Influence of Planting Density on Sweet Potato Storage Root Formation by Regulating Carbohydrate and Lignin Metabolism
Abstract
:1. Introduction
2. Results
2.1. Sweet Potato Storage Root Yields, Yield Components, and Appearance Quality
2.2. Commercial Storage Root Characteristics
2.3. Storage Root Traits at Closure Period
2.4. Effect on Root Development System and Potential Storage Root Traits
2.5. Effect on Dry Biomass Accumulation and Allocation
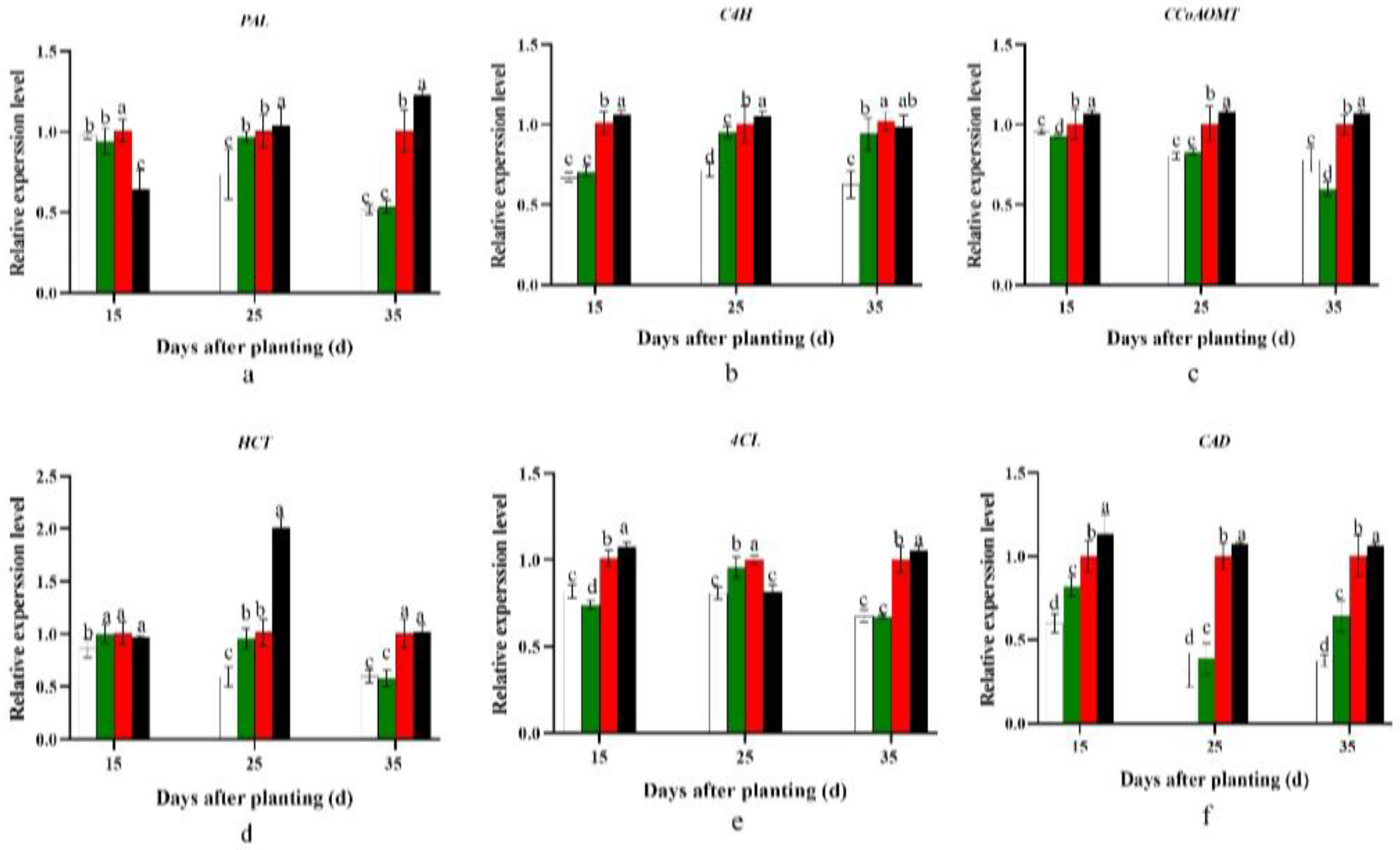
2.6. Influence on Expression of Genes Associated with Carbohydrate and Lignin Metabolism
2.7. Effect on Carbohydrate and Lignin Content
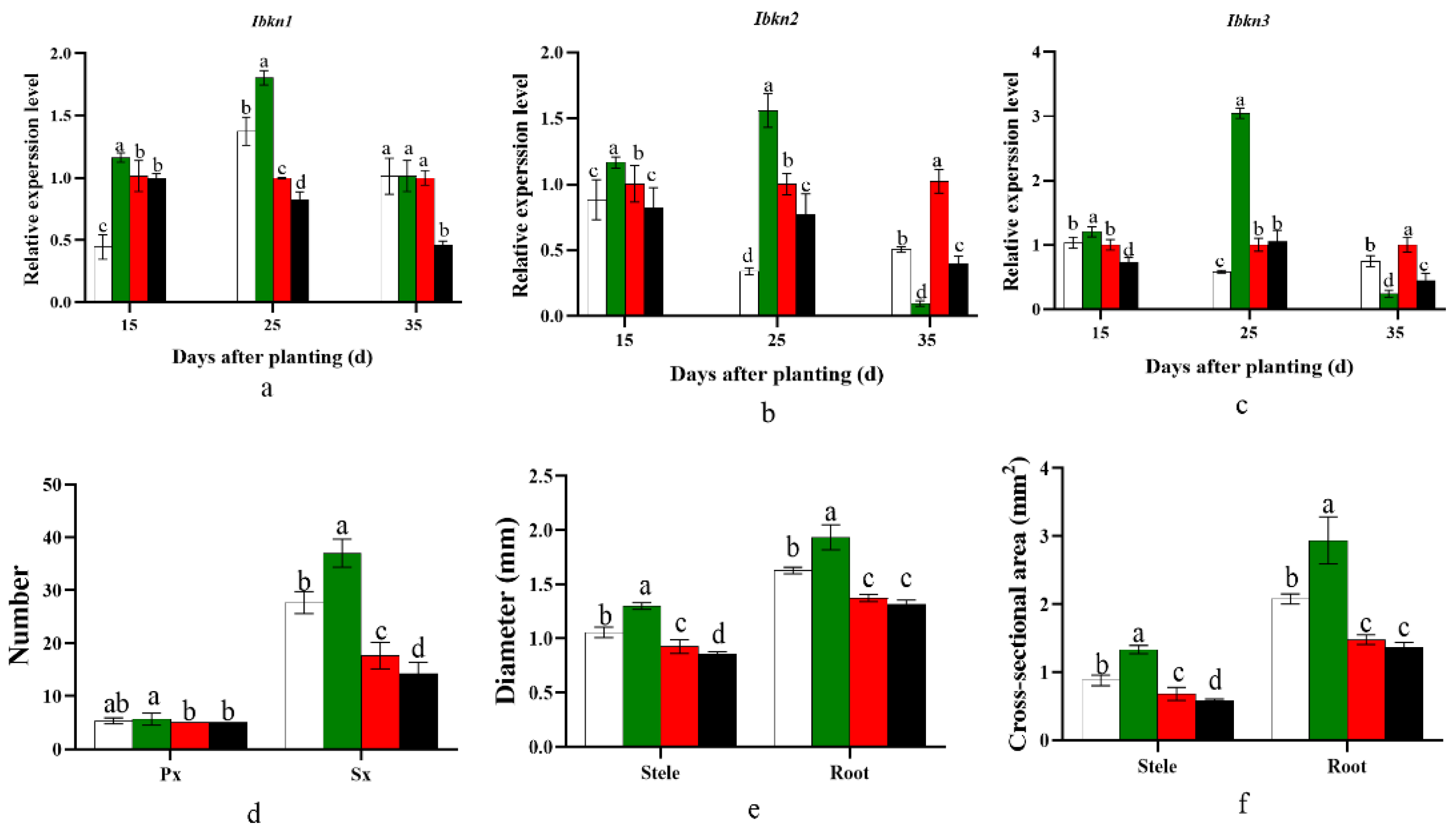
2.8. Influence on Genes Invloved in Cambium Development and Potential Storage Root Anatomy
2.9. Correlation Analysis of Storage Root Number Per Plant, Fresh Weight, and Yield with Relative Gene Expression
3. Discussion
3.1. Effect of Planting Density on Carbohydrate and Lignin Metabolism and Cambium Cell Development
3.2. Effect of Planting Density on Plant Dry Matter Dynamic and Root Development
3.3. Effect of Planting Density on Storage Root Yield, Components, and Commercial Characteristics
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Experimental Design
4.2.2. Sampling and Measurements
- Meanwhile, several parameters were obtained as follows:
- Root/shoot ratio = root dry weight/shoot dry weight × 100%;
- Ratio of root dry biomass allocation = root dry weight/total plant dry biomass × 100%;
- Ratio of shoot dry biomass allocation = shoot dry biomass/total plant dry biomass × 100%;
- Potential SR weight ratio = potential storage fresh weight/total root fresh weight × 100%.
4.2.3. Carbohydrate Content Determination
4.2.4. Lignin Content Determination
4.2.5. Root Anatomical Observation and Histochemical Analysis
4.2.6. Real-Time Quantitative PCR Performance
4.2.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Karan, Y.B.; Şanli, Ö. The assessment of yield and quality traits of sweet potato (Ipomoea batatas L.) genotypes in middle Black Sea region, Turkey. PLoS ONE 2021, 16, e0257703. [Google Scholar] [CrossRef] [PubMed]
- Rukundo, P.; Shimelis, H.; Laing, M.; Gahakwa, D. Combining ability; maternal effects, and hertability of drought tolerance, yield and yield components in sweetpotato. Front. Plant Sci. 2017, 7, 1981. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.D.; Avula, R.Y.; Pecota, K.V.; Yencho, G.C. Sweetpotato production; processing; nutritional quality. Handb. Veg. Veg. Process. 2018, 2, 811–838. [Google Scholar]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin promotes sweetpotato root vascular lignification and reduces storage-root formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Dong, H.T.; Li, Y.; Henderson, C.; Brown, P.; Xu, C.Y. Optimum Nitrogen Application Promotes Sweetpotato Storage Root Initiation. Horticulturae 2022, 8, 710. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Wang, N.; Si, C.C. Glutamate promotes sweet potato storage root swelling by enhancing starch accumulation. Acta Physiol. Plant. 2020, 42, 58. [Google Scholar]
- Meng, Y.Y.; Wang, N.; Si, C.C. The Application of Nitrogen Source in Regulating Lignin Biosynthesis, Storage Root Development and Yield of Sweet Potato. Agronomy 2022, 12, 2317. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Han, R.; Wang, H.; Liu, W. Comparative analysis of anatomical structure, assimilate accumulation and gene expression in lignin and carbohydrate metabolism pathway during taproot thickening of taicai and pak choi. Sci. Hortic. 2022, 301, 111046. [Google Scholar] [CrossRef]
- Bilska-Kos, A.; Mytych, J.; Suski, S.; Magoń, J.; Ochodzki, P.; Zebrowski, J. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus× giganteus and Zea mays at low temperature. Planta 2020, 252, 23. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T. Proteomic dissection of endosperm starch granule associated proteins reveals a network coordinating starch biosynthesis and amino acid metabolism and glycolysis in rice endosperms. Front. Plant Sci. 2016, 7, 707. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “Fiber Gap” development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef]
- Du, F.; Liu, H.; Yin, X.; Zhao, Q.; Shi, C. Potassium-mediated regulation of sucrose metabolism and storage root formation in sweet potato. Arch. Agron. Soil Sci. 2021, 67, 703–713. [Google Scholar] [CrossRef]
- Si, C.; Shi, C.; Liu, H.; Zhan, X.; Liu, Y.; Wang, D.; Meng, D.; Tang, L. Influence of two nitrogen forms on hormone metabolism in potential storage roots and storage root number of sweetpotato. Crop Sci. 2018, 58, 2558–2568. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in sucrose cleavage pattern and rapid starch accumulation govern lily shoot-to-bulblet transition in vitro. Front. Plant Sci. 2021, 11, 564713. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Escamilla-Treviño, L.L.; Shen, H.; Hernandez, T.; Yin, Y.; Xu, Y.; Dixon, R.A. Early lignin pathway enzymes and routes to chlorogenic acid in switchgrass (Panicum virgatum L.). Plant Mol. Biol. 2014, 84, 565–576. [Google Scholar] [CrossRef]
- Xu, R.X.; Ni, R.; Gao, S.; Fu, J.; Xiong, R.L.; Zhu, T.T.; Liu, H.X.; Cheng, A.X. Molecular cloning and characterization of two distinct caffeoyl CoA O-methyltransferases (CCoAOMTs) from the liverwort Marchantia paleacea. Plant Sci. 2022, 314, 111102. [Google Scholar] [CrossRef]
- Chao, N.; Huang, S.; Kang, X.; Yidilisi, K.; Dai, M.; Liu, L. Systematic functional characterization of cinnam yl alcohol dehydrogenase family members revealed their functional divergence in lignin biosynthesis and stress responses in mulberry. Plant Physiol. Biochem. 2022, 186, 145–156. [Google Scholar] [CrossRef]
- Li, L.; Yang, K.; Wang, S.; Lou, Y.; Zhu, C.; Gao, Z. Genome-wide analysis of laccase genes in moso bamboo highlights PeLAC10 involved in lignin biosynthesis and in response to abiotic stresses. Plant Cell Rep. 2020, 39, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cai, Z.; Huang, J.; Wang, A.; Ntambiyukuri, A.; Chen, B.; Zheng, G.; Li, H.; Huang, Y.; Zhan, J.; et al. Transcriptomic analysis of tuberous root in two sweet potato varieties reveals the important genes and regulatory pathways in tuberous root development. BMC Genom. 2022, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, J.; Zhang, M.; Fan, W.; Firon, N.; Pattanaik, S.; Yuan, L.; Zhang, P. Altered phenylpropanoid metabolism in the maize Lc-expressed sweet potato (Ipomoea batatas) affects storage root development. Sci. Rep. 2016, 6, 18645. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Xia, T.; Wu, D.; Chen, F.; Mi, G. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Chai, Q.; Feng, F.; Zhao, C.; Yu, A. Interspecies interactions in relation to root distribution across the rooting profile in wheat-maize intercropping under different plant densities. Front. Plant Sci. 2018, 9, 483. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Xin, Y.; Chen, G.; Wu, Q.; Liang, X.; Zhai, Y. Effects of Planting Density on Root Spatial and Temporal Distribution and Yield of Winter Wheat. Agronomy 2022, 12, 3014. [Google Scholar] [CrossRef]
- Muñoz-Parra, E.; Pelagio-Flores, R.; Raya-González, J.; Salmerón-Barrera, G.; Ruiz-Herrera, L.F.; Valencia-Cantero, E.; López-Bucio, J. Plant–plant interactions influence developmental phase transitions, grain productivity and root system architecture in Arabidopsis via auxin and PFT1/MED25 signalling. Plant Cell Environ. 2017, 40, 1887–1899. [Google Scholar] [CrossRef]
- Sukumaran, S.; Reynolds, M.P.; Lopes, M.S.; Crossa, J. Genome-wide association study for adaptation to agronomic plant density: A component of high yield potential in spring wheat. Crop Sci. 2015, 55, 2609–2619. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, B.; Wang, B.; Wang, Q.; Dong, S.; Li, A.; Hou, F.; Duan, W.; Zhang, L. Effects of Planting Density on Yield and Source-sink Characteristics of Sweet Potato [Lpomoea batatas (L.) Lam]. Agric. Sci. Technol. 2015, 16, 1628–1642. [Google Scholar]
- Firon, N.; LaBonte, D.; Villordon, A.; Kfir, Y.; Solis, J.; Lapis, E.; Perlman, T.S.; Doron-Faigenboim, A.; Hetzroni, A.; Althan, L.; et al. Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genom. 2013, 14, 460. [Google Scholar] [CrossRef]
- Si, C.; Shi, C.; Liu, H.; Zhan, X.; Liu, Y. Effects of nitrogen forms on carbohydrate metabolism and storage-root formation of sweet potato. J. Plant Nutr. Soil Sci. 2018, 181, 419–428. [Google Scholar] [CrossRef]
- Singh, V.; Zemach, H.; Shabtai, S.; Aloni, R.; Yang, J.; Zhang, P.; Sergeeva, L.; Ligterink, W.; Firon, N. Proximal and distal parts of sweetpotato adventitious roots display differences in root architecture, lignin, and starch metabolism and their developmental fates. Front. Plant Sci. 2021, 11, 609923. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hui, K.; Guo, Z.; Li, Y.; Fan, X. Cellulose and lignin contents are negatively correlated with starch accumulation, and their correlation characteristics vary across cassava varieties. J. Plant Growth Regul. 2023, 42, 658–669. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Tang, Y.; Min, Y.; Wang, D.; Ma, X.; Li, H.; Li, J.; Chen, Y.; Chen, S.; et al. Understanding the changes of phenylpropanoid metabolism and lignin accumulation in wounded cassava root during postharvest storage. Sci. Hortic. 2023, 310, 111765. [Google Scholar] [CrossRef]
- Townsley, B.T.; Sinha, N.R.; Kang, J. KNOX1 genes regulate lignin deposition and composition in monocots and dicots. Front. Plant Sci. 2013, 4, 121. [Google Scholar] [CrossRef]
- Tanaka, M. Recent progress in molecular studies on storage root formation in sweetpotato (Ipomoea batatas). Jpn. Agric. Res. Q. 2016, 50, 293–299. [Google Scholar] [CrossRef]
- Ravi, V.; Chakrabarti, S.K.; Makeshkumar, T.; Saravanan, R. Molecular regulation of storage root formation and development in sweet potato. In Horticultural Reviews: Volume 42; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 157–208. [Google Scholar]
- Si, C.C.; Liang, Q.G.; Liu, H.J.; Wang, N.; Kumar, S.; Chen, Y.L.; Zhu, G.P. Response Mechanism of Endogenous Hormones of Potential Storage Root to Phosphorus and Its Relationship with Yield and Appearance Quality of Sweetpotato. Front. Plant Sci. 2022, 13, 872422. [Google Scholar] [CrossRef]
- Villordon, A.Q.; Clark, C.A. Variation in virus symptom development and root architecture attributes at the onset of storage root initiation in ‘Beauregard’sweetpotato plants grown with or without nitrogen. PLoS ONE 2014, 9, e107384. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Liu, W. High nitrogen application rate and planting density reduce wheat grain yield by reducing filling rate of inferior grain in middle spikelets. Crop J. 2021, 9, 412–426. [Google Scholar] [CrossRef]
- Severini, A.D.; Borrás, L.; Westgate, M.E.; Cirilo, A.G. Kernel number and kernel weight determination in dent and popcorn maize. Field Crops Res. 2011, 120, 360–369. [Google Scholar] [CrossRef]
- Villordon, A.; LaBonte, D.; Firon, N.; Carey, E. Variation in nitrogen rate and local availability alter root architecture attributes at the onset of storage root initiation in ‘Beauregard’sweetpotato. HortScience 2013, 48, 808–815. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef] [PubMed]



| Years | Cultivar | Treatment † | SR Diameter (mm) | L/D Ratio | Average SR Weight (g) | CV (%) | Average SR Number Per Plant | Yield (kg·hm−2) | Yield Increment (%) ‡ |
|---|---|---|---|---|---|---|---|---|---|
| 2021 (Haikou) | YS-25 | D15 | 36.95 b | 2.7 | 104.56 d | 5.64 | 3.33 c | 29,017.72 b | 5.45 |
| D20 | 38.75 a | 2.5 | 111.13 c | 1.77 | 4.67 a | 33,030.38 a | 19.73 | ||
| D25 | 39.39 a | 2.6 | 127.26 b | 11.87 | 4.33 ab | 27,565.56 c | - | ||
| D30 | 39.15 a | 3.0 | 133.97 ab | 5.49 | 4.33 ab | 24,154.95 e | −14.11 | ||
| PS-32 | D15 | 32.00 c | 2.6 | 103.94 d | 11.37 | 3.00 d | 25,968.37 d | 1.56 | |
| D20 | 34.09 bc | 2.5 | 113.57 c | 5.74 | 4.00 b | 28,401.60 bc | 10.93 | ||
| D25 | 39.09 a | 2.7 | 127.82 b | 6.76 | 4.00 b | 25,576.78 d | - | ||
| D30 | 38.56 a | 2.9 | 141.53 a | 7.47 | 3.67 bc | 21,628.44 f | −18.51 | ||
| ANOVA | |||||||||
| C | 7.22 * | - | 2.46 * | - | 2.78 ns | 10.10 ** | - | ||
| T | 5.21 ** | - | 3.43 * | - | 3.07 * | 11.39 *** | - | ||
| C × T | 1.66 ns | - | 0.93 ns | - | 0.41 ns | 0.47 ns | - | ||
| 2022 (Sanya) | YS-25 | D15 | 38.04 c | 2.6 | 88.50 d | 7.69 | 5.26 bc | 38,795.60 b | 3.46 |
| D20 | 39.81 b | 2.3 | 110.81c | 2.90 | 6.19 a | 42,883.33 a | 14.13 | ||
| D25 | 39.72 b | 2.4 | 131.66 b | 6.71 | 5.71b | 37,494.90 bc | - | ||
| D30 | 42.71 a | 2.7 | 170.61a | 7.64 | 5.01 c | 35,592.04 c | −5.34 | ||
| PS-32 | D15 | 36.68 c | 2.4 | 91.91 d | 5.00 | 4.67 d | 35,569.17 c | 9.87 | |
| D20 | 38.14 b | 2.3 | 99.40 cd | 2.06 | 5.76 b | 35,795.45 c | 10.18 | ||
| D25 | 38.97 b | 2.6 | 131.42 b | 4.40 | 4.93 c | 32,437.80 d | - | ||
| D30 | 40.47 b | 3.1 | 160.09 a | 6.61 | 4.83 cd | 32,197.00 d | −0.62 | ||
| ANOVA | |||||||||
| C | 3.95 ns | - | 1.65 ns | - | 9.27 ** | 10.63 ** | - | ||
| T | 3.14 * | - | 86.25 *** | - | 10.11 ** | 12.36 ** | - | ||
| C × T | 8.36 ns | - | 1.12 ns | - | 0.61 ns | 0.56 * | - | ||
| Years | Cultivars | Treatment † | Commercial SR Weight Per Plant (g) | Commercial SR Number Per Plant | Large SR Number | Medium SR Number | Small SR Number | Commercial SR Yield (kg·hm−2) | Increment (%) ‡ |
|---|---|---|---|---|---|---|---|---|---|
| 2021 (Haikou) | YS-25 | D15 | 122.61 b | 2.33 d | 0.33 c | 1.67 c | 0.33 a | 19,558.23 b | −0.51 |
| D20 | 129.91 b | 3.00 b | 1.00 b | 2.00 b | - | 24,367.17 a | 23.35 | ||
| D25 | 158.45 a | 2.33 d | 1.33 a | 1.00 e | - | 19,697.51 b | - | ||
| D30 | 162.41 a | 2.67 c | 1.33 a | 1.33 d | - | 16,998.00 c | −16.16 | ||
| PS-32 | D15 | 115.20 c | 2.00 e | 1.00 b | 1.00 e | - | 19,202.65 b | 43.28 | |
| D20 | 119.26 c | 3.33 a | 1.00 b | 2.33 a | - | 22,368.40 a | 66.42 | ||
| D25 | 124.90 b | 2.33 d | 1.00 b | 1.33 d | - | 13,377.52 d | - | ||
| D30 | 133.31 b | 2.33 d | 1.33 a | 0.67 f | 0.33 a | 12,952.40 d | −3.24 | ||
| ANOVA | |||||||||
| C | 13.67 ** | 0.17 ns | 0.00 ns | 0.80 ns | 0.25 ns | 4.61 ns | - | ||
| T | 8.33 ** | 4.61 * | 0.67 ns | 2.93 ns | 2.92 ns | 5.35 * | - | ||
| C × T | 5.21 ns | 0.61 ns | 1.33 ns | 7.20 ** | 1.58 ns | 0.40 ns | - | ||
| 2022 (Sanya) | YS-25 | D15 | 98.89 bc | 4.00 c | - | 2.00 c | 2.00 a | 33,272.02 a | 29.60 |
| D20 | 117.89 bc | 4.67 b | 0.67 b | 3.00 a | 1.00 c | 34,342.08 a | 33.46 | ||
| D25 | 136.87 b | 4.00 c | 0.33 c | 2.00 c | 1.67 b | 25,676.50 de | - | ||
| D30 | 201.50 a | 3.00 e | 1.00 a | 1.33 d | 0.67 d | 25,198.06 e | −1.95 | ||
| PS-32 | D15 | 92.96 c | 3.67 cd | - | 1.67 c | 2.00 a | 28,412.07 c | 5.97 | |
| D20 | 102.05 bc | 5.00 a | 0.33 c | 3.00 a | 1.67 b | 31,899.79 b | 19.02 | ||
| D25 | 135.20 b | 4.00 c | 0.33 c | 2.67 b | 1.00 c | 26,851.42 d | - | ||
| D30 | 191.99 a | 3.33 d | 1.00 a | 2.00 c | 0.33 e | 26,533.47 d | −1.13 | ||
| ANOVA | |||||||||
| C | 0.88 ns | 0.40 ns | 0.25 ns | 3.00 ns | 0.25 ns | 0.13 ns | - | ||
| T | 26.02 *** | 7.07 ** | 6.25 ** | 17.2 *** | 13.58 *** | 7.23 ** | - | ||
| C × T | 0.12 ns | 0.13 ns | 0.25 ns | 3.00 ns | 2.92 ns | 0.41 ns | - | ||
| Years | Cultivar | Treatment † | SR Weight (g) | SR Number Per Plant | Young SR Number (2 < Φ < 5 mm) | Developing SR Number (5 < Φ < 20 mm) | Mature SR Number (Φ > 20 mm) | SR Diameter (mm) |
|---|---|---|---|---|---|---|---|---|
| 2021 (Haikou) | YS-25 | D15 | 1.35 f | 4.67 b | 2.33 a | 2.33 c | - | 5.14 d |
| D20 | 2.60 c | 5.67 a | 1.67 b | 4.00 a | - | 7.84 bc | ||
| D25 | 4.87 a | 4.33 c | 0.67 e | 3.67 ab | - | 8.07 bc | ||
| D30 | 4.70 a | 3.67 d | 0.67 e | 3.00 b | - | 10.62 a | ||
| PS-32 | D15 | 1.97 e | 3.67 d | 2.00 b | 1.67 e | - | 4.91 d | |
| D20 | 2.23 d | 4.33 c | 1.33 c | 2.33 c | 0.67a | 7.13 c | ||
| D25 | 3.15 bc | 4.00 bc | 2.00 b | 2.00 d | - | 8.71 b | ||
| D30 | 3.47 b | 3.33 e | 1.00 d | 2.33 c | - | 8.35 bc | ||
| ANOVA | ||||||||
| C | 3.30 ns | 8.33 ** | 1.80 ns | 39.20 *** | 4.00 ns | 0.79 ns | ||
| T | 10.38 *** | 4.56 ** | 8.73 ** | 6.93 ** | 4.00 * | 7.02 ** | ||
| C × T | 1.88 ns | 8.00 ns | 4.47 * | 4.00 * | 4.00 * | 0.73 ns | ||
| 2022 (Sanya) | YS-25 | D15 | 14.27 e | 5.00 c | 0.33 c | 3.67 c | 1.00 f | 13.00 e |
| D20 | 16.98 d | 7.00 a | 0.67 b | 4.33 b | 2.00 c | 15.10 de | ||
| D25 | 24.60 b | 5.00 cd | - | 2.67 e | 2.33b | 23.47 b | ||
| D30 | 34.08 a | 4.00 e | - | 1.00 f | 3.33 a | 27.54 a | ||
| PS-32 | D15 | 7.40 f | 5.67 b | 2.00 a | 2.67 e | 1.00 f | 13.38 e | |
| D20 | 13.35 e | 6.67a | - | 5.33 a | 1.33 d | 16.45 d | ||
| D25 | 19.34 c | 4.67 d | - | 3.00 d | 1.67 cd | 16.65 d | ||
| D30 | 20.53 c | 3.67 f | - | 1.33 f | 2.33 b | 19.28 c | ||
| ANOVA | ||||||||
| C | 4.56 ** | 10.36 * | 1.33 *** | 1.50 ns | 12.80 ** | 1.23 ns | ||
| T | 3.33 * | 8.33 ** | 1.20 *** | 49.50 *** | 17.33 *** | 2.05 ** | ||
| C × T | 2.69 ns | 6.23 ns | 1.33 *** | 4.61 * | 1.60 ns | 2.45 ns | ||
| DAP(d) | Cultivar | Treatment † | Adventitious Root Number | Total Root Fresh Weight (g) | Potential SR Diameter (mm) | Potential SR Weight (g) | Potential SR Weight Ratio (%) |
|---|---|---|---|---|---|---|---|
| 15 d | YS-25 | D15 | 13.60 b | 2.84 e | 1.03 c | 1.75 b | 61.62 a |
| D20 | 15.20 b | 3.44 c | 1.16 b | 1.96 ab | 56.97 ab | ||
| D25 | 15.20 b | 3.69 b | 1.12 b | 1.89 ab | 51.22 b | ||
| D30 | 13.80 b | 4.29 a | 1.05 c | 2.07 a | 48.25 b | ||
| PS-32 | D15 | 17.60 a | 2.95 de | 1.29 ab | 1.64 c | 55.59 a | |
| D20 | 19.00 a | 3.10 d | 1.41 a | 1.84 ab | 59.35 a | ||
| D25 | 18.20 a | 3.64 bc | 1.25 ab | 1.75 b | 48.08 b | ||
| D30 | 18.00 a | 3.80 b | 1.26 ab | 1.76 b | 46.31 b | ||
| ANOVA | |||||||
| C | 66.57 *** | 12.59 *** | 35.50 *** | 20.55 *** | 6.30 * | ||
| T | 2.28 ns | 81.94 *** | 2.86 * | 7.38 ** | 14.15 *** | ||
| C × T | 0.33 ns | 6.21 ** | 0.67 ns | 1.62 ns | 1.13 ns | ||
| 25 d | YS-25 | D15 | 14.00 bc | 6.11 b | 1.64 c | 3.96 a | 64.32 a |
| D20 | 15.00 b | 6.59 a | 2.39 a | 3.84 ab | 58.27 b | ||
| D25 | 13.20 c | 6.74 a | 2.00 b | 3.21 c | 47.62 c | ||
| D30 | 14.00 bc | 6.94 a | 1.96 b | 2.75 d | 39.62 d | ||
| PS-32 | D15 | 16.60 ab | 5.19 c | 1.59 c | 3.40 b | 65.51 a | |
| D20 | 17.40 a | 5.37 c | 2.20 a | 3.47 b | 64.61 a | ||
| D25 | 15.40 b | 5.41c | 2.19 a | 3.44 b | 63.58 a | ||
| D30 | 15.20 b | 6.18b | 2.19 a | 3.76 ab | 60.84 a | ||
| ANOVA | |||||||
| C | 55.98 *** | 112.96 *** | 1.86 ns | 0.61 ns | 0.92 ns | ||
| T | 5.14 ** | 14.29 *** | 16.12 *** | 4.61 ** | 8.51 *** | ||
| C × T | 0.38 ns | 12.04 *** | 3.94 * | 1.81 ns | 5.60 *** | ||
| D15 | - | 28.90 cd | 8.77 c | 4.32 c | 14.94 c | ||
| D20 | - | 36.94 b | 8.81 c | 8.21 a | 22.22 a | ||
| YS-25 | D25 | - | 39.83 ab | 10.75 b | 8.69 a | 21.81 a | |
| D30 | - | 39.79 ab | 9.66 b | 7.38 ab | 18.54 b | ||
| 35 d | D15 | - | 21.19 d | 9.27 bc | 4.39 c | 20.72 a | |
| D20 | - | 31.53 c | 10.67 b | 6.77 b | 21.47 a | ||
| PS-32 | D25 | - | 35.80 b | 11.13 ab | 7.83 ab | 21.87 a | |
| D30 | - | 41.26 a | 13.43 a | 7.70 ab | 18.66 b | ||
| ANOVA | |||||||
| C | - | 2.12 ns | 14.91 *** | 2.25 ns | 0.64 ns | ||
| T | - | 12.43 *** | 6.92 *** | 49.01 *** | 20.86 *** | ||
| C × T | - | 2.96 ** | 2.67 ns | 1.64 ns | 6.21 ** | ||
| DAP (d) | Cultivar | Treatment † | Total Plant Dry Weight (g) | Root Dry Weight (g) | Shoot Dry Weight (g) | Root Dry Weight Allocation (%) | Shoot Dry Weight Allocation (%) | Root/Shoot Ratio |
|---|---|---|---|---|---|---|---|---|
| 15 d | YS-25 | D15 | 1.98 c | 0.21 d | 1.77 d | 10.61bc | 89.39 a | 0.12 b |
| D20 | 2.40 b | 0.32 a | 2.08 bc | 13.33 a | 86.67 a | 0.15 a | ||
| D25 | 2.55 a | 0.24 c | 2.31 a | 9.41 cd | 90.59 a | 0.10 c | ||
| D30 | 2.56 a | 0.28 b | 2.28 a | 9.94 c | 89.06 a | 0.11 c | ||
| PS-32 | D15 | 2.04 c | 0.23 c | 1.86 cd | 8.83 d | 91.17 a | 0.10 c | |
| D20 | 2.29 bc | 0.31 a | 1.98 c | 13.54 a | 86.46 a | 0.16 a | ||
| D25 | 2.42 b | 0.27 b | 2.15 b | 11.16 b | 88.84 a | 0.13 b | ||
| D30 | 2.25 bc | 0.28 b | 2.05 bc | 8.89 d | 91.11 a | 0.10 c | ||
| ANOVA | ||||||||
| C | 4.87 * | 2.15 ns | 6.59 * | 10.39 ** | 8.75 ** | 11.37 ** | ||
| T | 3.43 * | 27.47 *** | 2.02 ns | 21.20 *** | 22.02 *** | 19.72 *** | ||
| C × T | 19.17 *** | 1.73 ns | 20.93 ** | 10.25 *** | 10.49 *** | 8.52 *** | ||
| 25 d | YS-25 | D15 | 4.23 c | 0.62 b | 3.61 c | 14.80 ab | 85.20 a | 0.17 b |
| D20 | 4.49 c | 0.71 a | 3.78 c | 15.82 a | 84.12 a | 0.19 a | ||
| D25 | 5.06 b | 0.67 a | 4.39 b | 13.00 c | 87.00 a | 0.15 c | ||
| D30 | 5.67 b | 0.63 b | 5.04 a | 11.20 d | 88.20 a | 0.12 d | ||
| PS-32 | D15 | 4.14 c | 0.58 c | 3.56 c | 14.00 b | 86.00 a | 0.16 bc | |
| D20 | 4.27 c | 0.61 b | 3.66 c | 14.20 b | 85.80 a | 0.16 bc | ||
| D25 | 4.51 c | 0.62 b | 3.89 c | 11.00 d | 89.00 a | 0.16 bc | ||
| D30 | 6.20 a | 0.69 a | 5.51 a | 11.20 d | 88.80 a | 0.12 d | ||
| ANOVA | ||||||||
| C | 0.52 ns | 5.95 * | 0.20 ns | 0.64 ns | 0.64 ns | 1.29 ns | ||
| T | 47.21 ** | 4.64 ** | 46.85 *** | 20.81 *** | 20.81 *** | 21.56 *** | ||
| C × T | 3.96 * | 6.61 *** | 3.17 * | 2.29 ns | 2.29 ns | 1.56 ns | ||
| D15 | 13.44 e | 3.58 d | 9.88 d | 26.60 c | 73.40 b | 0.36 c | ||
| D20 | 18.23 c | 4.91 c | 13.32 c | 27.00 c | 73.00 b | 0.37 c | ||
| YS-25 | D25 | 23.01 b | 5.04 c | 17.96 b | 22.00 d | 78.00 a | 0.28 d | |
| D30 | 25.30a | 5.34 bc | 19.95 a | 21.20 d | 78.80 a | 0.27 d | ||
| 35 d | D15 | 15.34 d | 4.76 c | 10.08 d | 31.00 b | 69.00 c | 0.47 b | |
| D20 | 15.69 d | 5.95 b | 9.74 d | 37.60 a | 62.40 d | 0.61 a | ||
| PS-32 | D25 | 23.62 b | 7.08 a | 16.53 b | 30.00 b | 70.00 bc | 0.43 bc | |
| D30 | 24.34 ab | 6.84 a | 17.49 b | 28.20 c | 71.80 bc | 0.39 c | ||
| ANOVA | ||||||||
| C | 0.51 ns | 72.60 *** | 24.68 *** | 83.33 *** | 83.33 *** | 28.50 *** | ||
| T | 208.66 *** | 28.38 *** | 150.35 *** | 16.80 *** | 16.80 *** | 5.09 ** | ||
| C × T | 7.75 ** | 1.69 ns | 7.11 *** | 2.44 ns | 2.44 ns | 1.67 ns | ||
| SuSy | SPS | SSS | AGPase | GBSS | SBE | PAL | 4CL | |
|---|---|---|---|---|---|---|---|---|
| SRN † | 0.44 | 0.61 *‡ | 0.165 | 0.31 | 0.72 ** | 0.30 | 0.27 | 0.33 |
| SRFW | −0.39 | −0.39 | −0.32 | −0.23 | −0.09 | −0.38 | −0.26 | −0.05 |
| Yield | 0.44 | 0.62 * | 0.43 | 0.29 | 0.33 | 0.47 | −0.11 | 0.00 |
| C4H | CCoAOMT | CAD | HCT | Ibkn1 | Ibkn2 | Ibkn3 | - | |
| SRN | −0.20 | 0.62 | 0.17 | −0.14 | 0.41 | 0.39 | 0.25 | - |
| SRFW | −0.21 | −0.41 | −0.04 | −0.03 | −0.35 | −0.10 | −0.27 | - |
| Yield | −0.24 | 0.16 | −0.43 | −0.25 | 0.51 | 0.08 | 0.32 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Chen, H.; Chang, H.; Liu, Y.; Wang, Q.; Wu, J.; Liu, Y.; Kumar, S.; Chen, Y.; Chen, Y.; et al. Influence of Planting Density on Sweet Potato Storage Root Formation by Regulating Carbohydrate and Lignin Metabolism. Plants 2023, 12, 2039. https://doi.org/10.3390/plants12102039
Liang Q, Chen H, Chang H, Liu Y, Wang Q, Wu J, Liu Y, Kumar S, Chen Y, Chen Y, et al. Influence of Planting Density on Sweet Potato Storage Root Formation by Regulating Carbohydrate and Lignin Metabolism. Plants. 2023; 12(10):2039. https://doi.org/10.3390/plants12102039
Chicago/Turabian StyleLiang, Qinggan, Hongrong Chen, Hailong Chang, Yi Liu, Qinnan Wang, Jiantao Wu, Yonghua Liu, Sunjeet Kumar, Yue Chen, Yanli Chen, and et al. 2023. "Influence of Planting Density on Sweet Potato Storage Root Formation by Regulating Carbohydrate and Lignin Metabolism" Plants 12, no. 10: 2039. https://doi.org/10.3390/plants12102039







