Cytogenetic Study and Pollen Viability of Phalaenopsis Queen Beer ‘Mantefon’
Abstract
:1. Introduction
2. Results
2.1. FISH Karyotype
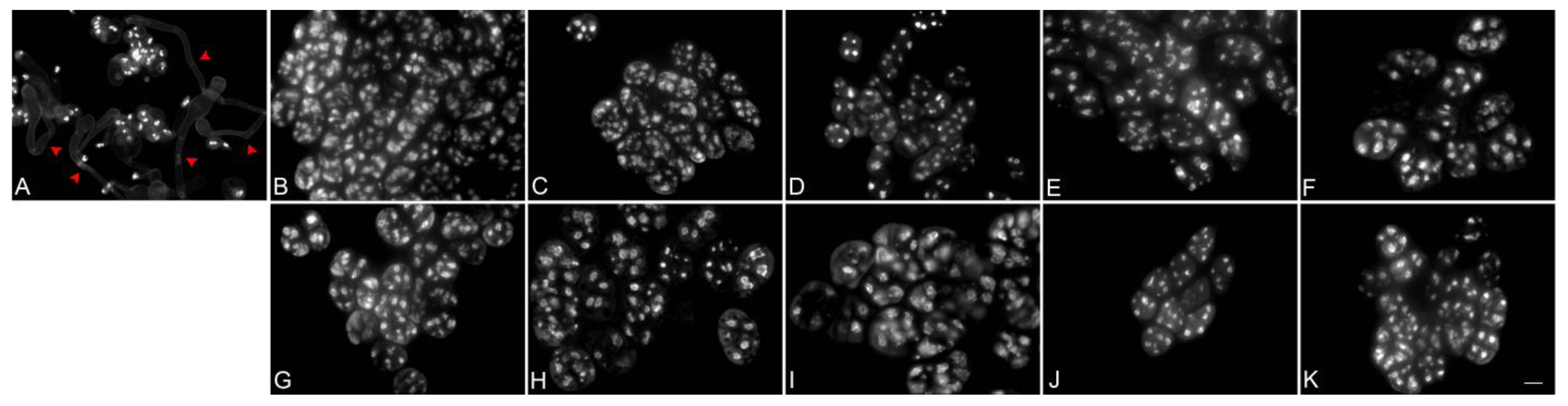
2.2. Pollen Viability Evaluation by Staining and In Vitro Germination
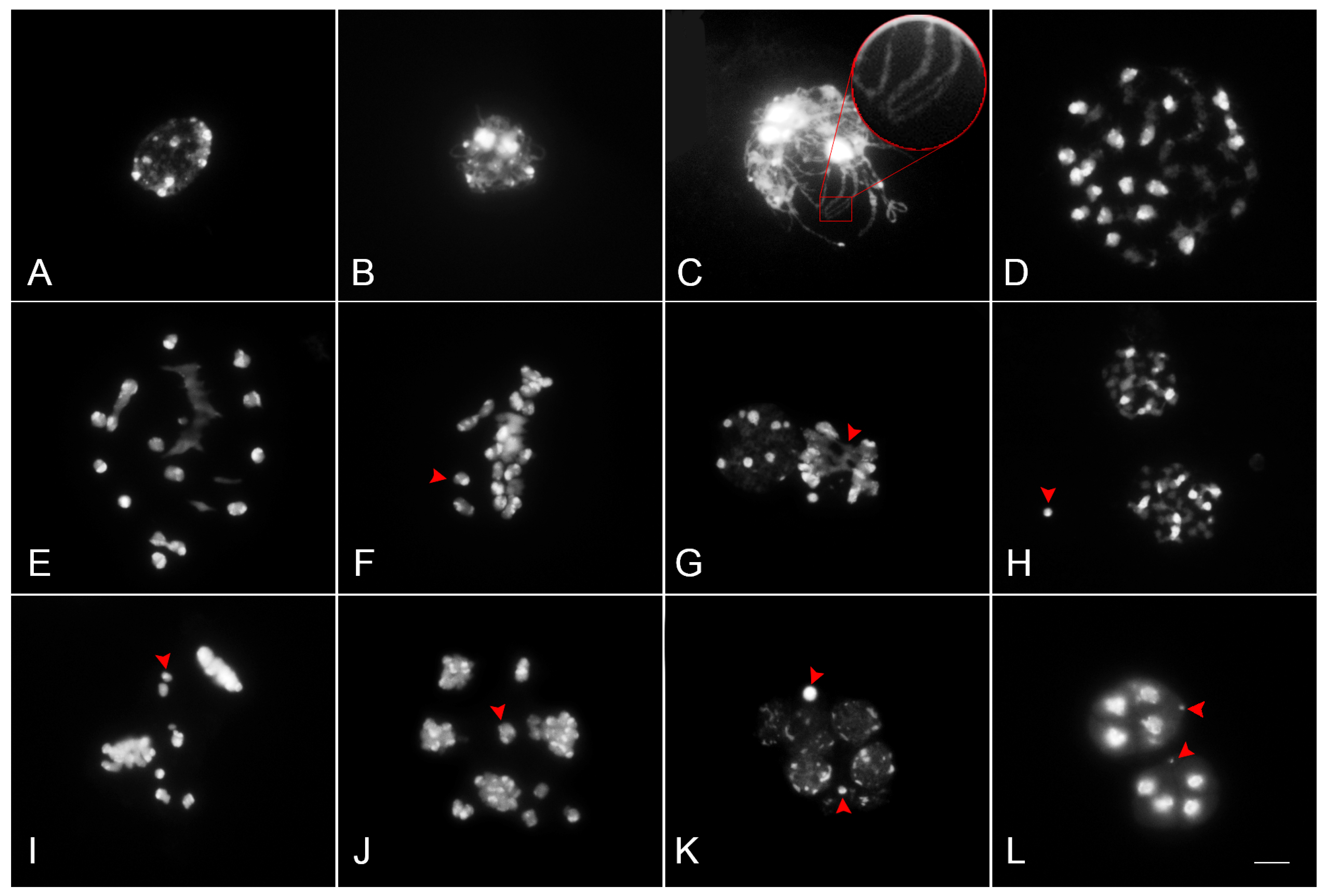
2.3. Meiotic Chromosome Behavior of P. Queen Beer ‘Mantefon’
2.4. Sporad Quantification in PMCs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Somatic Chromosome Preparation
4.3. Meiotic Chromosome Preparation
4.4. Fluorescence in Situ Hybridization
4.5. Sporad Quantification in Pollen Mother Cells
4.6. Pollen Viability by TTC Staining
4.7. In Vitro Pollen Germination
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, W.J.; Lin, Y.Z.; Lee, N. Photosynthetic light requirements and effects of low irradiance and daylength on Phalaenopsis amabilis. J. Am. Soc. Hortic. Sci. 2012, 137, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.J.; Hsu, B.D. Photosynthetic plasticity of Phalaenopsis in response to different light environments. J. Plant Physiol. 2004, 161, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Floriculture Crops 2017 Summary; United States Department of Agriculture: Washington, DC, USA, 2018. [Google Scholar]
- United States Department of Agriculture (USDA). Floriculture Crops 2020 Summary; United States Department of Agriculture: Washington, DC, USA, 2021. [Google Scholar]
- Cho, A.R.; Chung, S.W.; Kim, Y.J. Shortening the vegetative growth stage of Phalaenopsis Queen Beer ‘Mantefon’ by controlling light with calcium ammonium nitrate levels under enriched CO2. Horticulturae 2022, 8, 157. [Google Scholar] [CrossRef]
- Lee, Y.I.; Tseng, Y.F.; Lee, Y.C.; Chung, M.C. Chromosome constitution and nuclear DNA content of Phalaenopsis hybrids. Sci. Hortic. 2020, 262, 109089. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chen, W.H. Breeding and development of new varieties in Phalaenopsis. In Orchid Biotechnology, 1st ed.; Chen, W.H., Chen, H.H., Eds.; World Scientific Publishing Co., Pte. Ltd.: Toh Tuck Link, Singapore, 2007; pp. 1–22. [Google Scholar]
- Lin, C.; Chen, Y.; Chen, W.; Chen, C.; Kao, Y. Genome organization and relationships of Phalaenopsis orchids inferred from genomic in situ hybridization. Bot. Bull. Acad. Sin. 2005, 46, 339–345. [Google Scholar]
- Christenson, E.A. Phalaenopsis: A monograph; Timber Press: Portland, ON, USA, 2001. [Google Scholar]
- Singh, R.J. Plant cytogenetics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 163–164. [Google Scholar]
- Shin, H.; Park, H.R.; Park, J.E.; Yu, S.H.; Yi, G.; Kim, J.H.; Koh, W.; Kim, H.H.; Lee, S.S.; Huh, J.H. Reduced fertility caused by meiotic defects and micronuclei formation during microsporogenesis in xBrassicoraphanus. Genes. Genom. 2021, 43, 251–258. [Google Scholar] [CrossRef]
- Mercier, R.; Mézard, C.; Jenczewski, E.; Macaisne, N.; Grelon, M. The molecular biology of meiosis in plants. Ann. Rev. Plant Biol. 2015, 66, 297–327. [Google Scholar] [CrossRef]
- Gray, S.; Cohen, P.E. Control of meiotic crossovers: From double-strand break formation to designation. Ann. Rev. Genet. 2016, 50, 175. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; van Rengs, W.M.; Zaidan, M.W.A.M.; Underwood, C.J. Meiosis in crops: From genes to genomes. J. Exp. Bot. 2021, 72, 6091–6109. [Google Scholar] [CrossRef]
- Boff, T.; Schifino-Wittmann, M.T. Pollen fertility and meiotic behaviour in accessions and species of Lleucaena. Trop. Grass 2002, 36, 54–58. [Google Scholar]
- Cifuentes, M.; Eber, F.; Lucas, M.O.; Lode, M.; Chèvre, A.M.; Jenczewski, E. Repeated polyploidy drove different levels of crossover suppression between homoeologous chromosomes in Brassica napus allohaploids. Plant Cell 2010, 22, 2265–2276. [Google Scholar] [CrossRef] [Green Version]
- Szadkowski, E.; Eber, F.; Huteau, V.; Lode, M.; Huneau, C.; Belcram, H.; Coriton, O.; Manzanares-Dauleux, M.J.; Delourme, R.; King, G.J.; et al. The first meiosis of resynthesized Brassica napus, a genome blender. New Phytol. 2010, 186, 102–112. [Google Scholar] [CrossRef]
- Lee, Y.H. Cytology and fertility of an intergeneric orchid hybrid. J. Hered. 1987, 78, 319–322. [Google Scholar] [CrossRef]
- Kamemoto, H.; Amore, T.D.; Kuehnle, A.R. Breeding Dendrobium Orchids in Hawaii; University of Hawai’i Press: Honolulu, HI, USA, 1999; pp. 3–43. [Google Scholar]
- Bolanos-Villegas, P.; Chin, S.W.; Chen, F.C. Meiotic chromosome behavior and capsule setting in Doritaenopsis hybrids. J. Am. Soc. Hortic. Sci. 2008, 133, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.I.; Chang, F.C.; Chung, M.C. Chromosome pairing affinities in interspecific hybrids reflect phylogenetic distances among lady’s slipper orchids (Paphiopedilum). Ann. Bot. 2011, 108, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Robertson, J.S.; Paterson, A.H. Inference of subgenomic origin of BACs in an interspecific hybrid sugarcane cultivar by overlapping oligonucleotide hybridizations. Genome 2011, 54, 727–737. [Google Scholar] [CrossRef]
- Li, C.; Dong, N.; Zhao, Y.; Wu, S.; Liu, Z. A review for the breeding of orchids: Current achievements and prospects. Hortic. Plant J. 2021, 7, 380–392. [Google Scholar] [CrossRef]
- Kopecký, D.; Martis, M.; Číhalíková, J.; Hřibová, E.; Vrána, J.; Barto¡, J.; Kopecká, J.; Cattonaro, F.; Stočes, Š.; Novák, P.; et al. Flow sorting and sequencing meadow fescue chromosome 4F. Plant Physiol. 2013, 163, 1323–1337. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.; Armstead, I.; Thomas, A.; James, C.; Gasior, D.; Bisaga, M.; Roberts, L.; King, I.; King, J. Alien introgression in the grasses Lolium perenne (perennial ryegrass) and Festuca pratensis (meadow fescue): The development of seven monosomic substitution lines and their molecular and cytological characterization. Ann. Bot. 2011, 107, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Moscone, E.A.; Matzke, M.A.; Matzke, A.J.M. The use of combined FISH/GISH in conjunction with DAPI counterstaining to identify chromosomes containing transgene inserts in amphidiploid tobacco. Chromosoma 1996, 105, 321–326. [Google Scholar] [CrossRef]
- Jacobsen, E.; De Jong, J.H.; Kamstra, S.A.; Van den Berg, P.M.; Ramanna, M.S. Genomic in situ hybridization (GISH) and RFLP analysis for the identification of alien chromosomes in the backcross progeny of potato (+) tomato fusion hybrids. Heredity 1995, 74, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Palma-Silva, C.; dos Santos, D.G.; Kaltchuk-Santos, E.; Bodanese-Zanettini, M.H. Chromosome numbers, meiotic behavior, and pollen viability of species of Vriesea and Aechmea genera (Bromeliaceae) native to Rio Grande do Sul, Brazil. Am. J. Bot. 2004, 91, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Boldrini, K.R.; Agostinho, K.F.; Corrêa, B.J.S.; Donazzolo, J. Cytogenetic study and pollen viability of Diatenopteryx sorbifolia Radlk. Ciênc. Florest. 2022, 32, 233–246. [Google Scholar] [CrossRef]
- Griesbach, R.J. Polypioidy in Phalaenopsis orchid improvement. J. Hered. 1985, 76, 74–75. [Google Scholar] [CrossRef]
- Lim, K.B.; Wennekes, J.; Jong, J.H.D.; Jacobsen, E.; Van Tuyl, J.M. Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 2001, 44, 911–918. [Google Scholar] [CrossRef]
- Maluszynska, J.; Heslop-Harrison, J.S. Physical mapping of rDNA loci in Brassica species. Genome 1993, 36, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Maluszynska, J.; Heslop-Harrison, J.S. Molecular cytogenetics of the genus Arabidopsis: In situ localization of rDNA sites, chromosome numbers and diversity in centromeric heterochromatin. Ann. Bot. 1993, 71, 479–484. [Google Scholar] [CrossRef]
- Galasso, I.; Schmidt, T.; Pignone, D.; Heslop-Harrison, J.S. The molecular cytogenetics of Vigna unguiculata (L.) Walp: The physical organization and characterization of 18 s-5.8 s-25 s rRNA genes, 5 s rRNA genes, telomere-like sequences, and a family of centromeric repetitive DNA sequences. Theor. Appl. Genet. 1995, 91, 928–935. [Google Scholar] [CrossRef]
- Pacini, E. Tapetum character states: Analytical keys for tapetum types and activities. Can. J. Bot. 1997, 75, 1448–1459. [Google Scholar] [CrossRef]
- Pacini, E. Orchid pollen dispersal units and reproductive consequences. Orchid. Biol. Rev. Perspect. 2009, 10, 185–218. [Google Scholar]
- Pacini, E.; Hesse, M. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Ann. Bot. 2002, 89, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.D.; Pereira, T.N.S.; Martins, E.R. Microsporogenesis and microgametogenesis associated with flower bud and anther size and pollen viability in yellow passion fruit (Passiflora edulis Sims f. flavicarpa Degener). Sci. Agrotechnol 2002, 26, 1209–1217. [Google Scholar]
- Ottaviano, E.; Mulcahy, D.L. Genetics of angiosperm pollen. Adv. Genet. 1989, 26, 1–64. [Google Scholar]
- Shivanna, K.R.; Ram, H.M. Pollination biology: Contributions to fundamental and applied aspects. Curr. Sci. 1993, 65, 226–233. [Google Scholar]
- Sulusoglu, M.; Cavusoglu, A. In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci. World J. 2014, 2014, 657123. [Google Scholar] [CrossRef]
- Mattson, A.M.; Jensen, C.O.; Dutcher, R.A. Triphenyltetrazolium chloride as a dye for vital tissues. Science 1947, 106, 294–295. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. The estimation of pollen viability in rice. J. Exp. Bot. 1995, 46, 151–154. [Google Scholar] [CrossRef]
- Sorkheh, K.; Shiran, B.; Rouhi, V.; Khodambashi, M. Influence of temperature on the in vitro pollen germination and pollen tube growth of various native Iranian almonds (Prunus L. spp.) species. Trees 2011, 25, 809–822. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Johnson, S.D.; Van Staden, J. Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae). S. Afr. J. Bot. 2012, 79, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain. Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Van Der Walt, I.D.; Littlejohn, G.M. Storage and viability testing of Protea pollen. J. Am. Soc. Hortic. Sci. 1996, 121, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Bellusci, F.; Musacchio, A.; Stabile, R.; Pellegrino, G. Differences in pollen viability in relation to different deceptive pollination strategies in Mediterranean orchids. Ann. Bot. 2010, 106, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junillon, T.; Morand, L.; Flandrois, J.P. Enhanced tetrazolium violet reduction of Salmonella spp. by magnesium addition to the culture media. Food Microbiol. 2014, 42, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Lopez Del Egido, L.; Navarro-Miró, D.; Martinez-Heredia, V.; Toorop, P.E.; Iannetta, P.P. A spectrophotometric assay for robust viability testing of seed batches using 2, 3, 5-triphenyl tetrazolium chloride: Using Hordeum vulgare L. as a model. Front. Plant Sci. 2017, 8, 747. [Google Scholar] [CrossRef] [Green Version]
- Arends, J.C. Cytological observations on genome homology in eight interspecific hybrids of Phalaenopsis. Genetica 1970, 41, 88–100. [Google Scholar] [CrossRef]
- McClintock, B. Chromosome morphology in Zea mays. Science 1929, 69, 629. [Google Scholar] [CrossRef]
- Conceição, S.I.; Róis, A.S.; Caperta, A.D. Nonreduction via meiotic restitution and pollen heterogeneity may explain residual male fertility in triploid marine halophyte Limonium algarvense (Plumbaginaceae). Caryologia 2019, 72, 53–62. [Google Scholar]
- Kao, Y.Y.; Chang, S.B.; Lin, T.Y.; Hsieh, C.H.; Chen, Y.H.; Chen, W.H.; Chen, C.C. Differential accumulation of heterochromatin as a cause for karyotype variation in Phalaenopsis orchids. Ann. Bot. 2001, 87, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Del Bosco, S.F.; Tusa, N.; Conicella, C. Microsporogenesis in a Citrus interspecific tetraploid somatic hybrid and its fusion parents. Heredity 1999, 83, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Tel-Zur, N.; Abbo, S.; Mizrahi, Y. Cytogenetics of semi-fertile triploid and aneuploid intergeneric vine cacti hybrids. J. Hered. 2005, 96, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huo, B.; Liu, W.; Li, D.; Liao, L. Abnormal meiosis in an intersectional allotriploid of Populus L. and segregation of ploidy levels in 2x× 3x progeny. PLoS ONE 2017, 12, e0181767. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Chen, H.H.; Chen, W.H. Phalaenopsis. In Ornamental Crops; Springer: Cham, Switzerland, 2018; pp. 567–625. [Google Scholar]
- Chen, W.H.; Tang, C.Y.; Kao, Y.L. Polyploidy and variety improvement of Phalaenopsis orchids. I Int. Orchid. Symp. 2010, 878, 133–138. [Google Scholar] [CrossRef]
- Lim, K.B.; De Jong, H.; Yang, T.J.; Park, J.Y.; Kwon, S.J.; Kim, J.S.; Lim, M.H.; Kim, J.A.; Jin, M.; Jin, Y.M.; et al. 2005. Characterization of rDNAs and tandem repeats in the heterochromatin of Brassica rapa. Mol. Cells 2005, 19, 436–444. [Google Scholar]
- Park, H.R.; Park, J.E.; Kim, J.H.; Shin, H.; Yu, S.H.; Son, S.; Yi, G.; Lee, S.S.; Kim, H.H.; Huh, J.H. Meiotic chromosome stability and suppression of crossover between non-homologous chromosomes in x Brassicoraphanus, an intergeneric allotetraploid derived from a cross between Brassica rapa and Raphanus sativus. Front. Plant Sci. 2020, 11, 851. [Google Scholar] [CrossRef]
- Waminal, N.E.; Pellerin, R.J.; Kim, N.S.; Jayakodi, M.; Park, J.Y.; Yang, T.J.; Kim, H.H. Rapid and efficient FISH using pre-labeled oligomer probes. Sci. Rep. 2018, 8, 8224. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.B.; Yang, T.J.; Hwang, Y.J.; Kim, J.S.; Park, J.Y.; Kwon, S.J.; Kim, J.; Choi, B.S.; Lim, M.H.; Jin, M.; et al. Characterization of the centromere and peri-centromere retrotransposons in Brassica rapa and their distribution in related Brassica species. Plant J. 2007, 49, 173–183. [Google Scholar] [CrossRef]
- Singh, R.N. Chromosomal abnormalities and fertility in induced autotetraploid Helianthus annuus in the C1 and C2 generations. Cytologia 1992, 57, 277–281. [Google Scholar] [CrossRef] [Green Version]



| Chromosome No. | No. of Metaphase I Cells | Pairing Configuration | ||
|---|---|---|---|---|
| Univalents | Bivalents | Trivalents | ||
| 57 | 35 | 7 (1–19) | 20.3 (19–28) | 1.3 (1–4) |
| Stage | Abnormality (%) | Normality (%) |
|---|---|---|
| Prophase I | 64.93% (87/134) 1 | 35.07% (47/134) 2 |
| Metaphase I | 98.77% (80/81) | 1.23% (1/81) |
| Anaphase I | 83.72% (36/43) | 16.28% (7/43) |
| Metaphase II | 80% (8/10) | 20% (2/10) |
| Anaphase II | 92.6% (25/27) | 7.40% (2/27) |
| Tetrad Stage | 21.30% (69/324) | 78.70% (255/324) |
| Total Number of Sporocytes | Pollen Mother Cells (Percentage %) | ||||||
|---|---|---|---|---|---|---|---|
| Monad | Dyad | Triad | Tetrad | Polyad | Tetrad with Micronucleus | Sporads with 1 Micronucleus | |
| 324 | 5 (2%) | 6 (2%) | 18 (6%) | 69 (21%) | 35 (11%) | 141 (44%) | 50 (15%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevilleno, S.S.; An, H.R.; Cabahug-Braza, R.A.M.; Ahn, Y.-J.; Hwang, Y.-J. Cytogenetic Study and Pollen Viability of Phalaenopsis Queen Beer ‘Mantefon’. Plants 2023, 12, 2828. https://doi.org/10.3390/plants12152828
Sevilleno SS, An HR, Cabahug-Braza RAM, Ahn Y-J, Hwang Y-J. Cytogenetic Study and Pollen Viability of Phalaenopsis Queen Beer ‘Mantefon’. Plants. 2023; 12(15):2828. https://doi.org/10.3390/plants12152828
Chicago/Turabian StyleSevilleno, Samantha Serafin, Hye Ryun An, Raisa Aone M. Cabahug-Braza, Yun-Jae Ahn, and Yoon-Jung Hwang. 2023. "Cytogenetic Study and Pollen Viability of Phalaenopsis Queen Beer ‘Mantefon’" Plants 12, no. 15: 2828. https://doi.org/10.3390/plants12152828






