Analysis of MsTERT Gene Expression Profile in Alfalfa (Medicago sativa) Indicates Their Response to Abiotic Stress and Seed Aging
Abstract
:1. Introduction
2. Results
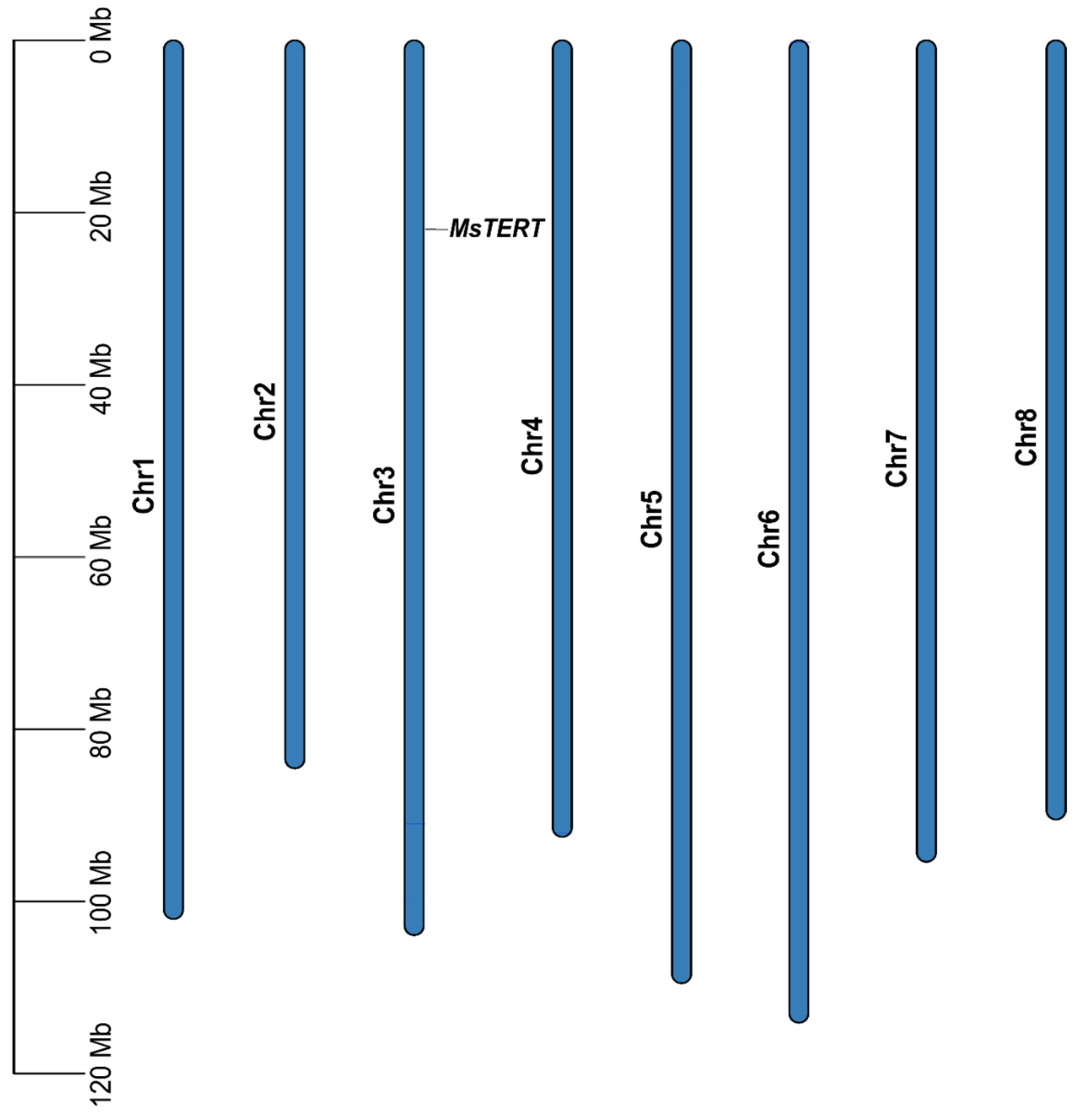
2.1. Identification and Chromosomal Mapping of MsTERT Gene
2.2. Physico-Chemical Properties of MsTERT Protein
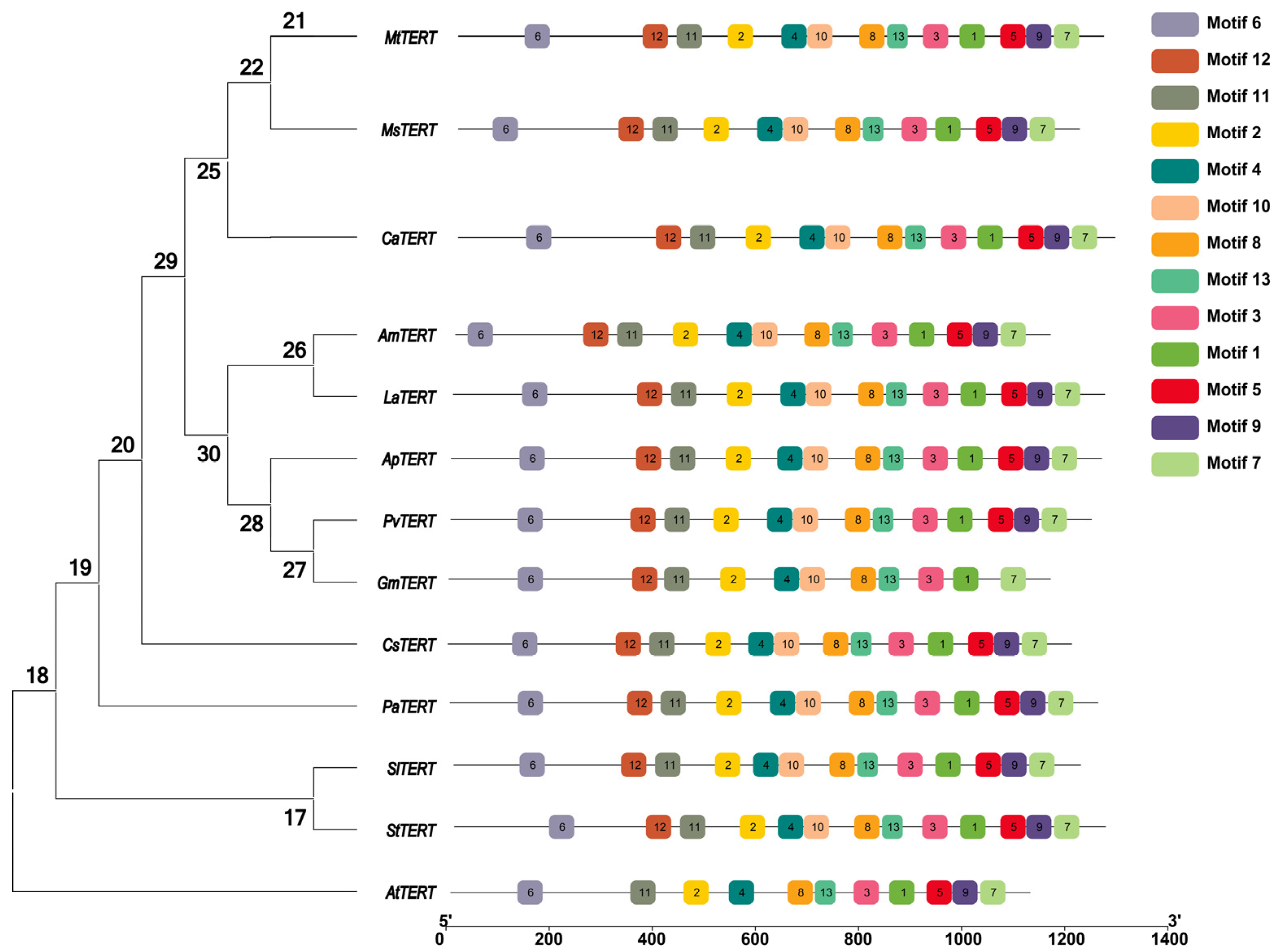
2.3. Phylogenetic and Motif Pattern Analysis of TERT Proteins
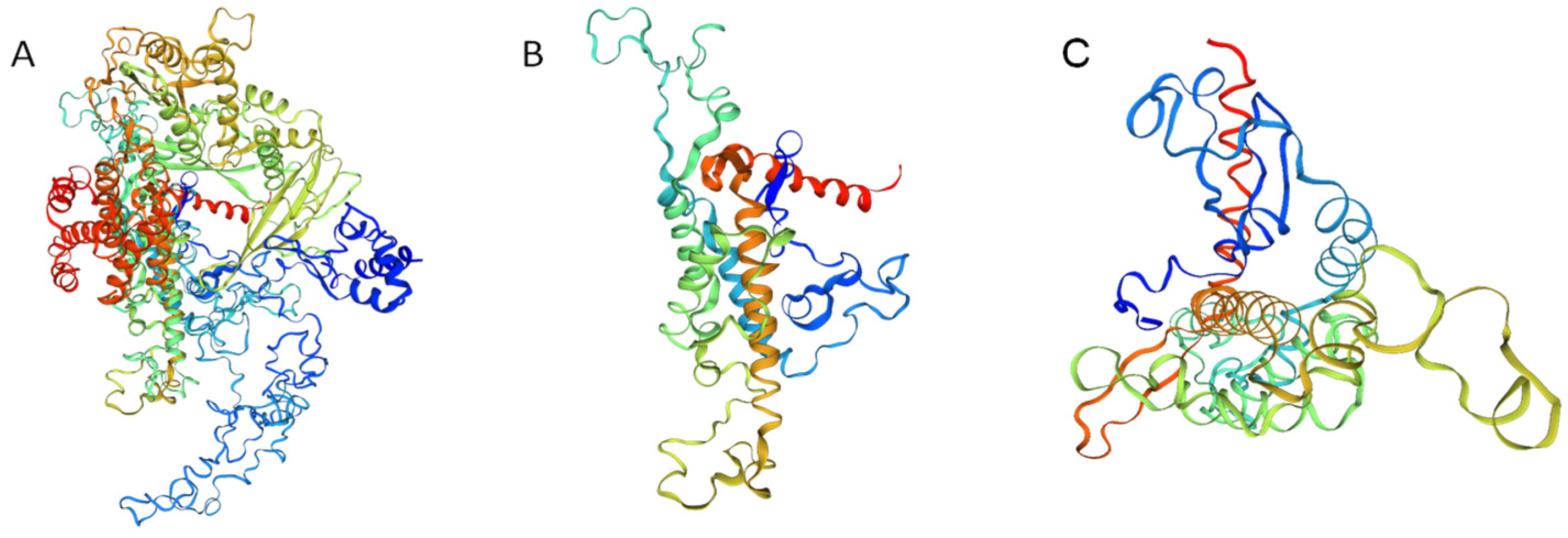
2.4. Structural Analysis of MsTERT
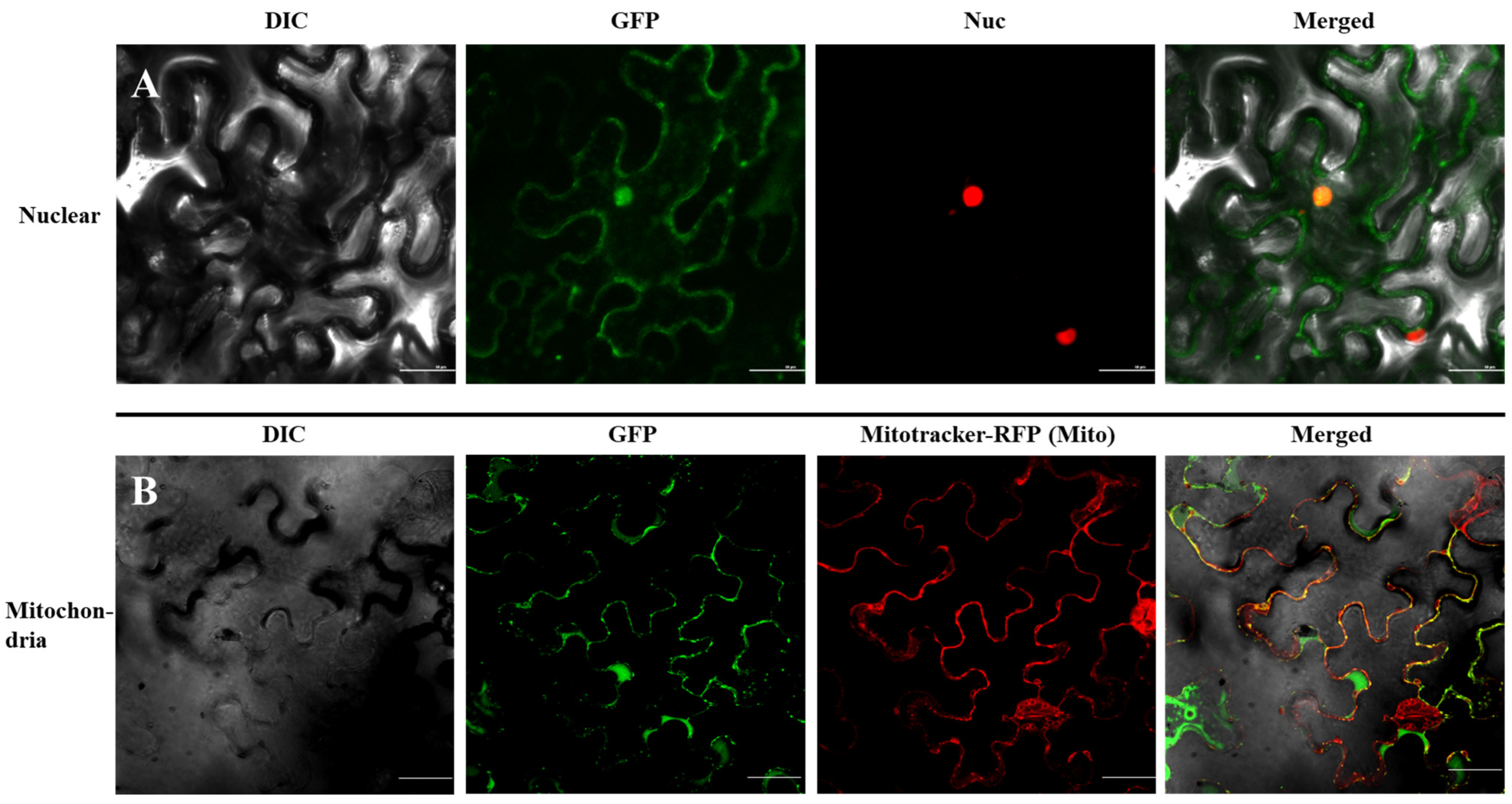
2.5. MsTERT–GFP Protein Was Localized in the Nucleus and Mitochondrion
2.6. Identification of Cis-Elements in the Promoter Region of MsTERT Genes
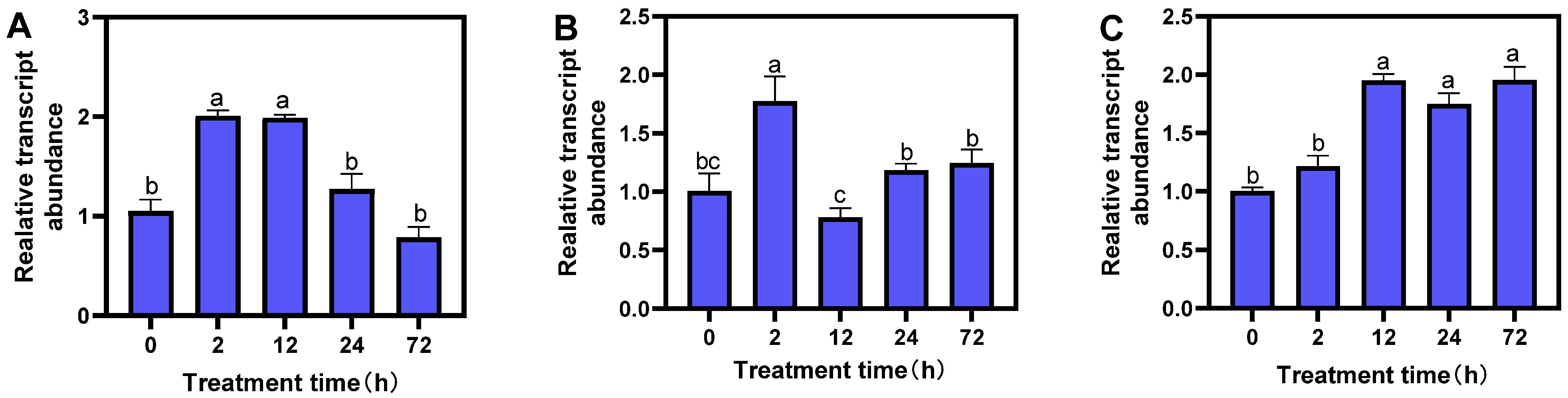
2.7. Expression Analysis of MsTERT Genes under Hormone Treatment
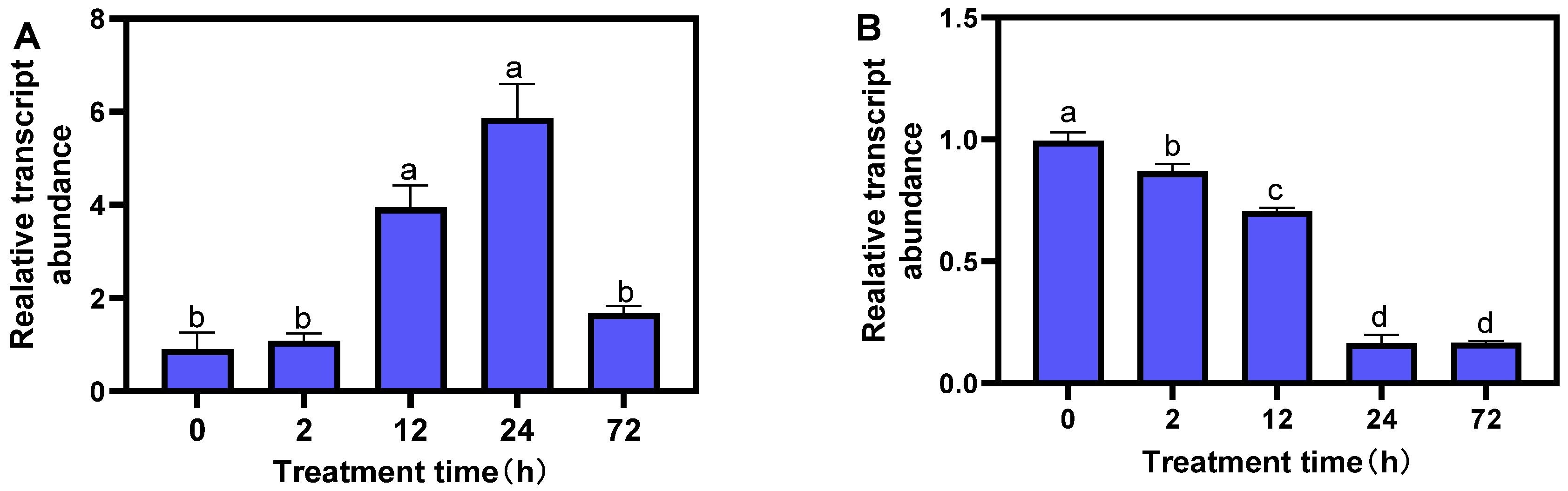
2.8. Response of MsTERT to Drought and Salt Stress
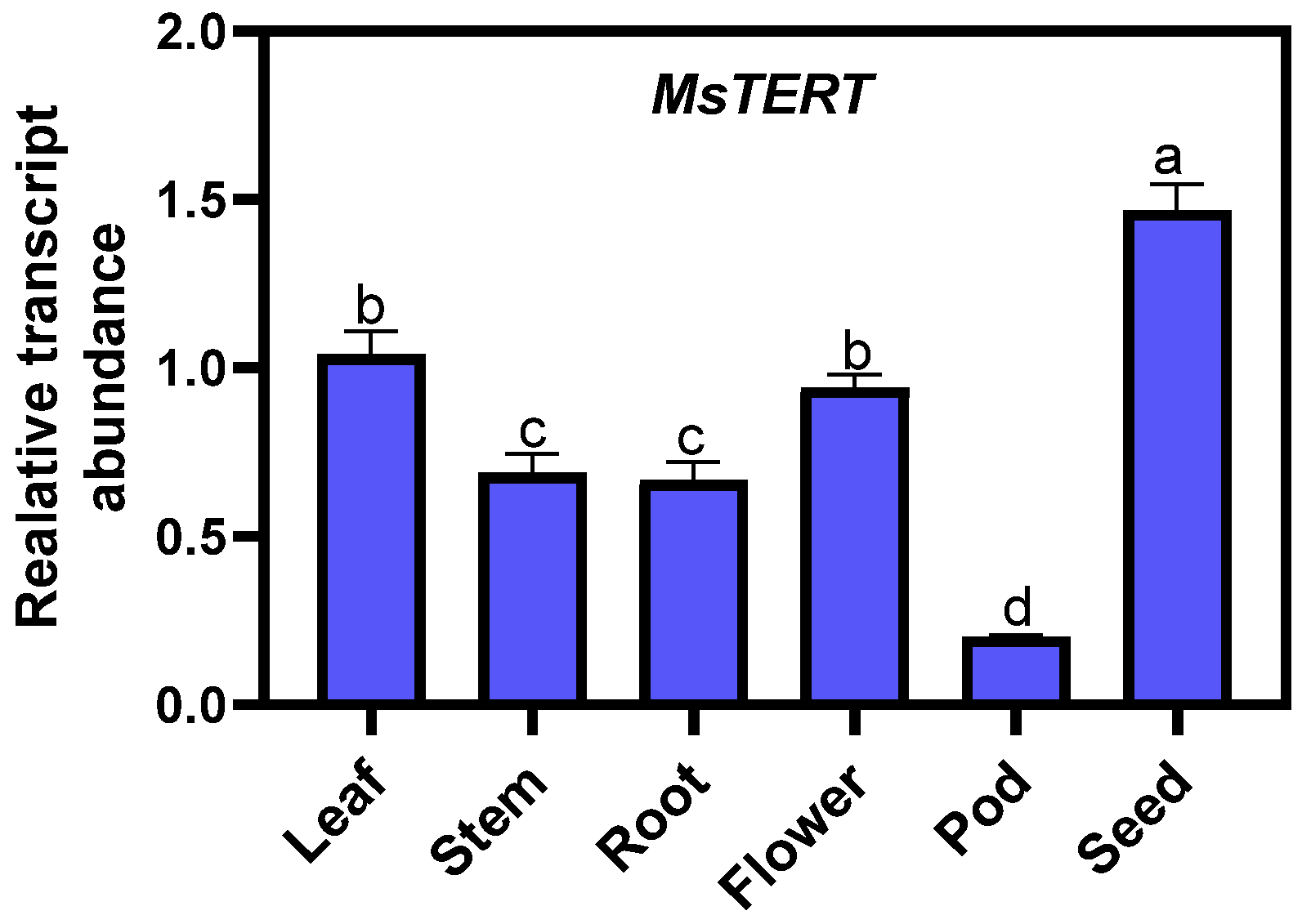
2.9. Tissue-Specific Expression Analysis of MsTERT Gene
2.10. Response to Seed Aging Stress of MsTERT
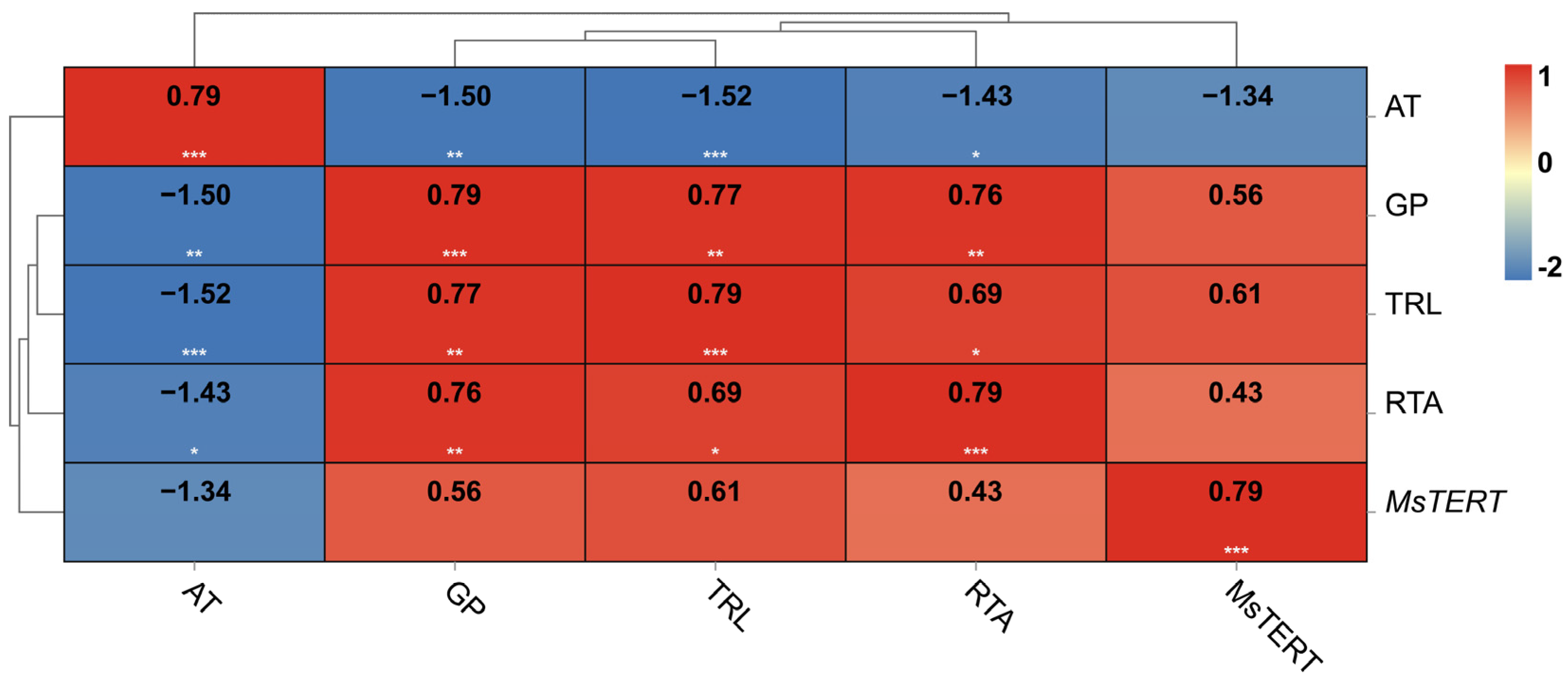
2.11. Correlation Analysis between the Five Indicators
3. Discussion
3.1. Identification of MsTERT Bioinformatics
3.2. MsTERT Response Patterns to Abiotic Stress and Hormone Treatments
3.3. Promising Indicators in Seed Germination and Seed Age Evaluation
4. Materials and Methods
4.1. Preparation of Seeds
4.2. Seed Aging Treatment and Germination Parameter Tests
4.3. Subcellular Localization Assay
4.4. Determination of Telomerase Activity and Telomere Length
4.5. Abiotic Stress and Hormone Treatments of Alfalfa Seeds
4.6. MsTERT Bioinformatics Analysis Method
4.7. qRT-PCR and Statistical Analyses of MsTERT Gene
4.8. Data Analysis and Figure Construction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Dong, X.; Tong, J. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity. Int. J. Biol. Macromol. 2013, 62, 387–396. [Google Scholar] [CrossRef]
- Burr, B.; Burr, F.A.; Matz, E.C.; Romero-Severson, J. Pinning down loose ends: Mapping telomeres and factors affecting their length. Plant Cell 1992, 4, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Blackburn, E.H. Cloning yeast telomeres on linear plasmid vectors. Cell 1982, 29, 245–255. [Google Scholar] [CrossRef]
- Mikhelson, V.M.; Gamaley, I.A. Telomere shortening: The main mechanism of natural and radiation aging. Biophysics 2010, 55, 848–853. [Google Scholar] [CrossRef]
- Chan, H.; Wang, Y.; Feigon, J. Progress in Human and Tetrahymena Telomerase Structure Determination. Annu. Rev. Biophys. 2017, 46, 199–225. [Google Scholar] [CrossRef]
- Shirgahi Talari, F.; Bagherzadeh, K.; Golestanian, S.; Jarstfer, M.; Amanlou, M. Potent Human Telomerase Inhibitors: Molecular Dynamic Simulations, Multiple Pharmacophore-Based Virtual Screening, and Biochemical Assays. J. Chem. Inf. Model. 2015, 55, 2596–2610. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G. Telomerase, mitochondria and oxidative stress. Exp. Gerontol. 2009, 44, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, K.; Liu, H.; Tamura, K.; Takahashi, H. Molecular cloning and characterization of AtTERT, a telomerase reverse transcriptase homolog in Arabidopsis thaliana. FEBS Lett. 1999, 457, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, K.; Tamura, K.; Takahashi, H. Characterization of Oryza sativa telomerase reverse transcriptase and possible role of its phosphorylation in the control of telomerase activity. Gene 2004, 342, 57–66. [Google Scholar] [CrossRef]
- Sun, L.C.; Yang, Y.; Yu, T.Q. Cloning and expression analysis of telomerase reverse transcriptase (AmTERT) gene from Ammopiptanthus mongolicus. Chin. J. Cell Biol. 2018, 40, 1088–1100. [Google Scholar]
- Correia, J.S. Molecular Mechanisms Associated to Thermotolerance in Plants. Ph.D. Thesis, University of Minho, Braga, Portugal, 2009. [Google Scholar]
- Teymouri, M.; Shahri, M.P.K.; Darvishzadeh, R. Salt-induced differences during the gene expression of telomerase enzyme in sunflower. Iran. J. Biotechnol. 2021, 19, e2579. [Google Scholar] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Doksani, Y.; de Lange, T. Telomere-internal double-strand breaks are repaired by homologous recombination and PARP1/Lig3-dependent end-joining. Cell Rep. 2016, 17, 1646–1656. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Sušac, L.; Chan, H.; Basu, R.; Zhou, Z.H.; Feigon, J. Structure of telomerase with telomeric DNA. Cell 2018, 173, 1179–1190. [Google Scholar] [CrossRef]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef]
- Yang, J.-H.; Sun, Y.-P.; Lu, Y.-N. Abiotic Stress Resistance of Escherichia coli Transformed with Arabidopsis thaliana AtTERT Gene. Biotechnol. Bull. 2022, 38, 1–9. [Google Scholar]
- Zhang, T.-C.; Schmitt, M.T.; Mumford, J.L. Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis 2003, 24, 1811–1817. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- TeKrony, D.M.; Egli, D.B. Relationship of seed vigor to crop yield: A review. Crop Sci. 1991, 31, 816–822. [Google Scholar] [CrossRef]
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Schlotz, N.; Schreiner, M.; Lamy, E. Short-term dietary intervention with cooked but not raw brassica leafy vegetables increases telomerase activity in CD8+ lymphocytes in a randomized human trial. Nutrients 2019, 11, 786. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Hudon, S.F.; Palencia Hurtado, E.; Beck, J.D.; Burden, S.J.; Bendixsen, D.P.; Callery, K.R.; Sorensen Forbey, J.; Waits, L.P.; Miller, R.A.; Nielsen, Ó.K. Primers to highly conserved elements optimized for qPCR--based telomere length measurement in vertebrates. Mol. Ecol. Resour. 2021, 21, 59–67. [Google Scholar] [CrossRef]
- Aronen, T.; Ryynänen, L. Variation in telomeric repeats of Scots pine (Pinus sylvestris L.). Tree Genet. Genomes 2012, 8, 267–275. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Wang, X.; Sun, M.; Song, R.; Mao, P.; Jia, S. Genome-wide identification of GRAS gene family and their responses to abiotic stress in Medicago sativa. Int. J. Mol. Sci. 2021, 22, 7729. [Google Scholar] [CrossRef]

| Regulatory Elements | Strand | Number | Sequence | Function |
|---|---|---|---|---|
| CAAT-box | + − | 20 | CAAAT CCAAT | Common cis-acting elements in promoter and enhancer regions |
| TATA-box | + − | 63 | ATATAA ATTATA TACAAAA TACATAAA TATA TATAA TATAAA TATAAAA TATAAAT TATACA TATATA TATATAA TATTTAAA | Core promoter elements around −30 of transcription start |
| LTR | + − | 3 | CCGAAA | Cis-acting element involved in low-temperature responsiveness |
| MBS | − | 1 | CAACTG | MYB binding site involved in drought-inducibility |
| ARE | + − | 3 | AAACCA | Cis-acting regulatory element essential for the anaerobic induction |
| TATC-box | + | 1 | TATCCCA | Cis-acting element involved in gibberellin-responsiveness |
| TGA | − | 1 | AACGAC | Auxin-responsive element |
| TCA | + | 2 | CCATCTTTTT | Cis-acting element involved in salicylic acid responsiveness |
| Gene | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|
| MsTERT | MsTERT-F | CAGGGTTGGAGATGATTA |
| MsTERT-R | TAGAGACTGATTGGAGGA | |
| Actin | Actin-F | CAAAAGATGGCAGATGCTGAGGAT |
| Actin-R | CATGACACCAGTATGAGAGGTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Ma, W.; Mao, P. Analysis of MsTERT Gene Expression Profile in Alfalfa (Medicago sativa) Indicates Their Response to Abiotic Stress and Seed Aging. Plants 2023, 12, 2036. https://doi.org/10.3390/plants12102036
Sun S, Ma W, Mao P. Analysis of MsTERT Gene Expression Profile in Alfalfa (Medicago sativa) Indicates Their Response to Abiotic Stress and Seed Aging. Plants. 2023; 12(10):2036. https://doi.org/10.3390/plants12102036
Chicago/Turabian StyleSun, Shoujiang, Wen Ma, and Peisheng Mao. 2023. "Analysis of MsTERT Gene Expression Profile in Alfalfa (Medicago sativa) Indicates Their Response to Abiotic Stress and Seed Aging" Plants 12, no. 10: 2036. https://doi.org/10.3390/plants12102036





