Identification of the Plant Family Caryophyllaceae in Korea Using DNA Barcoding
Abstract
:1. Introduction
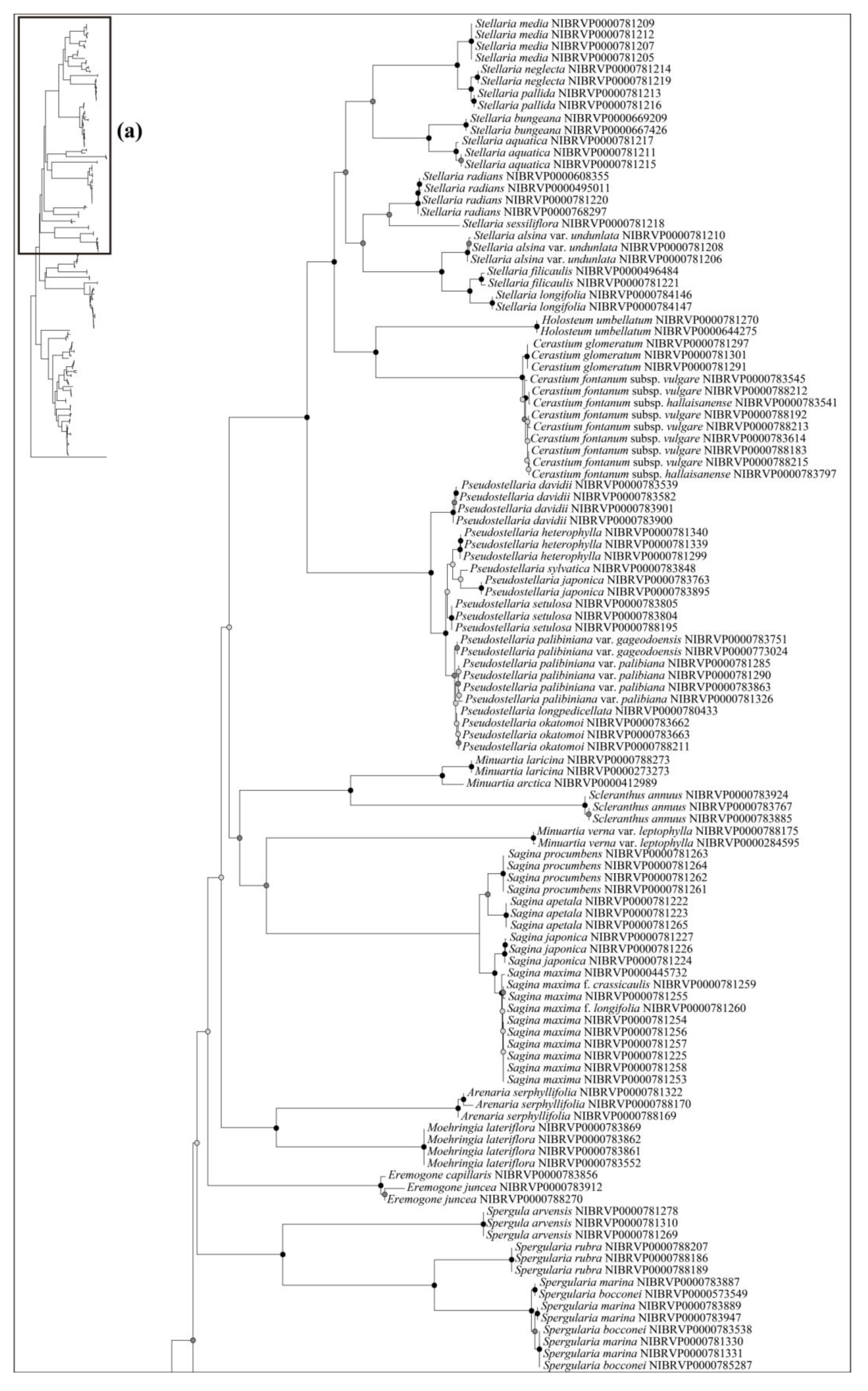
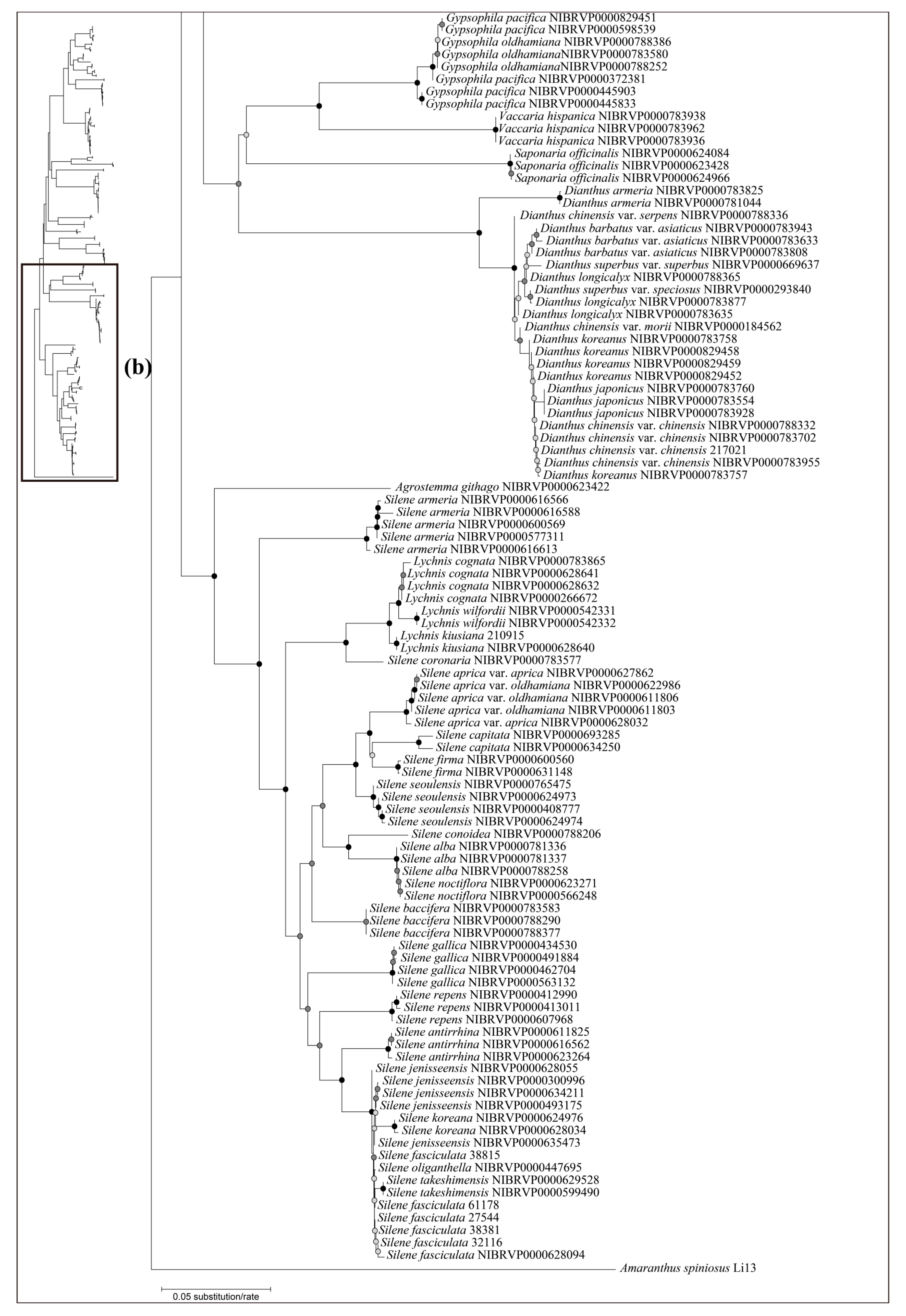
2. Results
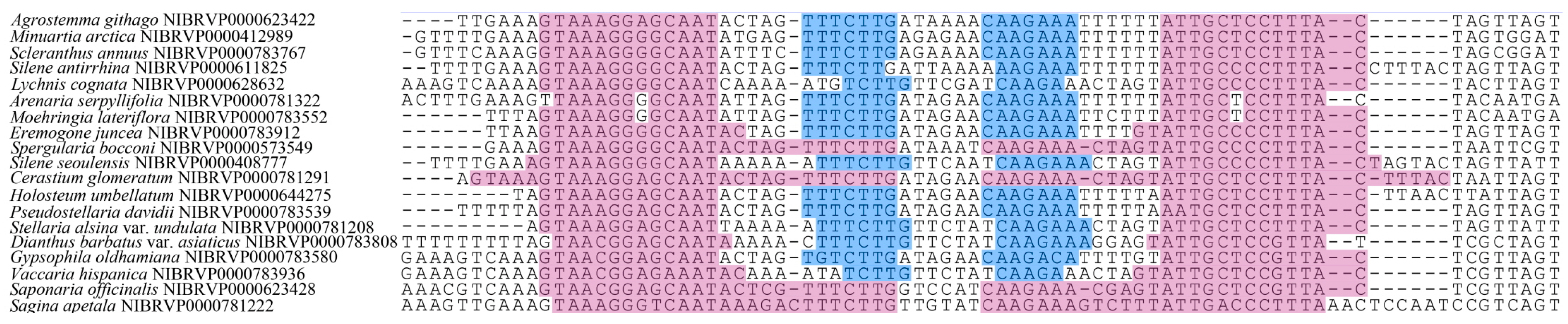
2.1. Characteristics of DNA Barcode Regions
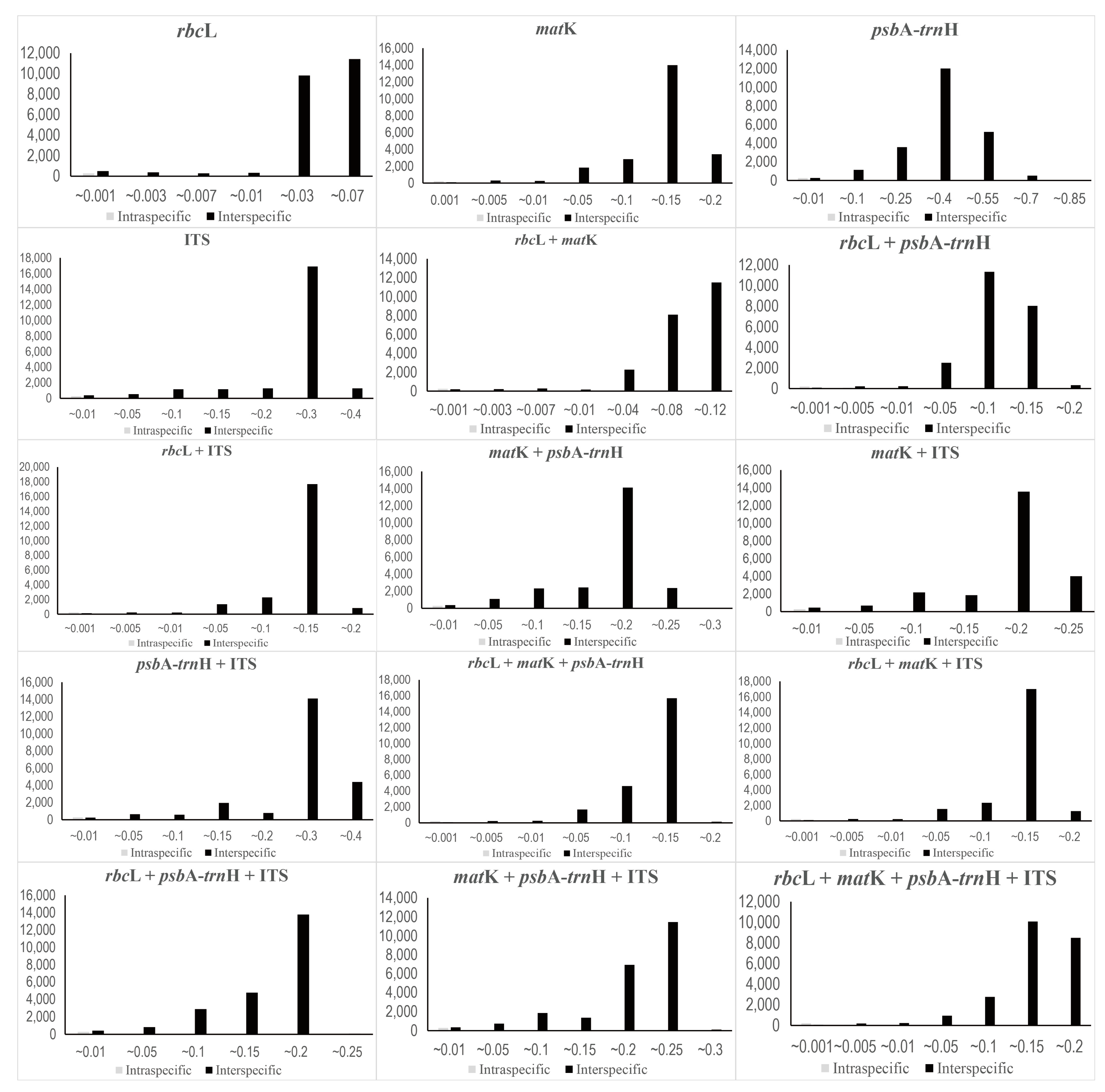
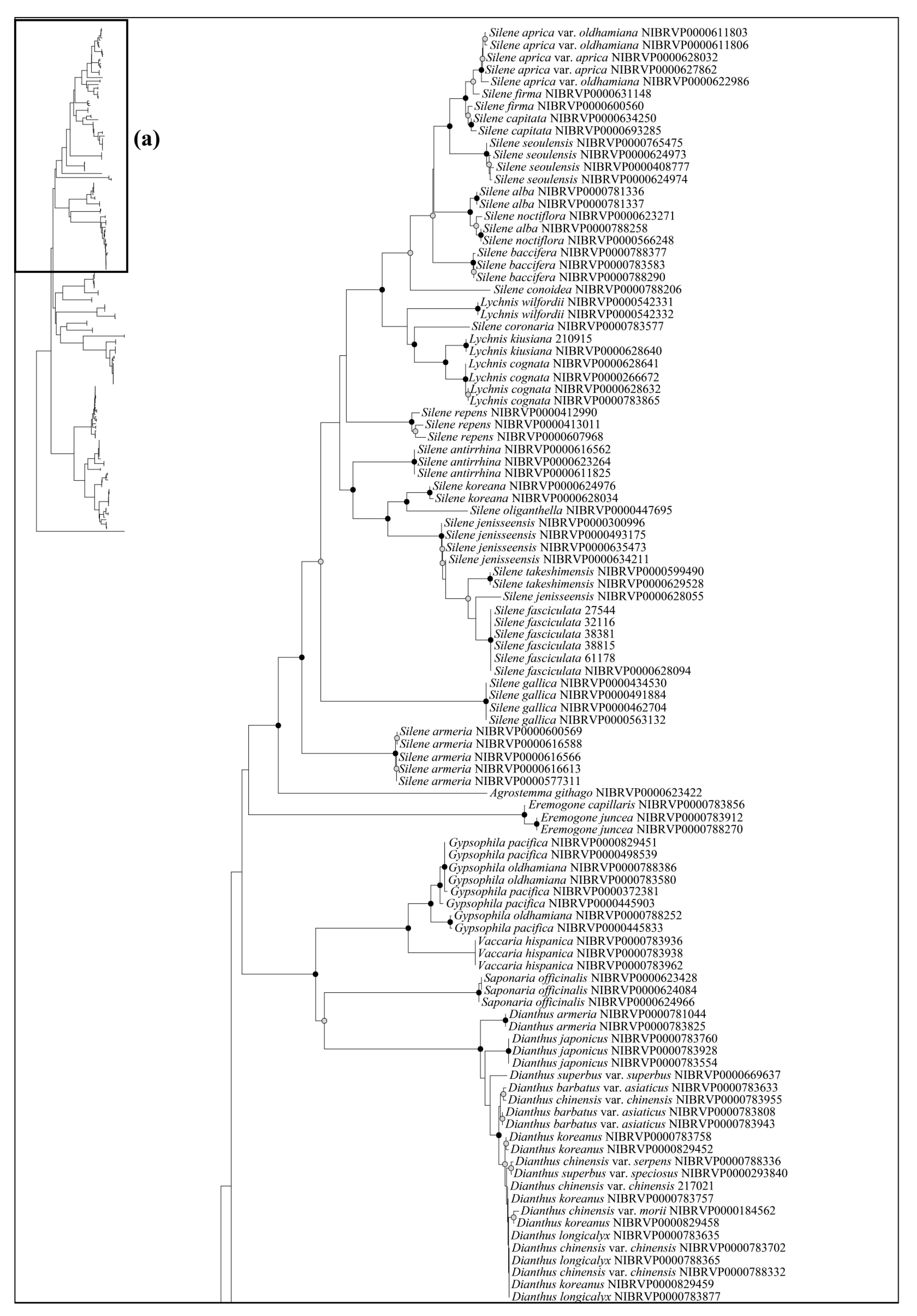
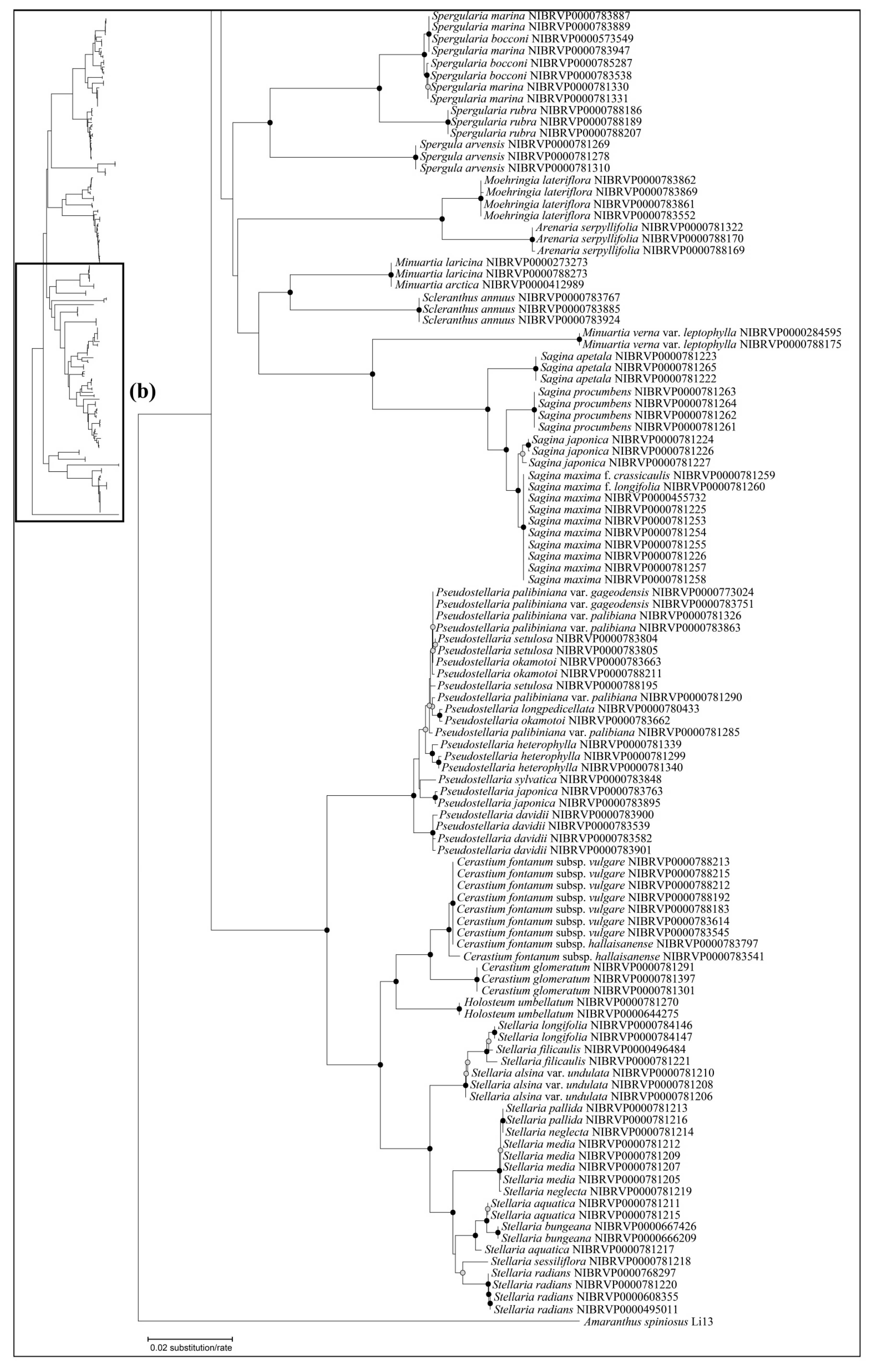
2.2. Evaluation of DNA Barcodes
3. Discussion
4. Materials and Methods
4.1. Taxon Sampling
4.2. DNA Extraction, Polymerase Chain Reaction (PCR), and Sequencing
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bittrich, V. Caryophyllaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Bittrich, V., Rohwer, J., Eds.; Springer: Berlin, Germany, 1993; Volume 2, pp. 206–230. [Google Scholar]
- Chandra, S.; Rawat, D.S. Medicinal plants of the family Caryophyllaceae: A review of ethno-medicinal uses and pharmacological properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Choi, K. Caryophyllaceae. In Flora of Korea; Flora of Korea Editorial Committee, Ed.; National Institute of Biological Resources: Incheon, Republic of Korea, 2018; Volume 3, pp. 30–61. [Google Scholar]
- National Institute of Biological Resources. National List of Species of Korea. 2021. Available online: https://species.nibr.go.kr/index.do (accessed on 7 February 2023).
- Han, Y.S.; Yim, D.S.; Lee, S.Y. Studies on biological activities of the roots of Pseudostellaria heterophylla. Saengyak Hakhoe Chi 2000, 31, 45–50, (In Korean with English Abstract). [Google Scholar]
- Xie, L.X.; Zhang, H.C.; Wang, H.Y.; Wang, Y.; Wang, F.L.; Sun, J.Y. Two new triterpenoids from Gypsophila oldhamiana. Nat. Prod. Res. 2016, 30, 1068–1074. [Google Scholar] [CrossRef]
- Cho, H.D.; Kang, W.S.; Kim, D.H.; Ku, J.J.; Seo, K.I. Comparison of biological activity between Stellaria aquatica seed extracts. Korean J. Food Preserv. 2019, 26, 228–237. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Widyowati, R.; Sulistyowaty, M.I.; Sugimoto, S.; Yamano, Y.; Kawakami, S.; Otsuka, H.; Matsunami, K. Firmosides A and B: Two new sucrose ferulates from the aerial parts of Silene firma and evaluation of radical scavenging activities. J. Nat. Med. 2020, 74, 796–803. [Google Scholar]
- Gil, M.; Kwon, H.H.; Kwon, Y.H.; Jung, M.J.; Kim, S.Y.; Rhie, Y.H. Growth of Minuartia laricina, Arenaria juncea, and Corydalis speciose in field with various soil water contents. J. Bio-Envrion. Control 2020, 29, 344–353, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- National Institute of Ecology. Endangered Wildlife. Available online: https://www.nie.re.kr/nieEng/main/contents.do?menuNo=400052 (accessed on 7 February 2023).
- Kim, S.C.; Oh, Y.J.; Kwon, Y.W. Weed flora of agricultural area in Korea. KJWS 1992, 12, 317–334. [Google Scholar]
- Lee, I.Y.; Oh, Y.J.; Hong, S.H.; Heo, S.J.; Lee, C.Y.; Park, K.W.; Cho, S.H.; Kwon, O.D.; Im, I.B.; Kim, S.K. Occurrence of weed flora and changes in weed vegetation in orchard fields of Korea. Weed Turf. Sci. 2017, 6, 21–27, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, S.H.; Choi, H.S.; Yang, J.C.; Nam, G.H.; Chung, G.Y.; Choi, H.J. Floristic study of Dalmasan and its adjacent regions. Korean J. Environ. Ecol. 2009, 23, 1–21, (In Korean with English Abstract). [Google Scholar]
- Bak, G.J.; Koh, J.K.; Pak, J.H. Newly recorded naturalized species in Korea, Silene Antirrhina L. (Caryophyllaceae). Korean J. Plant Taxon. 2011, 41, 171–174, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Jung, S.Y.; Hong, J.K.; Park, S.H.; Yang, J.C.; Yun, S.M.; Kang, Y.S. Two unrecorded alien plants of South Korea: Geranium Dissectum L. (Geraniaceae) and Dianthus Armeria L. Korean J. Plant Taxon. 2015, 45, 272–277, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, J.H.; Hong, J.K.; Kim, J.S. Two new naturalized species from South Korea, Polygonum ramosissimum Michx. (Polygonaceae) and Spergularia Bocconi (Scheele) Graebn. (Caryophyllaceae). Korean J. Plant Taxon. 2016, 46, 326–330, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Hong, J.K.; Shim, S.D.; Kim, H.S.; Sim, S.; Hyun, C.W.; Kim, J.H. New record of an alien plant, Petrorhagia nanteuilii (Caryophyllaceae) in Korea. Korean J. Plant Taxon. 2021, 51, 386–390. [Google Scholar] [CrossRef]
- Rembold, K.; Mangopo, H.; Tjitrosoedirdjo, S.S.; Kreft, H. Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 2017, 213, 234–242. [Google Scholar] [CrossRef]
- Son, S.H.; Jo, A.R.; Kim, D.E. Current status of alert alien species management for the establishment of proactive management systems in Korea. J. Ecol. Environ. 2021, 45, 26. [Google Scholar] [CrossRef]
- Brysting, A.K.; Elven, R. The Cerastium alpinum-C. arcticum complex (Caryophyllaceae): Numerical analyses of morphological variation and a taxonomic revision of C. arcticum Lange sl. Taxon 2000, 49, 189–216. [Google Scholar] [CrossRef]
- Farsi, M.; Behroozian, M.; Vaezi, J.; Joharchi, M.R.; Memariani, F. The evolution of Dianthus polylepis complex (Caryophyllaceae) inferred from morphological and nuclear DNA sequence data: One or two species? Plant Syst. Evol. 2013, 299, 1419–1431. [Google Scholar] [CrossRef]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. DNA barcodes: Genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. USA 2008, 105, 2761–2762. [Google Scholar] [CrossRef]
- Meusnier, I.; Singer, G.A.C.; Landry, J.F.; Hickey, D.A.; Hebert, P.D.N.; Hajibabaei, M. A universal DNA mini-barcode for biodiversity analysis. BMC Genom. 2008, 9, 214. [Google Scholar] [CrossRef]
- Naeem, A.; Khan, A.A.; Cheema, H.M.N.; Khan, I.A.; Buerkert, A. DNA barcoding for species identification in the Palmae family. Genet. Mol. Res. 2014, 13, 10341–10348. [Google Scholar] [CrossRef] [PubMed]
- Gernandt, D.S.; López, G.G.; García, S.O.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kumar, A.; Nagireddy, A.; Mani, D.N.; Shukla, A.K.; Tiwari, R.; Sundaresan, V. DNA barcoding: An efficient tool to overcome authentication challenges in the herbal market. Plant Biotechnol. J. 2016, 14, 8–21. [Google Scholar] [CrossRef]
- Gesto-Borroto, R.; Medina-Jiménez, K.; Lorence, A.; Villarreal, M.L. Application of DNA barcoding for quality control of herbal drugs and their phytopharmaceuticals. Rev. Bras. Farmacogn. 2021, 31, 127–141. [Google Scholar] [CrossRef]
- Hartvig, I.; Czako, M.; Kjær, E.D.; Nielsen, L.R.; Theilade, I. The use of DNA barcoding in identification and conservation of rosewood (Dalbergia spp.). PLoS ONE 2015, 10, e0138231. [Google Scholar] [CrossRef]
- Van De Wiel, C.C.M.; Van Der Schoot, J.; Van Valkenburg, J.; Duistermaat, H.; Smulders, M.J.M. DNA barcoding discriminates the noxious invasive plant species, floating pennywort (Hydrocotyle ranunculoides L.f.), from non-invasive relatives. Mole. Ecol. Resour. 2009, 9, 1086–1091. [Google Scholar] [CrossRef]
- Jin, D.P.; Kim, J.H.; Sim, S.; Suh, H.J.; Kim, J.S. New record of an alien plant, Desmodium paniculatum (Fabaceae), in Korea based on a morphological examination and DNA barcoding. Korean J. Plant Taxon. 2021, 51, 133–140. [Google Scholar] [CrossRef]
- Mosa, K.A.; Gairola, S.; Jamdade, R.; El-Keblawy, A.; Al Shaer, K.I.; Al Harthi, E.K.; Shabana, H.A.; Mahmoud, T. The promise of molecular and genomic techniques for biodiversity research and DNA barcoding of the Arabian Peninsula flora. Front. Plant Sci. 2019, 9, 1929. [Google Scholar] [CrossRef]
- Pax, F.; Hoffmann, K. Caryophyllaceae. In Die Natürlichen Pflanzenfamilien, 2nd ed.; Engler, A., Prantl, K., Eds.; Engelmann: Leipzig, Germany, 1934; pp. 275–364. [Google Scholar]
- Rabeler, R.K.; Bittrich, V. Suprageneric nomenclature in the Caryophyllaceae. Taxon 1993, 42, 857–863. [Google Scholar] [CrossRef]
- Fior, S.; Karis, P.O.; Casazza, G.; Minuto, L.; Sala, F. Molecular phylogeny of the Caryophyllaceae (Caryophyllales) inferred from chloroplast matK and nuclear rDNA ITS sequences. Am. J. Bot. 2006, 93, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Harbaugh, D.T.; Nepokroeff, M.; Rabeler, R.K.; McNeill, J.; Zimmer, E.A.; Wagner, W.L. A new lineage-based tribal classification of the family Caryophyllaceae. Int. J. Plant Sci. 2010, 171, 185–198. [Google Scholar] [CrossRef]
- Greenberg, A.K.; Donoghue, M.J. Molecular systematics and character evolution in Caryophyllaceae. Taxon 2011, 60, 1637–1652. [Google Scholar] [CrossRef]
- Frajman, B.; Thollesson, M.; Oxelman, B. Taxonomic revision of Atocion and Viscaria (Sileneae, Caryophyllaceae). Bot. J. Linn. Soc. 2013, 173, 194–210. [Google Scholar] [CrossRef]
- Madhani, H.; Rabeler, R.; Pirani, A.; Oxelman, B.; Heubl, G.; Zarre, S. Untangling phylogenetic patterns and taxonomic confusion in tribe Caryophylleae (Caryophyllaceae) with special focus on generic boundaries. Taxon 2018, 67, 83–112. [Google Scholar] [CrossRef]
- Sharples, M.T.; Tripp, E.A. Phylogenetic relationships within and delimitation of the cosmopolitan flowering plant genus Stellaria L. (Caryophyllaceae): Core stars and fallen stars. Syst. Bot. 2019, 44, 857–876. [Google Scholar] [CrossRef]
- Jafari, F.; Zarre, S.; Gholipour, A.; Eggens, F.; Rabeler, R.K.; Oxelman, B. A new taxonomic backbone for the infrageneric classification of the species-rich genus Silene (Caryophyllaceae). Taxon 2020, 69, 337–368. [Google Scholar] [CrossRef]
- Luo, K.; Chen, S.; Chen, K.; Song, J.; Yao, H.; Ma, X.; Zhu, Y.; Pang, X.; Yu, H.; Li, X.; et al. Assessment of candidate plant DNA barcodes using the Rutaceae family. Sci. China Life Sci. 2010, 53, 701–708. [Google Scholar] [CrossRef]
- Jin, D.P.; Park, J.W.; Park, J.S.; Choi, B.H. DNA barcode and phylogenetic study of the tribe Desmodieae (Fabaceae) in Korea. Korean J. Plant Taxon. 2019, 49, 224–239. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, B. Species identification in complex groups of medicinal plants based on DNA barcoding: A case study on Astragalus Spp. (Fabaceae) from Southwest China. Conserv. Genet. Resour. 2020, 12, 469–478. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, Y.; Wang, S.; Jiang, M.; Chen, Z.; Ying, Q.; Wang, H. Molecular identification of Dendrobium species (Orchidaceae) based on the DNA barcode ITS2 region and its application for phylogenetic study. Int. J. Mol. Sci. 2015, 16, 21975–21988. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Ji, Y.; Choi, G.; Kang, Y.M.; Yang, S.; Moon, B.C. Molecular identification and phylogenetic analysis of important medicinal plant species in genus Paeonia based on rDNA-ITS, matK, and rbcL DNA barcode sequences. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Yao, P.C.; Gao, H.Y.; Wei, Y.N.; Zhang, J.H.; Chen, X.Y.; Li, H.Q. Evaluating sampling strategy for DNA barcoding study of coastal and inland halo-tolerant Poaceae and Chenopodiaceae: A case study for increased sample size. PLoS ONE 2017, 12, e0185311. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xie, W.; Li, X.; Shi, S.; Guo, Z. Establishing community-wide DNA barcode references for conserving mangrove forests in China. BMC Plant Biol. 2021, 21, 571. [Google Scholar] [CrossRef] [PubMed]
- Aubriot, X.; Lowry, P.P., II; Cruaud, C.; Couloux, A.; Haevermans, T. DNA barcoding in a biodiversity hot spot: Potential value for the identification of Malagasy Euphorbia L. listed in CITES Appendices I and II. Mole. Ecol. Resour. 2013, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ng, W.L.; Mahat, M.N.; Nazre, M.; Mohamed, R. DNA barcoding of the endangered Aquilaria (Thymelaeaceae) and its application in species authentication of agarwood products traded in the market. PLoS ONE 2016, 11, e0154631. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Z.; Magnus, L.; Bengt, O. Silene Linnaeus. In Flora of China; Wu, C.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 6, pp. 66–100. [Google Scholar]
- Edwards, D.; Horn, A.; Taylor, D.; Savolainen, V.; Hawkins, J.A. DNA barcoding of a large genus, Aspalathus L. (Fabaceae). Taxon 2008, 57, 1317–1327. [Google Scholar] [CrossRef]
- Castro, C.; Hernandez, A.; Alvarado, L.; Flores, D. DNA barcodes in fig cultivars (Ficus carica L.) using ITS regions of ribosomal DNA, the psbA–trnH spacer and the matK coding sequence. Am. J. Plant Sci. 2015, 6, 95–102. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Kuzmina, M.L.; Newmaster, S.G.; Hollingsworth, P.M. DNA barcoding methods for land plants. In DNA Barcodes: Methods and Protocols; Kress, W.J., Erickson, D.L., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 223–252. [Google Scholar]
- Ma, X.Y.; Xie, C.X.; Liu, C.; Song, J.Y.; Yao, H.; Luo, K.; Zhu, Y.J.; Gao, T.; Pang, X.H.; Qian, J.; et al. Species identification of medicinal Pteridophytes by a DNA barcode marker, the chloroplast psbA–trnH intergenic region. Biol. Phar. Bull. 2010, 33, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Štorchová, H.; Olson, M.S. The architecture of the chloroplast psbA–trnH non-coding region in angiosperms. Plant Syst. Evol. 2007, 268, 235–256. [Google Scholar] [CrossRef]
- Dillenberger, M.S.; Kadereit, J.W. Maximum polyphyly: Multiple origins and delimitation with plesiomorphic characters require a new circumscription of Minuartia (Caryophyllaceae). Taxon 2014, 63, 64–88. [Google Scholar] [CrossRef]
- Moore, A.J.; Dillenberger, M.S. A conspectus of the genus Cherleria (Minuartia s.l., Caryophyllaceae). Willdenowia 2017, 47, 5–14. [Google Scholar] [CrossRef]
- Lu, D.; Morton, J.K. Cerastium Linnaeus. In Flora of China; Wu, C.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 6, pp. 31–37. [Google Scholar]
- Hong, S.P.; Han, M.J.; Kim, K.J. Systematic significance of seed coat morphology in Silene L. s. str. (Sileneae-Caryophyllaceae) from Korea. J. Plant Biol. 1999, 42, 146–150. [Google Scholar] [CrossRef]
- Onozaki, T. Dianthus. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 349–381. [Google Scholar]
- Adams, L.G.; West, J.G.; Cowley, K.J. Revision of Spergularia (Caryophyllaceae) in Australia. Aust. Syst. Bot. 2008, 21, 251–270. [Google Scholar] [CrossRef]
- Alonso, M.Á.; Crespo, M.B.; Martínez-Azorín, M.; Mucina, L. Taxonomic identity and evolutionary relationships of South African taxa related to the Spergularia media group (Caryophyllaceae). Plant Syst. Evol. 2021, 307, 24. [Google Scholar] [CrossRef]
- Kolodziej, B.; Okon, S.; Nucia, A.; Ociepa, T.; Luchowska, K.; Sugier, D.; Gevrenova, R.; Henry, M. Morphological, chemical, and genetic diversity of Gypsophila L. (Caryophyllaceae) species and their potential use in the pharmaceutical industry. Turk. J. Botany 2018, 42, 257–270. [Google Scholar]
- Muir, G.; Schlötterer, C. Limitations to the Phylogenetic Use of ITS Sequences in Closely Related Species and Populations—A Case Study in Quercus Petraea (Matt.) Liebl. Available online: http://webdoc.sub.gwdg.de/ebook/y/1999/whichmarker/m11/Chap11.htm (accessed on 7 February 2023).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Fay, M.F.; Swensen, S.M.; Chase, M.W. Taxonomic affinities of Medusagyne oppositifolia (Medusagynaceae). Kew Bull. 1997, 52, 111–120. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef]
- Tate, J.A. Systematics and Evolution of Tarasa (Malvaceae): An Enigmatic Andean Polyploid Genus. Ph.D. Dissertation, The University of Texas at Austin, Austin, TX, USA, 2002. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP: Phylogenetic Analysis Using Parsimony, Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Desper, R.; Gascuel, O. Fast and accurate phylogeny reconstruction algorithms based on the minimum-evolution principle. J. Comput. Biol. 2002, 9, 687–705. [Google Scholar] [CrossRef]
- Meier, R.; Kwong, S.; Vaidya, G.; Ng, P.K.L. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]


| rbcL | matK | psbA–trnH | ITS | Total | |
|---|---|---|---|---|---|
| Sequence length (bp) | 689 | 746–782 | 160–404 | 606–637 | - |
| Aligned length (bp) | 689 | 784 | 600 | 672 | 2747 |
| G + C ratio (%) | 43.0–44.3 | 29.6–33.4 | 21.1–33.3 | 50.7–63.2 | - |
| Number of variable sites (% variable sites) | 124 (18.0) | 431 (55.0) | 398 (66.3) | 393 (58.3) | 1346 (49.0) |
| Number of informative sites (% variable sites) | 115 (16.7) | 411 (52.4) | 371 (61.8) | 381 (56.5) | 1278 (46.5) |
| Region | Intraspecific K2P Distance | Interspecific K2P Distance | ||||
|---|---|---|---|---|---|---|
| Min. | Mean | Max. | Min. | Mean | Max. | |
| rbcL | 0 | 0.0004 | 0.0313 | 0 | 0.0291 | 0.0576 |
| matK | 0 | 0.0019 | 0.1296 | 0 | 0.1140 | 0.1754 |
| psbA–trnH | 0 | 0.0078 | 0.4690 | 0 | 0.3245 | 0.7045 |
| ITS | 0 | 0.0037 | 0.2478 | 0 | 0.2259 | 0.3531 |
| rbcL + matK | 0 | 0.0012 | 0.0817 | 0 | 0.0726 | 0.1126 |
| rbcL + psbA–trnH | 0 | 0.0022 | 0.1284 | 0 | 0.0888 | 0.1888 |
| rbcL + ITS | 0 | 0.0019 | 0.1246 | 0 | 0.1141 | 0.1657 |
| matK + psbA–trnH | 0 | 0.0033 | 0.2015 | 0 | 0.1548 | 0.2599 |
| matK + ITS | 0 | 0.0008 | 0.0072 | 0 | 0.1609 | 0.2340 |
| psbA–trnH + ITS | 0 | 0.0048 | 0.3077 | 0 | 0.2502 | 0.3754 |
| rbcL + matK + psbA–trnH | 0 | 0.0021 | 0.1289 | 0 | 0.1003 | 0.1658 |
| rbcL + matK + ITS | 0 | 0.0019 | 0.1265 | 0 | 0.1140 | 0.1622 |
| rbcL + psbA–trnH + ITS | 0 | 0.0028 | 0.1724 | 0 | 0.1405 | 0.2113 |
| matK + psbA–trnH + ITS | 0 | 0.0011 | 0.0110 | 0 | 0.1809 | 0.2666 |
| rbcL + matK + psbA–trnH + ITS | 0 | 0.0008 | 0.0077 | 0 | 0.1314 | 0.1936 |
| Region | Phylogenetic Analysis | Best Close Match | |||
|---|---|---|---|---|---|
| Correct | Ambiguous | Incorrect | No Match | ||
| rbcL | 44.74% | 84 (39.06%) | 121 (56.27%) | 10 (4.65%) | 0 (0.00%) |
| matK | 56.58% | 115 (53.48%) | 74 (34.41%) | 25 (11.62%) | 1 (0.46%) |
| psbA–trnH | 59.21% | 139 (64.65%) | 50 (23.25%) | 23 (10.69%) | 3 (1.39%) |
| ITS | 76.32% | 149 (69.30%) | 48 (22.32%) | 16 (7.44%) | 2 (0.93%) |
| ITS + matK | 73.68% | 160 (74.41%) | 34 (15.81%) | 20 (9.30%) | 1 (0.46%) |
| ITS + psbA–trnH | 76.32% | 164 (76.27%) | 18 (8.37%) | 30 (13.95%) | 3 (1.39%) |
| ITS + rbcL | 77.63% | 152 (70.69%) | 45 (20.93%) | 17 (7.90%) | 1 (0.46%) |
| matK + psbA–trnH | 61.84% | 141 (65.58%) | 43 (20.00%) | 28 (13.02%) | 3 (1.39%) |
| rbcL + matK | 60.53% | 124 (57.67%) | 64 (29.76%) | 27 (12.55%) | 0 (0.00%) |
| rbcL + psbA–trnH | 61.84% | 141 (65.58%) | 44 (20.46%) | 28 (13.02%) | 2 (0.93%) |
| ITS + matK + psbA–trnH | 73.68% | 165 (76.74%) | 20 (9.30%) | 28 (13.02%) | 2 (0.93%) |
| ITS + rbcL + matK | 73.68% | 163 (75.81%) | 28 (13.02%) | 23 (10.69%) | 1 (0.46%) |
| ITS + rbcL + psbA–trnH | 76.32% | 164 (76.27%) | 17 (7.90%) | 32 (14.88%) | 2 (0.93%) |
| rbcL + matK + psbA–trnH | 59.21% | 146 (67.9%) | 32 (14.88%) | 36 (16.74%) | 1 (0.46%) |
| ITS + rbcL + matK + psbA–trnH | 75.00% | 165 (76.74%) | 18 (8.37%) | 31 (14.41%) | 1 (0.46%) |
| Region | Primer Name | Primer Sequence (′5-′3) | Reference |
|---|---|---|---|
| ITS | ITS18S-A | CCTTMTCATYTAGAGGAAGGAG | Muir & Schlötterer [69] |
| ITS4 | TCCTCCGCTTATTGATATGC | White et al. [70] | |
| rbcL | rbcL 1F | ATGTCACCACAAACAGAAAC | Fay et al. [71] |
| rbcL 724R | TCGCATGTACCTGCAGTAGC | ||
| matK | matK_3F | CGTACAGTACTTTTGTGTTTACGAG | Kim K.J. (unpublished work) |
| matK_1R | ACCCAGTCCATCTGGAAATCTTGGTTC | ||
| psbA–trnH | psbA_sang | GTTATGCATGAACGTAATGCTC | Sang et al. [72] |
| trnH_tate | CGCGCATGGTGGATTCACAATCC | Tate [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.-P.; Sim, S.; Park, J.-W.; Choi, J.-E.; Yoon, J.; Lim, C.E.; Kim, M.-H. Identification of the Plant Family Caryophyllaceae in Korea Using DNA Barcoding. Plants 2023, 12, 2060. https://doi.org/10.3390/plants12102060
Jin D-P, Sim S, Park J-W, Choi J-E, Yoon J, Lim CE, Kim M-H. Identification of the Plant Family Caryophyllaceae in Korea Using DNA Barcoding. Plants. 2023; 12(10):2060. https://doi.org/10.3390/plants12102060
Chicago/Turabian StyleJin, Dong-Pil, Sunhee Sim, Jong-Won Park, Ji-Eun Choi, Jiwon Yoon, Chae Eun Lim, and Min-Ha Kim. 2023. "Identification of the Plant Family Caryophyllaceae in Korea Using DNA Barcoding" Plants 12, no. 10: 2060. https://doi.org/10.3390/plants12102060






