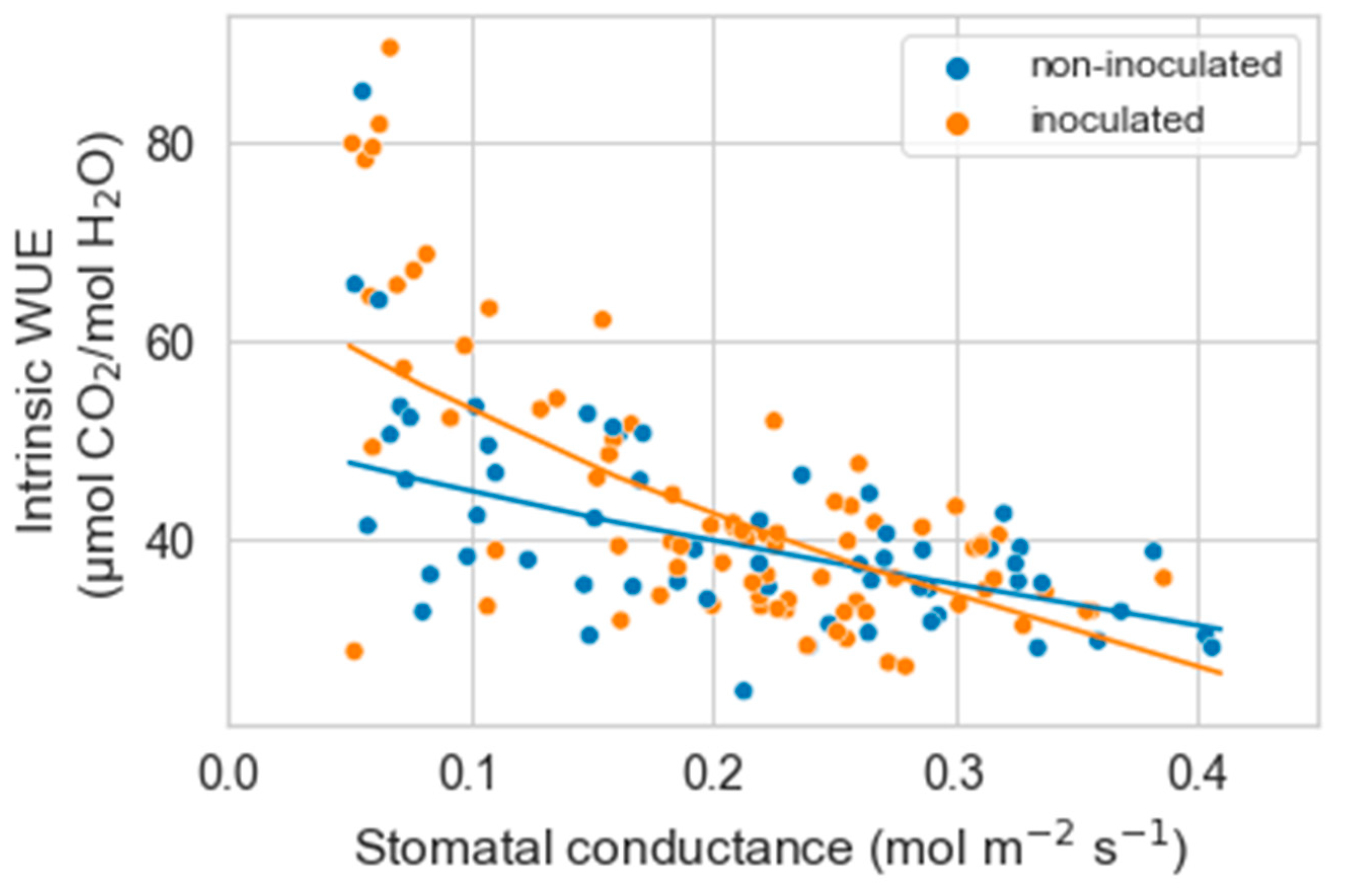
2.1. Greenhouse Experiment 1: Inoculation with Spores
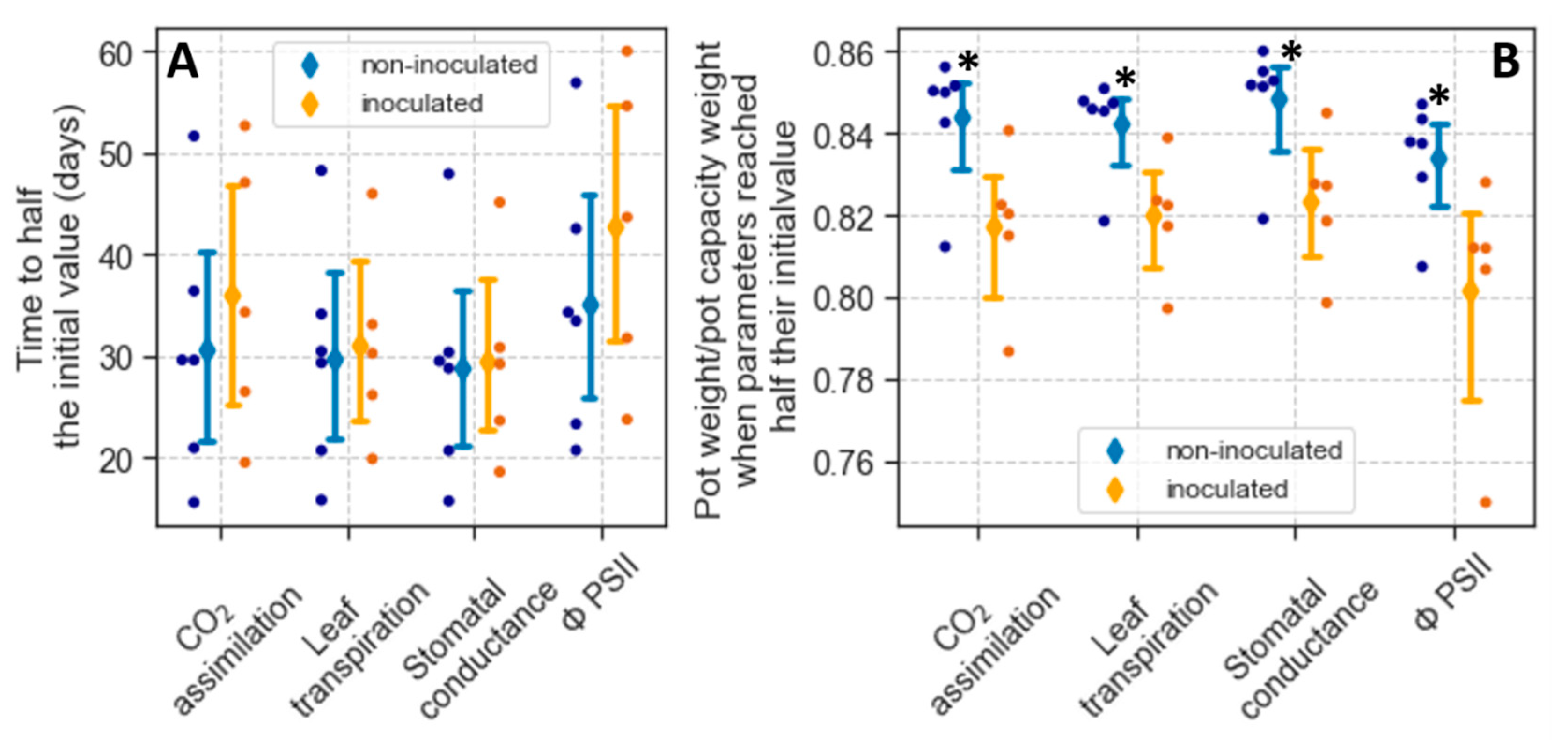
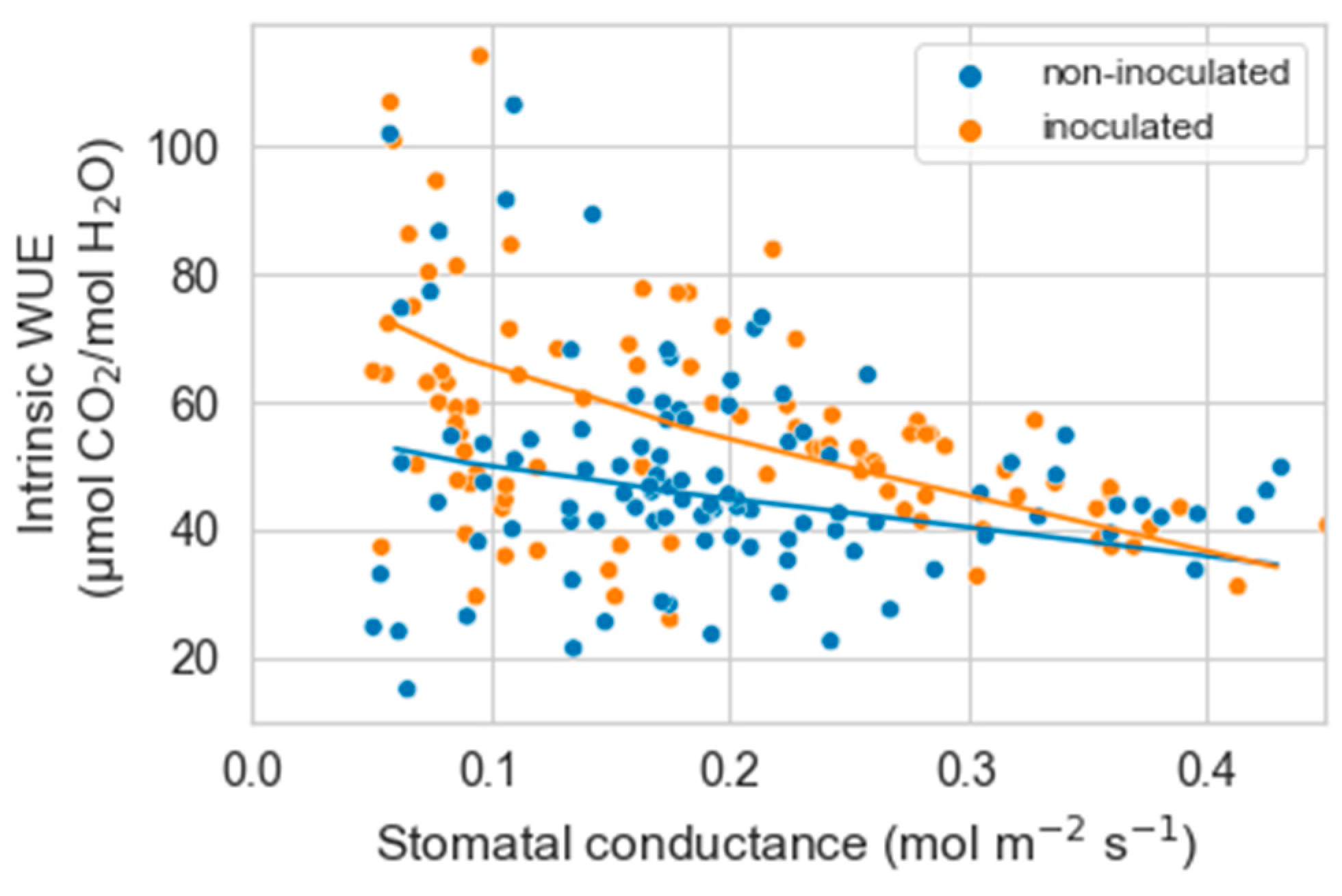
2.2. Greenhouse Experiment 2: Inoculation with Soil and Roots from Trap Cultures
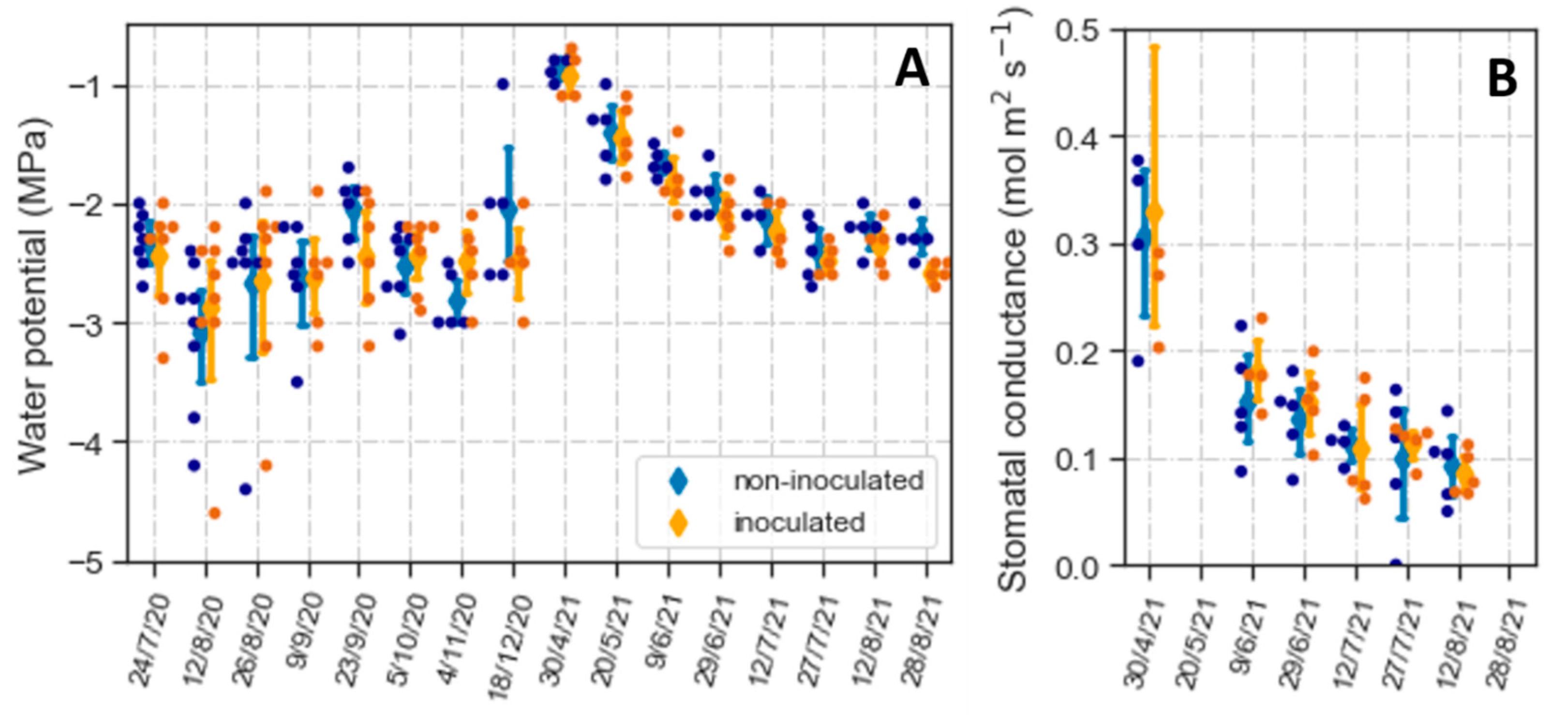
2.3. Field Experiment
3. Discussion
3.1. Greenhouse Experiments
3.2. Field Experiment
4. Materials and Methods
4.1. Greenhouse Experiment 1: Inoculation with Extracted Spores
4.1.1. Fungal and Plant Material
4.1.2. Growing Conditions, Experimental Approach, and Data Collection
4.1.3. Data Analyses
4.2. Greenhouse Experiment 2: Inoculation with Soil and Root from the Trap Cultures
4.2.1. Plant and Fungal Material
4.2.2. Growing Conditions, Experimental Approach, and Data Collection
4.2.3. Data Analyses
4.3. Field Experiment
4.3.1. Fungal and Plant Material, Experimental Approach, and Data Collection
4.3.2. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Gardes, M.; Dahlberg, A. Mycorrhizal Diversity in Arctic and Alpine Tundra: An Open Question. New Phytol. 1996, 133, 147–157. [Google Scholar] [CrossRef]
- Xu, Z.; Ban, Y.; Jiang, Y.; Zhang, X.; Liu, X. Arbuscular Mycorrhizal Fungi in Wetland Habitats and Their Application in Constructed Wetland: A Review. Pedosphere 2016, 26, 592–617. [Google Scholar] [CrossRef]
- Vasar, M.; Davison, J.; Sepp, S.-K.; Öpik, M.; Moora, M.; Koorem, K.; Meng, Y.; Oja, J.; Akhmetzhanova, A.A.; Al-Quraishy, S.; et al. Arbuscular Mycorrhizal Fungal Communities in the Soils of Desert Habitats. Microorganisms 2021, 9, 229. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.-M.; Klingl, V.; von Röpenack-Lahaye, E.; Wang, T.L.; et al. Lipid Transfer from Plants to Arbuscular Mycorrhiza Fungi. eLife 2017, 6, e29107. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M. Arbuscular Mycorrhizal Symbiosis and Alleviation of Osmotic Stress. New Perspectives for Molecular Studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sikes, B.A. When Do Arbuscular Mycorrhizal Fungi Protect Plant Roots from Pathogens? Plant Signal. Behav. 2010, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Hyperaccumulators, Arbuscular Mycorrhizal Fungi and Stress of Heavy Metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.; Porcel, R.; Bárzana, G.; Azcón, R.; Aroca, R. Contribution of Arbuscular Mycorrhizal Symbiosis to Plant Drought Tolerance: State of the Art. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin, Heidelberg, 2012; pp. 335–362. ISBN 978-3-642-32653-0. [Google Scholar]
- Jayne, B.; Quigley, M. Influence of Arbuscular Mycorrhiza on Growth and Reproductive Response of Plants under Water Deficit: A Meta-Analysis. Mycorrhiza 2014, 24, 109–119. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular Mycorrhizal Fungi Mediated Enhanced Biomass, Root Morphological Traits and Nutrient Uptake under Drought Stress: A Meta-Analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Suriyagoda, L.D.B.; Ryan, M.H.; Renton, M.; Lambers, H. Plant Responses to Limited Moisture and Phosphorus Availability: A Meta-Analysis. Adv. Agron. 2014, 124, 143–200. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The Arbuscular Mycorrhizal Symbiosis Enhances the Photosynthetic Efficiency and the Antioxidative Response of Rice Plants Subjected to Drought Stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef]
- Barzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Manuel Ruiz-Lozano, J. New Insights into the Regulation of Aquaporins by the Arbuscular Mycorrhizal Symbiosis in Maize Plants Under Drought Stress and Possible Implications for Plant Performance. Mol. Plant. Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Arbuscular Mycorrhiza Improves Substrate Hydraulic Conductivity in the Plant Available Moisture Range Under Root Growth Exclusion. Front. Plant Sci. 2018, 9, 301. [Google Scholar] [CrossRef]
- Augé, R.M. Water Relations, Drought and Vesicular-Arbuscular Mycorrhizal Symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Khalvati, M.A.; Hu, Y.; Mozafar, A.; Schmidhalter, U. Quantification of Water Uptake by Arbuscular Mycorrhizal Hyphae and Its Significance for Leaf Growth, Water Relations, and Gas Exchange of Barley Subjected to Drought Stress. Plant Biol. 2005, 7, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative Importance of an Arbuscular Mycorrhizal Fungus (Rhizophagus intraradices) and Root Hairs in Plant Drought Tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Charest, C.; Dwyer, L.M.; Hamilton, R.I. Effects of Arbuscular Mycorrhizae on Leaf Water Potential, Sugar Content, and P Content during Drought and Recovery of Maize. Can. J. Bot. 1997, 75, 1582–1591. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Torrecillas, A.; Rodríguez, P.; Morte, A.; Sánchez-Blanco, M.J. Responses of Tomato Plants Associated with the Arbuscular Mycorrhizal Fungus Glomus clarum during Drought and Recovery. J. Agric. Sci. 2002, 138, 387–393. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular Mycorrhizal Influence on Leaf Water Potential, Solute Accumulation, and Oxidative Stress in Soybean Plants Subjected to Drought Stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-29638-4. [Google Scholar]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular Mycorrhizal Symbiosis Alters Stomatal Conductance of Host Plants More under Drought than under Amply Watered Conditions: A Meta-Analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-Taking Plants: Anisohydric Behavior as a Stress-Resistance Trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Mathimaran, N. Mycosphere Essay 17 Arbuscular Mycorrhizal Symbiosis and Drought Tolerance in Crop Plants. Mycosphere 2017, 8, 361–376. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Barea, J.M.; Allen, M.F.; Caravaca, F.; Roldán, A. Differential Response of δ13C and Water Use Efficiency to Arbuscular Mycorrhizal Infection in Two Aridland Woody Plant Species. Oecologia 2003, 135, 510–515. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular Mycorrhizal Fungi Enhance Photosynthesis, Water Use Efficiency, and Growth of Frankincense Seedlings under Pulsed Water Availability Conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Unravelling the Role of Arbuscular Mycorrhizal Fungi in Mitigating the Oxidative Burst of Plants under Drought Stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Hovland, M.; Mata-González, R.; Schreiner, R.P.; Rodhouse, T.J. Fungal Facilitation in Rangelands: Do Arbuscular Mycorrhizal Fungi Mediate Resilience and Resistance in Sagebrush Steppe? Rangel. Ecol. Manag. 2019, 4, 678–691. [Google Scholar] [CrossRef]
- Davies, K.W.; Boyd, C.S.; Beck, J.L.; Bates, J.D.; Svejcar, T.J.; Gregg, M.A. Saving the Sagebrush Sea: An Ecosystem Conservation Plan for Big Sagebrush Plant Communities. Biol. Conserv. 2011, 144, 2573–2584. [Google Scholar] [CrossRef]
- Baker, W.L. Fire and Restoration of Sagebrush Ecosystems. Wildl. Soc. Bull. 2006, 34, 177–185. [Google Scholar] [CrossRef]
- Dettweiler-Robinson, E.; Bakker, J.D.; Evans, J.R.; Newsome, H.; Davies, G.M.; Wirth, T.A.; Pyke, D.A.; Easterly, R.T.; Salstrom, D.; Dunwiddie, P.W. Outplanting Wyoming Big Sagebrush Following Wildfire: Stock Performance and Economics. Rangel. Ecol. Manag. 2013, 66, 657–666. [Google Scholar] [CrossRef]
- Clements, C.D.; Harmon, D. Survivability of Wyoming Big Sagebrush Transplants. Rangelands 2019, 41, 88–93. [Google Scholar] [CrossRef]
- Maier, A.M.; Perryman, B.L.; Olson, R.A.; Hild, A.L. Climatic Influences on Recruitment of 3 Subspecies of Artemisia tridentata. J. Range Manag. 2001, 54, 699–703. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Lauenroth, W.K.; Bradford, J.B. Natural Regeneration Processes in Big Sagebrush (Artemisia tridentata). Rangel. Ecol. Manag. 2014, 67, 344–357. [Google Scholar] [CrossRef]
- Stahl, P.D.; Williams, S.E.; Christensen, M. Efficacy of Native Vesicular Arbuscular Mycorrhizal Fungi after Severe Soil Disturbance. New Phytol. 1988, 110, 347–354. [Google Scholar] [CrossRef]
- Busby, R.R.; Stromberger, M.E.; Rodriguez, G.; Gebhart, D.L.; Paschke, M.W. Arbuscular Mycorrhizal Fungal Community Differs between a Coexisting Native Shrub and Introduced Annual Grass. Mycorrhiza 2013, 23, 129–141. [Google Scholar] [CrossRef]
- Davidson, B.E.; Novak, S.J.; Serpe, M.D. Consequences of Inoculation with Native Arbuscular Mycorrhizal Fungi for Root Colonization and Survival of Artemisia tridentata ssp. wyomingensis Seedlings after Transplanting. Mycorrhiza 2016, 26, 595–608. [Google Scholar] [CrossRef]
- Prado-Tarango, D.E.; Mata-González, R.; Hovland, M.; Schreiner, R.P. Assessing Commercial and Early-Seral Arbuscular Mycorrhizal Fungi Inoculation to Aid in Restoring Sagebrush Steppe Shrubs. Rangel. Ecol. Manag. 2021, 79, 87–90. [Google Scholar] [CrossRef]
- Busby, R.R.; Gebhart, D.L.; Stromberger, M.E.; Meiman, P.J.; Paschke, M.W. Early Seral Plant Species’ Interactions with an Arbuscular Mycorrhizal Fungi Community Are Highly Variable. Appl. Soil Ecol. 2011, 48, 257–262. [Google Scholar] [CrossRef]
- Carpenter, C.L.; White, M.; Serpe, M.D. Co-Inoculation with a Dark Septate Endophyte Alters Arbuscular Mycorrhizal Colonization of Two Widespread Plants of the Sagebrush Steppe. Symbiosis 2021, 85, 343–357. [Google Scholar] [CrossRef]
- Stahl, P.D.; Schuman, G.E.; Frost, S.M.; Williams, S.E. Arbuscular Mycorrhizae and Water Stress Tolerance of Wyoming Big Sagebrush Seedlings. Soil Sci. Soc. Am. J. 1998, 62, 1309–1313. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is Plant Performance Limited by Abundance of Arbuscular Mycorrhizal Fungi? A Meta-Analysis of Studies Published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H. The Continuum Concept Remains a Useful Framework for Studying Mycorrhizal Functioning. Plant Soil 2013, 363, 411–419. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in Plant Response to Native and Exotic Arbuscular Mycorrhizal Fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Allen, M.F.; Caravaca, F.; Roldan, A. Differential Modulation of Host Plant Delta C-13 and Delta O-18 by Native and Nonnative Arbuscular Mycorrhizal Fungi in a Semiarid Environment. New Phytol. 2006, 169, 379–387. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource Limitation Is a Driver of Local Adaptation in Mycorrhizal Symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Weber, C.F.; King, G.M.; Aho, K. Relative Abundance of and Composition within Fungal Orders Differ between Cheatgrass (Bromus tectorum) and Sagebrush (Artemisia tridentata)-Associated Soils. PLoS ONE 2015, 10, e0117026. [Google Scholar] [CrossRef]
- Gehring, C.A.; Hayer, M.; Flores-Rentería, L.; Krohn, A.F.; Schwartz, E.; Dijkstra, P. Cheatgrass Invasion Alters the Abundance and Composition of Dark Septate Fungal Communities in Sagebrush Steppe. Botany 2016, 94, 481–491. [Google Scholar] [CrossRef]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The Dark Side Is Not Fastidious—Dark Septate Endophytic Fungi of Native and Invasive Plants of Semiarid Sandy Areas. PLoS ONE 2012, 7, e32570. [Google Scholar] [CrossRef] [PubMed]
- Barrow, J.R. Atypical Morphology of Dark Septate Fungal Root Endophytes of Bouteloua in Arid Southwestern USA Rangelands. Mycorrhiza 2003, 13, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark Septate Endophytes: Mutualism from by-Products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the Role of Dark Septate Endophytes in the Plant Resistance to Abiotic and Biotic Stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.-A. Sebacinales—One Thousand and One Interactions with Land Plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Mayerhofer, M.S.; Kernaghan, G.; Harper, K.A. The Effects of Fungal Root Endophytes on Plant Growth: A Meta-Analysis. Mycorrhiza 2013, 23, 119–128. [Google Scholar] [CrossRef]
- Fleege, C.D. National Proceedings: Forest and Conservation Nursery Associations-2009; US Department of Agriculture: Washington, DC, USA, 2010; p. 3. [Google Scholar]
- Mummey, D.L.; Antunes, P.M.; Rillig, M.C. Arbuscular Mycorrhizal Fungi Pre-Inoculant Identity Determines Community Composition in Roots. Soil Biol. Biochem. 2009, 41, 1173–1179. [Google Scholar] [CrossRef]
- Czaplicki, L. RDP LSU Taxonomic Training Data Formatted for DADA2 (Trainingset 11) 2017. Available online: https://zenodo.org/record/835855 (accessed on 15 July 2023).
- Krüger, M.; Krüger, C.; Walker, C.; Stockinger, H.; Schüßler, A. Phylogenetic Reference Data for Systematics and Phylotaxonomy of Arbuscular Mycorrhizal Fungi from Phylum to Species Level. New Phytol. 2012, 193, 970–984. [Google Scholar] [CrossRef]
- Earl, H.J. A Precise Gravimetric Method for Simulating Drought Stress in Pot Experiments. Crop Sci. 2003, 43, 1868–1873. [Google Scholar] [CrossRef]
- Liyanage, D.K.; Chathuranga, I.; Mori, B.A.; Thilakarathna, M.S. A Simple, Semi-Automated, Gravimetric Method to Simulate Drought Stress on Plants. Agronomy 2022, 12, 349. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular Mycorrhizas: A Promising Component of Plant Production Systems Provided Favorable Conditions for Their Growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Osmotic Adjustment in Leaves of VA Mycorrhizal and Nonmycorrhizal Rose Plants in Response to Drought Stress. Plant Physiol. 1986, 82, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-S.; Xia, R.-X. Arbuscular Mycorrhizal Fungi Influence Growth, Osmotic Adjustment and Photosynthesis of Citrus under Well-Watered and Water Stress Conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor Maintenance by Osmotic Adjustment: 40 Years of Progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Barzana, G.; Aroca, R.; Antonio Paz, J.; Chaumont, F.; Carmen Martinez-Ballesta, M.; Carvajal, M.; Manuel Ruiz-Lozano, J. Arbuscular Mycorrhizal Symbiosis Increases Relative Apoplastic Water Flow in Roots of the Host Plant under Both Well-Watered and Drought Stress Conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef]
- Bitterlich, M.; Sandmann, M.; Graefe, J. Arbuscular Mycorrhiza Alleviates Restrictions to Substrate Water Flow and Delays Transpiration Limitation to Stronger Drought in Tomato. Front. Plant Sci. 2018, 9, 154. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant Resistance to Drought Depends on Timely Stomatal Closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Levionnois, S.; Bonal, D.; Heuret, P.; Stahl, C.; Coste, S. Large Leaf Hydraulic Safety Margins Limit the Risk of Drought-Induced Leaf Hydraulic Dysfunction in Neotropical Rainforest Canopy Tree Species. Funct. Ecol. 2023, 37, 1717–1731. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula Phosphate Transporter Indispensable for the Arbuscular Mycorrhizal Symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular Mycorrhizal Fungi Mediated Alleviation of Drought Stress via Non-Enzymatic Antioxidants: A Meta-Analysis. Plants 2022, 11, 2448. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M. Arbuscular Mycorrhizal Fungi and Antioxidant Enzymes in Ameliorating Drought Stress: A Meta-Analysis. Plant Soil 2022, 480, 295–303. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought Stress and Reactive Oxygen Species: Production, Scavenging and Signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, F.; Barea, J.M.; Palenzuela, J.; Figueroa, D.; Alguacil, M.M.; Roldán, A. Establishment of Shrub Species in a Degraded Semiarid Site after Inoculation with Native or Allochthonous Arbuscular Mycorrhizal Fungi. Appl. Soil Ecol. 2003, 22, 103–111. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Azcón-Aguilar, C.; Barea, J.M.; Ruiz-Lozano, J.M. Importance of Native Arbuscular Mycorrhizal Inoculation in the Halophyte Asteriscus maritimus for Successful Establishment and Growth under Saline Conditions. Plant Soil 2013, 370, 175–185. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of Commercial Arbuscular Mycorrhizal Inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Quoreshi, A.M.; Khasa, D.P. Effectiveness of Mycorrhizal Inoculation in the Nursery on Root Colonization, Growth, and Nutrient Uptake of Aspen and Balsam Poplar. Biomass Bioenergy 2008, 32, 381–391. [Google Scholar] [CrossRef]
- Amerian, M.R.; Stewart, W.S.; Griffiths, H. Effect of Two Species of Arbuscular Mycorrhizal Fungi on Growth, Assimilation and Leaf Water Relations in Maize (Zea mays). Asp. Appl. Biol. 2001, 63, 71–76. [Google Scholar]
- Miller, R.F.; Shultz, L.M. Development and Longevity of Ephemeral and Perennial Leaves on Artemisia tridentata nutt. ssp. wyomingensis. Great Basin Nat. 1987, 47, 227–230. [Google Scholar]
- Kolb, K.J.; Sperry, J.S. Differences in Drought Adaptation Between Subspecies of Sagebrush (Artemisia tridentata). Ecology 1999, 80, 2373–2384. [Google Scholar] [CrossRef]
- AgriMet Columbia-Pacific Northwest Region|Bureau of Reclamation. Available online: https://www.usbr.gov/pn/agrimet/ (accessed on 30 July 2023).
- Geisler, M.; Buerki, S.; Serpe, M.D. Herbivory Amplifies Adverse Effects of Drought on Seedling Recruitment in a Keystone Species of Western North American Rangelands. Plants 2022, 11, 2628. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Samenus, R.J.; Fischer, L.E.; Hetrick, B.A.D. Plant Demographic Responses to Mycorrhizal Symbiosis in Tallgrass Prairie. Oecologia 1994, 99, 21–26. [Google Scholar] [CrossRef]
- Javaid, A.; Riaz, T. Mycorrhizal Colonization in Different Varieties of Gladiolus and Its Relation with Plant Vegetative and Reproductive Growth. Int. J. Agric. Biol. 2008, 10, 278–282. [Google Scholar]
- Vaingankar, J.D.; Rodrigues, B.F. Effect of Arbuscular Mycorrhizal (AM) Inoculation on Growth and Flowering in Crossandra infundibuliformis (L.) Nees. J. Plant Nutr. 2015, 38, 1478–1488. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From Root to Fruit: RNA-Seq Analysis Shows That Arbuscular Mycorrhizal Symbiosis May Affect Tomato Fruit Metabolism. BMC Genom. 2014, 15, 221. [Google Scholar] [CrossRef]
- Bennett, A.E.; Meek, H.C. The Influence of Arbuscular Mycorrhizal Fungi on Plant Reproduction. J. Chem. Ecol. 2020, 46, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-M.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi Regulate Flowering of Hyacinths orientalis L. Anna Marie. Emir. J. Food Agric. 2018, 30, 144–149. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z. How Do Arbuscular Mycorrhizas Affect Reproductive Functional Fitness of Host Plants? Front. Plant Sci. 2022, 13, 975488. [Google Scholar] [CrossRef] [PubMed]
- Maron, J.L.; Hajek, K.L.; Hahn, P.G.; Pearson, D.E. Seedling Recruitment Correlates with Seed Input across Seed Sizes: Implications for Coexistence. Ecology 2019, 100, e02848. [Google Scholar] [CrossRef]
- Zaiats, A.; Germino, M.J.; Serpe, M.D.; Richardson, B.A.; Caughlin, T.T. Intraspecific Variation Mediates Density Dependence in a Genetically Diverse Plant Species. Ecology 2021, 102, e03502. [Google Scholar] [CrossRef]
- INVAM. Available online: https://invam.ku.edu/node/1 (accessed on 21 January 2023).
- Morton, J.B.; Koske, R.E.; Stürmer, S.L.; Bentivenga, S.P. Mutualistic Arbuscular Endomycorrhizal Fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G., Bills, G., Foster, M., Eds.; Academic Press: Cambridge, MA, USA, 2004; pp. 317–336. [Google Scholar]
- Serpe, M.D.; Thompson, A.; Petzinger, E. Effects of a Companion Plant on the Formation of Mycorrhizal Propagules in Artemisia tridentata Seedlings. Rangel. Ecol. Manag. 2020, 73, 138–146. [Google Scholar] [CrossRef]
- SoilWeb: An Online Soil Survey Browser|California Soil Resource Lab. Available online: https://casoilresource.lawr.ucdavis.edu/gmap/ (accessed on 13 July 2022).
- Kobae, Y.; Ohtomo, R. An Improved Method for Bright-Field Imaging of Arbuscular Mycorrhizal Fungi in Plant Roots. Soil Sci. Plant Nutr. 2016, 62, 27–30. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Guyot, G.; Scoffoni, C.; Sack, L. Combined Impacts of Irradiance and Dehydration on Leaf Hydraulic Conductance: Insights into Vulnerability and Stomatal Control. Plant Cell Environ. 2012, 35, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Newville, M.; Stensitzki, T.; Allen, D.B.; Ingargiola, A. LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python 2014. Available online: https://zenodo.org/record/11813 (accessed on 15 June 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Cleophas, T.J.; Zwinderman, A.H. Mixed Linear Models for Repeated Measures. In Statistics Applied to Clinical Studies; Cleophas, T.J., Zwinderman, A.H., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 593–605. ISBN 978-94-007-2863-9. [Google Scholar]
- Bolker, B.M. Linear and Generalized Linear Mixed Models. In Ecological Statistics; Fox, G.A., Negrete-Yankelevich, S., Sosa, V.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 309–333. ISBN 978-0-19-967254-7. [Google Scholar]
- R-Development-Core-Team R: A Language and Environment for Statistical Computing 2022. Viena, Austria. Available online: https://www.R-Project.Org/ (accessed on 14 June 2023).
- Turner, N.C. Imposing and Maintaining Soil Water Deficits in Drought Studies in Pots. Plant Soil 2019, 439, 45–55. [Google Scholar] [CrossRef]
- Krueger, M.; Stockinger, H.; Krueger, C.; Schuessler, A. DNA-Based Species Level Detection of Glomeromycota: One PCR Primer Set for All Arbuscular Mycorrhizal Fungi. New Phytol. 2009, 183, 212–223. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using “Ggplot2” 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 15 June 2023).
- Hacke, U.G.; Sperry, J.S.; Pittermann, J. Drought Experience and Cavitation Resistance in Six Shrubs from the Great Basin, Utah. Basic Appl. Ecol. 2000, 1, 31–41. [Google Scholar] [CrossRef]
- Hoover, D.L.; Wilcox, K.R.; Young, K.E. Experimental Droughts with Rainout Shelters: A Methodological Review. Ecosphere 2018, 9, e02088. [Google Scholar] [CrossRef]


| Colonization | Non-Inoculated | Inoculated | p-Value |
|---|---|---|---|
| Total before | 0 (0, 18.2) | 65.0 (35.6, 75.7) | 0.004 |
| Arbuscular before | 0 (0, 15.3) | 33.8 (14.4, 64.0) | 0.015 |
| Vesicles before | 0 (0, 0) | 9.0 (1.7, 22.2) | 0.002 |
| Total after | 0 (0, 12.6) | 48.2 (39.7, 55.3) | 0.008 |
| Arbuscular after | 0 (0, 11.5) | 16.7 (7.1, 33.8) | 0.016 |
| Vesicles after | 0 (0, 0) | 6.0 (1.2, 21.1) | 0.008 |
| Parameter | Non-Inoculated | Inoculated | p-Value |
|---|---|---|---|
| A (µmol CO2 m−2 s−1) | 10.20 (±1.28) | 9.57 (±0.72) | 0.68 |
| Tr (mol H2O m−2 s−1) | 4.74 (±0.49) | 5.61 (±0.42) | 0.22 |
| gs (mol H2O m−2 s−1) | 0.29 (±0.03) | 0.28 (±0.03) | 0.77 |
| ΦPSII | 0.46 (±0.014) | 0.45 (±0.015) | 0.54 |
| Colonization | Non-Inoculated | Inoculated | p-Value |
|---|---|---|---|
| Total before | 0 (0, 0.9) | 20.6 (14.3, 22.3) | 0.008 |
| Arbuscular before | 0 (0, 0.8) | 6.9 (1.8, 14.3) | 0.007 |
| Vesicles before | 0 (0, 0) | 2.0 (0.9, 2.9) | 0.005 |
| Total after | 1.5 (0, 4.9) | 32.3 (17.0, 43.7 | 0.004 |
| Arbuscular after | 0 (0, 1.8) | 6.3 (0, 14.6) | 0.03 |
| Vesicles after | 0 (0, 0.4) | 2.0 (1.0, 2.1) | 0.004 |
| Total well-watered | 1.0 (0, 4.5) | 31.3 (22.1, 42.6) | 0.029 |
| Arbuscular well-watered | 0 (0, 0.9) | 17.5 (13.5, 30.3) | 0.029 |
| Vesicles well-watered | 0 (0, 0) | 2.1 (0.5, 4.6) | 0.014 |
| Parameter | Non-Inoculated | Inoculated | p-Value |
|---|---|---|---|
| A (µmol CO2 m−2 s−1) | 11.4 (±1.8) | 11.9 (±1.9) | 0.84 |
| Tr (mol H2O m−2 s−1) | 5.3 (±0.6) | 4.9 (±0.4) | 0.62 |
| gs (mol H2O m−2 s−1) | 0.27 (±0.04) | 0.26 (±0.03) | 0.87 |
| ΦPSII | 0.21 (±0.01) | 0.20 (±0.01) | 0.42 |
| Colonization | Non-Inoculated | Inoculated | p-Value |
|---|---|---|---|
| Total spring | 24.9 (±4.8) | 46.7 (±4.8) | 0.008 |
| Arbuscular spring | 7.2 (±2.9) | 17.0 (±2.9) | 0.027 |
| Vesicles spring | 8.1 (±3.1) | 8.4 (±3.1) | 0.950 |
| Total fall | 8.4 (±6.7) | 33.8 (±6.7) | 0.028 |
| Arbuscular fall | 3.0 (±4.1) | 9.2 (±4.1) | 0.302 |
| Vesicles fall | 0.3 (±5.5) | 11.8 (±5.5) | 0.1892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisler, M.; Buerki, S.; Serpe, M.D. Arbuscular Mycorrhizae Alter Photosynthetic Responses to Drought in Seedlings of Artemisia tridentata. Plants 2023, 12, 2990. https://doi.org/10.3390/plants12162990
Geisler M, Buerki S, Serpe MD. Arbuscular Mycorrhizae Alter Photosynthetic Responses to Drought in Seedlings of Artemisia tridentata. Plants. 2023; 12(16):2990. https://doi.org/10.3390/plants12162990
Chicago/Turabian StyleGeisler, Mathew, Sven Buerki, and Marcelo D. Serpe. 2023. "Arbuscular Mycorrhizae Alter Photosynthetic Responses to Drought in Seedlings of Artemisia tridentata" Plants 12, no. 16: 2990. https://doi.org/10.3390/plants12162990





