Functional Divergence Analysis of AGL6 Genes in Prunus mume
Abstract
:1. Introduction
2. Results
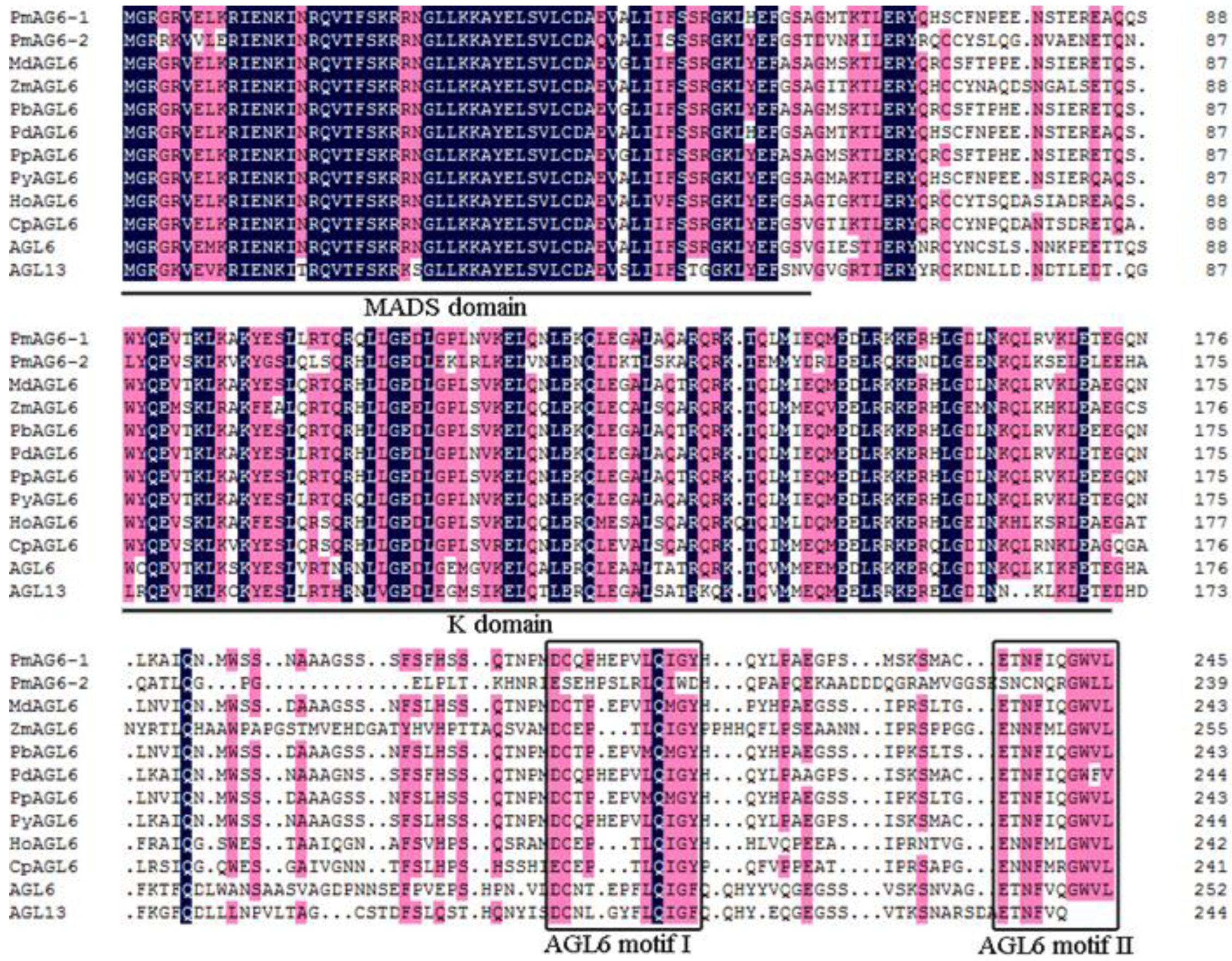
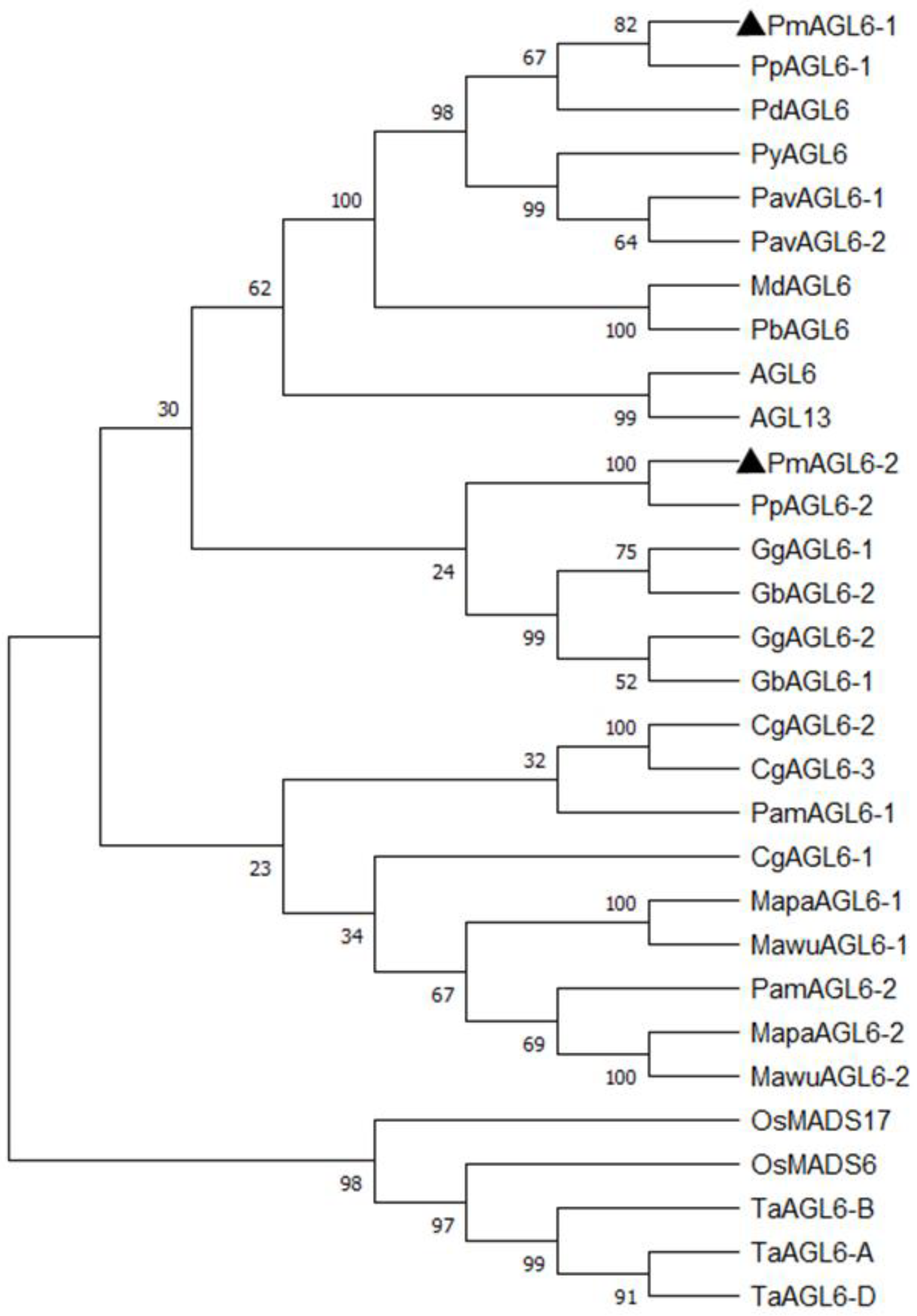
2.1. Cloning and Sequence Analysis of PmAGL6-1 and PmAGL6-2
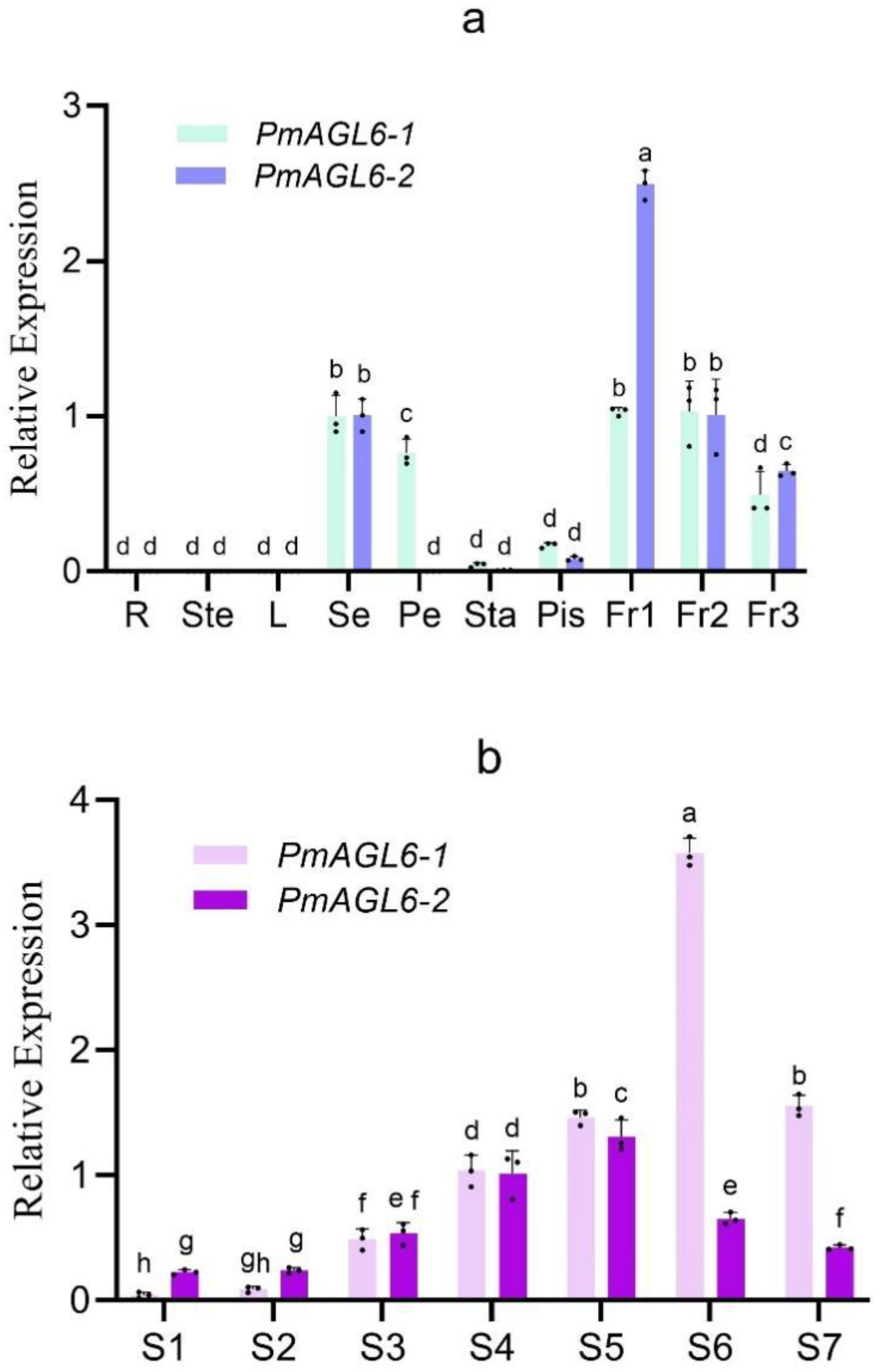
2.2. Expression Pattern Analysis of PmAGL6-1 and PmAGL6-2
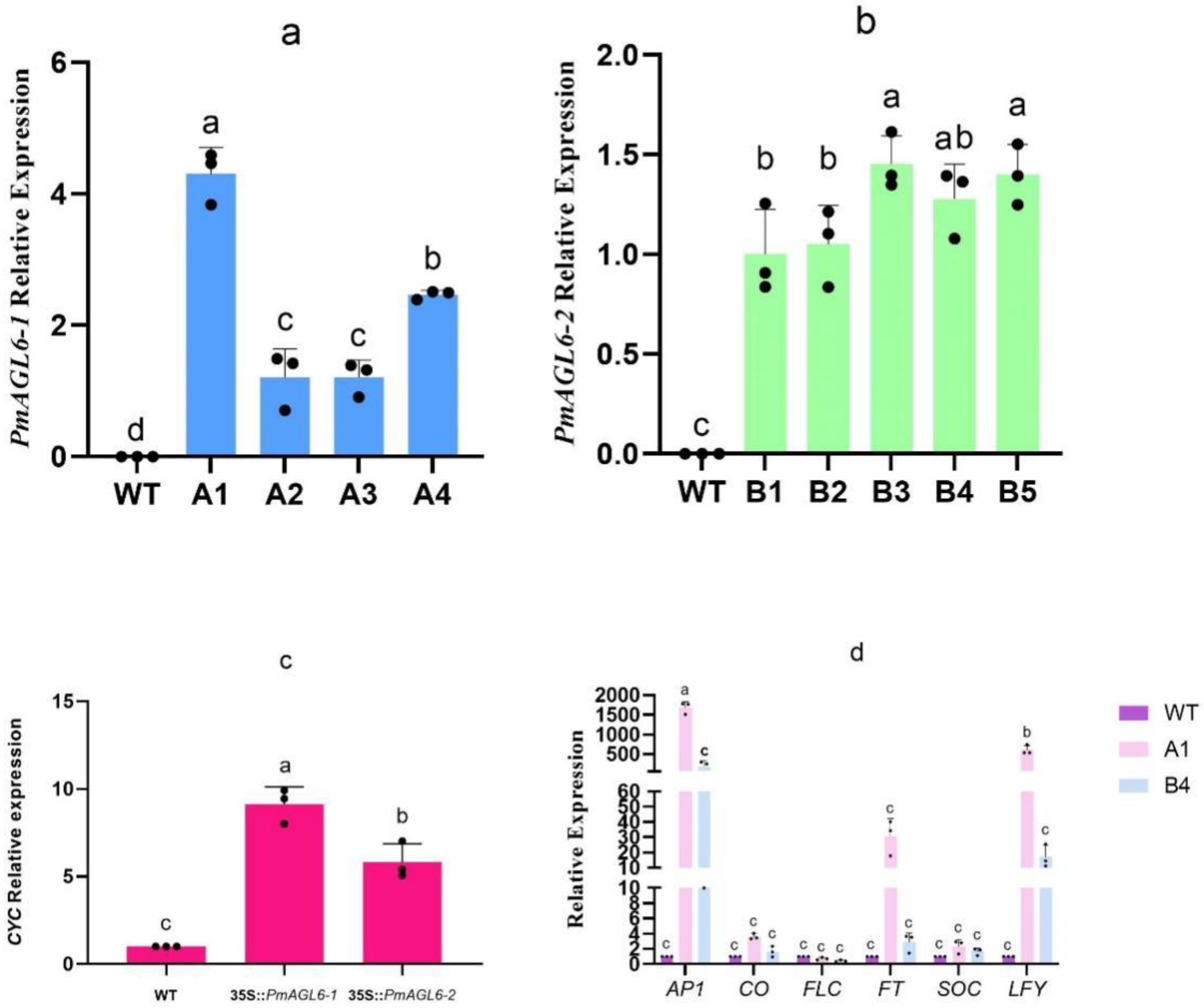
2.3. Ectopic Expression of PmAGL6-1 and PmAGL6-2 in Arabidopsis
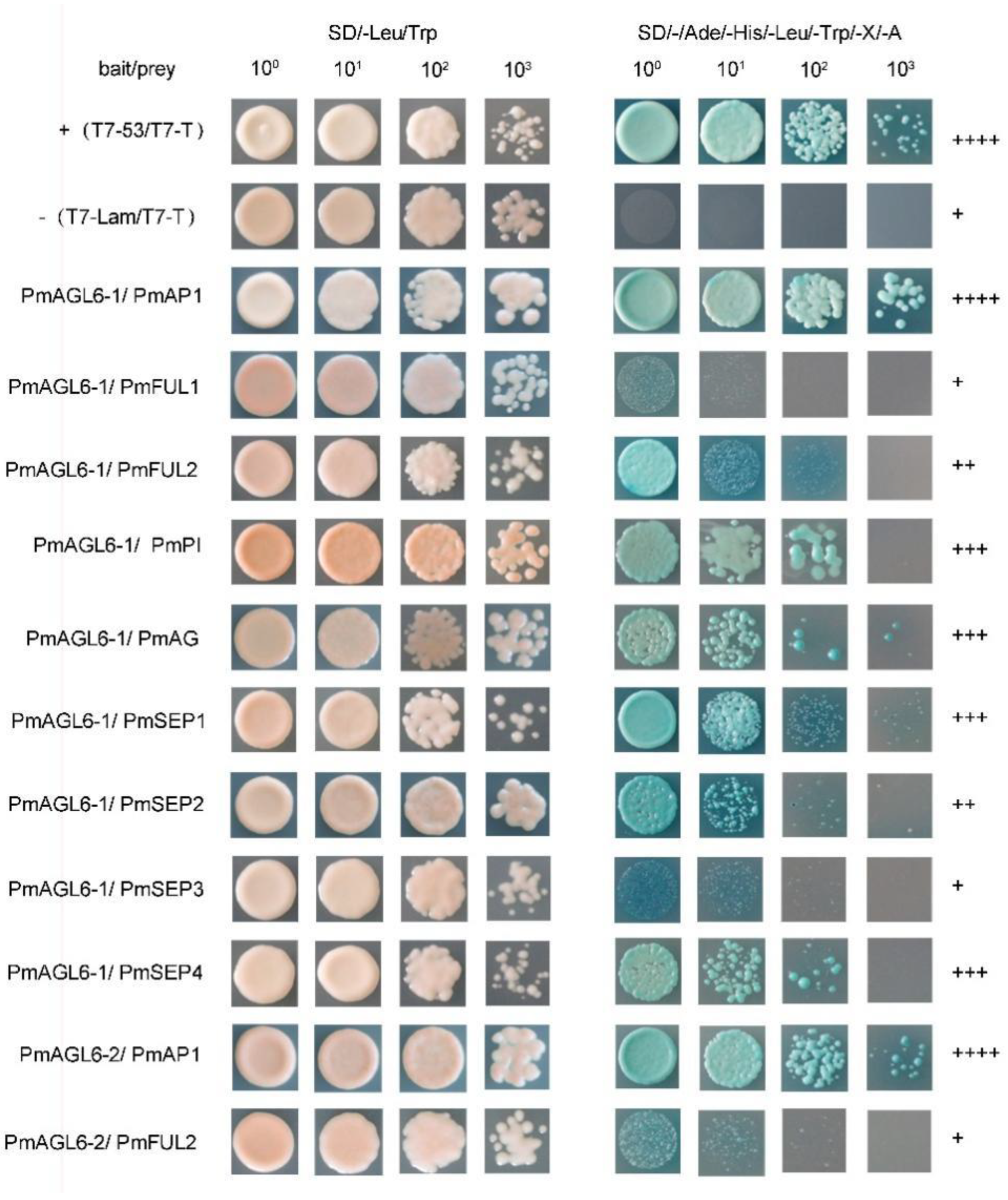
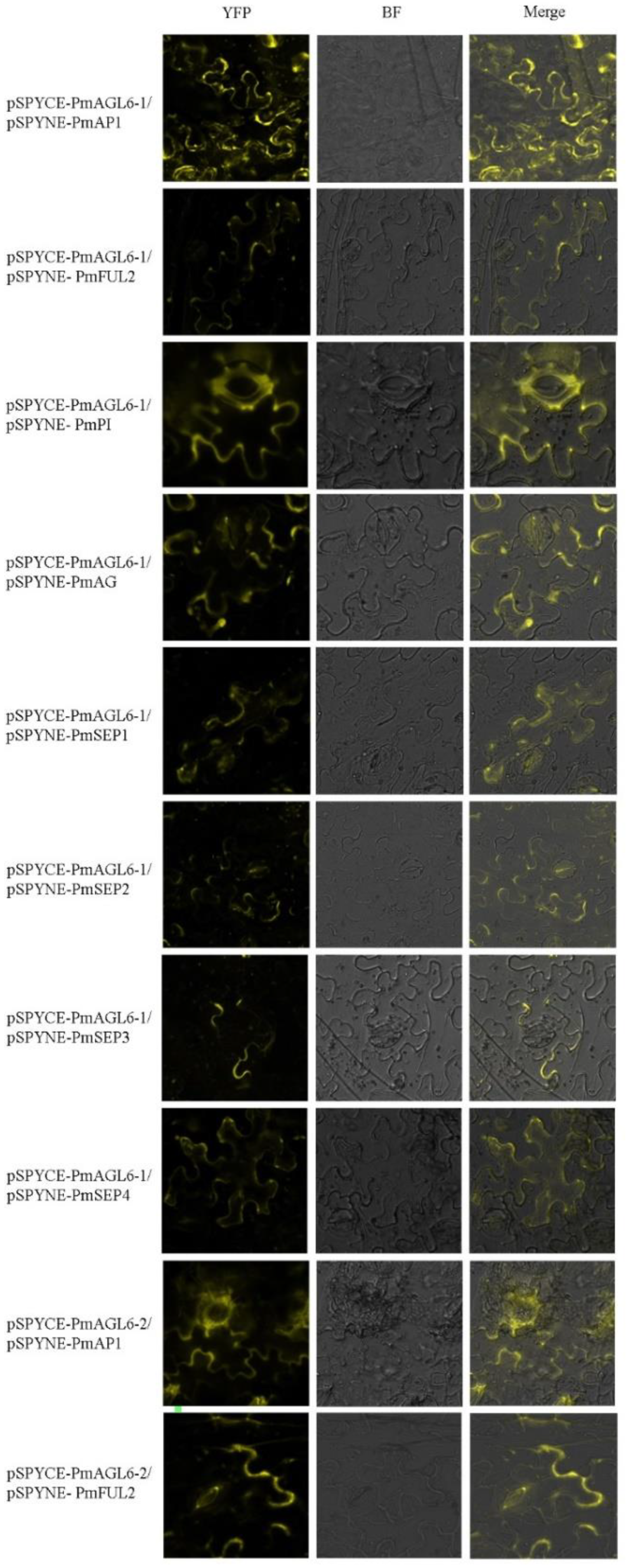
2.4. Protein–Protein Interactions among the Products of Two AGL6 Genes and Other Floral Organ Identity-Determining Genes in P. mume
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Screening of AGL6 Gene in Prunus mume
4.3. Extraction of Total RNA and Synthesis of First-Strand cDNA
4.4. Cloning of the Full-Length CDSs of PmAGL6-1 and PmAGL6-2
4.5. Sequence Alignment and Phylogenetic Analysis of PmAGL6-1 and PmAGL6-2
4.6. Real-Time Quantitative RT-PCR
4.7. Vector Construction and Arabidopsis Transformation
4.8. Yeast Two-Hybrid Assay
4.9. Bimolecular Fluorescence Complementation Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef] [Green Version]
- Theißen, G.; Saedler, H. Floral quartets. Nature 2001, 409, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Tandre, K.; Johanson, U.; Englund, M.; Engström, P. The MADS-box gene DAL1 is a potential mediator of the juvenile-to-adult transition in Norway spruce (Picea abies). Plant J. 2004, 40, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Katahata, S.-I.; Futamura, N.; Igasaki, T.; Shinohara, K. Functional analysis of SOC1-like and AGL6-like MADS-box genes of the gymnosperm Cryptomeria japonica. Tree Genet. Genomes 2014, 10, 317–327. [Google Scholar] [CrossRef]
- Koo, S.C.; Bracko, O.; Park, M.S.; Schwab, R.; Chun, H.J.; Park, K.M.; Seo, J.S.; Grbic, V.; Balasubramanian, S.; Schmid, M.; et al. Control of lateral organ development and flowering time by the Arabidopsis thaliana MADS-box Gene AGAMOUS-LIKE6. Plant J. 2010, 62, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Yeh, T.-J.; Huang, K.-Y.; Li, J.-Y.; Chen, H.-Y.; Yang, C.-H. AGAMOUS-LIKE13, a putative ancestor for the E functional genes, specifies male and female gametophyte morphogenesis. Plant J. 2014, 77, 1–15. [Google Scholar] [CrossRef]
- Hatsuda, Y.; Nishio, S.; Komori, S.; Nishiyama, M.; Kanahama, K.; Kanayama, Y. Relationship between MdMADS11 Gene Expression and Juvenility in Apple. J. Jpn. Soc. Hortic. Sci. 2011, 80, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Viaene, T.; Vekemans, D.; Becker, A.; Melzer, S.; Geuten, K. Expression divergence of the AGL6 MADS domain transcription factor lineage after a core eudicot duplication suggests functional diversification. BMC Plant Biol. 2010, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Su, C.L.; Chen, W.C.; Lee, A.Y.; Chen, C.Y.; Chang, Y.C.; Chao, Y.T.; Shih, M.C. A modified ABCDE model of flowering in orchids based on gene expression profiling studies of the moth orchid Phalaenopsis aphrodite. PLoS ONE 2013, 8, e80462. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fan, T.; Song, J.; Sun, W.; Xia, K.; Liao, J.; Zhang, M. Functional conservation and divergence of four ginger AP1/AGL9 MADS-box genes revealed by analysis of their expression and protein-protein interaction, and ectopic expression of AhFUL gene in Arabidopsis. PLoS ONE 2014, 9, e114134. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, W.; Dong, X.; Guo, W.; Shu, H. Ectopic expression of a hyacinth AGL6 homolog caused earlier flowering and homeotic conversion in Arabidopsis. Sci. China C Life Sci. 2007, 50, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Rijpkema, A.S.; Zethof, J.; Gerats, T.; Vandenbussche, M. The petunia AGL6 gene has a SEPALLATA-like function in floral patterning. Plant J. 2009, 60, 1–9. [Google Scholar] [CrossRef]
- Wang, B.-G.; Zhang, Q.; Wang, L.-G.; Duan, K.; Pan, A.-H.; Tang, X.-M.; Sui, S.-Z.; Li, M.-Y. The AGL6-like Gene CpAGL6, a Potential Regulator of Floral Time and Organ Identity in Wintersweet (Chimonanthus praecox). J. Plant Growth Regul. 2011, 30, 343–352. [Google Scholar] [CrossRef]
- Duan, Y.; Xing, Z.; Diao, Z.; Xu, W.; Li, S.; Du, X.; Wu, G.; Wang, C.; Lan, T.; Meng, Z.; et al. Characterization of Osmads6-5, a null allele, reveals that OsMADS6 is a critical regulator for early flower development in rice (Oryza sativa L.). Plant Mol. Biol. 2012, 80, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.E.; Bartling, L.; Whipple, C.; Hall, D.H.; Sakai, H.; Schmidt, R.; Hake, S. bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell 2009, 21, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.F.; Huang, C.H.; Chou, L.T.; Yang, C.H. Ectopic expression of an orchid (Oncidium Gower Ramsey) AGL6-like gene promotes flowering by activating flowering time genes in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.K.; Wu, X.; Lee, J.S.; Ahn, J.H. AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis. Plant J. 2011, 65, 62–76. [Google Scholar] [CrossRef]
- Duarte, J.M.; Cui, L.; Wall, P.K.; Zhang, Q.; Zhang, X.; Leebens-Mack, J.; Ma, H.; Altman, N.; de Pamphilis, C.W. Expression Pattern Shifts Following Duplication Indicative of Subfunctionalization and Neofunctionalization in Regulatory Genes of Arabidopsis. Mol. Biol. Evol. 2005, 23, 469–478. [Google Scholar] [CrossRef]
- Airoldi, C.; Davies, B. Gene Duplication and the Evolution of Plant MADS-box Transcription Factors. J. Genet. Genom. 2012, 39, 157–165. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Ali, Z.; Raza, Q.; Atif, R.M.; Aslam, U.; Ajmal, M.; Chung, G. Genetic and Molecular Control of Floral Organ Identity in Cereals. Int. J. Mol. Sci. 2019, 20, 2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchimoto, S.; Mayama, T.; van der Krol, A.; Ohtsubo, E. The whorl-specific action of a petunia class B floral homeotic gene. Genes Cells 2000, 5, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, R.Z.; Guo, J.J.; Liu, D.M.; Li, A.L.; Fan, R.C.; Mao, L.; Zhang, X.Q. Genome-wide analysis of the MADS-box gene family in Brachypodium distachyon. PLoS ONE 2014, 9, e84781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yockteng, R.; Almeida, A.M.; Morioka, K.; Alvarez-Buylla, E.R.; Specht, C.D. Molecular evolution and patterns of duplication in the SEP/AGL6-like lineage of the Zingiberales: A proposed mechanism for floral diversification. Mol. Biol. Evol. 2013, 30, 2401–2422. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Deng, S.; Chen, L.; Jia, Z.; Sang, Z.; Zhu, Z.; Ma, L.; Chen, F. Gene duplication led to divergence of expression patterns, protein-protein interaction patterns and floral development functions of AGL6-like genes in the basal angiosperm Magnolia wufengensis (Magnoliaceae). Tree Physiol. 2019, 39, 861–876. [Google Scholar] [CrossRef]
- Ohmori, S.; Kimizu, M.; Sugita, M.; Miyao, A.; Hirochika, H.; Uchida, E.; Nagato, Y.; Yoshida, H. MOSAIC FLORAL ORGANS1, an AGL6-Like MADS Box Gene, Regulates Floral Organ Identity and Meristem Fate in Rice. Plant Cell 2009, 21, 3008–3025. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Liang, W.; An, G.; Zhang, D. OsMADS6 Controls Flower Development by Activating Rice FACTOR OF DNA METHYLATION LIKE1. Plant Physiol. 2018, 177, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Liu, J.; Liang, W.; Dou, Y.; Fu, R.; Li, W.; Feng, C.; Gao, C.; Zhang, D.; Kang, Z.; et al. Wheat AGAMOUS LIKE 6 transcription factors function in stamen development by regulating the expression of Ta APETALA3. Development 2019, 146, dev177527. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Chen, Y.; Chen, L.; Fu, X.; Zhao, K.; Zhang, J.; Sun, C. B and E MADS-box genes determine the perianth formation in Cymbidium goeringii Rchb.f. Physiol. Plant 2018, 162, 353–369. [Google Scholar] [CrossRef]
- Liu, J.; Fu, X.; Dong, Y.; Lu, J.; Ren, M.; Zhou, N.; Wang, C. MIKC(C)-type MADS-box genes in Rosa chinensis: The remarkable expansion of ABCDE model genes and their roles in floral organogenesis. Hortic. Res. 2018, 5, 25. [Google Scholar] [CrossRef]
- Busch, A.W.U.; Zachgo, S. Flower symmetry evolution: Towards understanding the abominable mystery of angiosperm radiation. BioEssays 2009, 31, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Hileman, L.C. Trends in flower symmetry evolution revealed through phylogenetic and developmental genetic advances. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.D. Functional Analysis of MADS-box Genes Related to Floral Organ Development in Prunus Mume; Beijing Forestry University: Beijing, China, 2015. [Google Scholar]
- Song, G.Q.; Chen, Q. Overexpression of the MADS-box gene K-domain increases the yield potential of blueberry. Plant Sci. 2018, 276, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jack, T. Defining subdomains of the K domain important for protein–protein interactions of plant MADS proteins. Plant Mol. Biol. 2004, 55, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Chiu, Y.-F.; Wu, J.-W.; Yang, C.-H. Four Orchid (Oncidium Gower Ramsey) AP1/AGL9-like MADS Box Genes Show Novel Expression Patterns and Cause Different Effects on Floral Transition and Formation in Arabidopsis thaliana. Plant Cell Physiol. 2009, 50, 1425–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbesier, L.; Coupland, G. The quest for florigen: A review of recent progress. J. Exp. Bot. 2006, 57, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Irish, V.F. Gene networks controlling petal organogenesis. J. Exp. Bot. 2015, 67, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, K.; Melzer, R.; Theissen, G. MIKC-type MADS-domain proteins: Structural modularity, protein interactions and network evolution in land plants. Gene 2005, 347, 183–198. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef] [Green Version]
- Searle, I.; He, Y.; Turck, F.; Vincent, C.; Fornara, F.; Kröber, S.; Amasino, R.A.; Coupland, G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006, 20, 898–912. [Google Scholar] [CrossRef]
- Irish, V.F. The Arabidopsis petal: A model for plant organogenesis. Trends Plant Sci. 2008, 13, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, M.; Pieper, B.; McKim, S.M.; Routier-Kierzkowska, A.L.; Kierzkowski, D.; Smith, R.S.; Hay, A. The role of APETALA1 in petal number robustness. eLife 2018, 7, e39399. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, S.; Jiang, J.; Chen, Y.; Chen, F.; Teng, N.; Yin, D.; Huang, C. The expression of floral organ identity genes in contrasting water lily cultivars. Plant Cell. Rep. 2011, 30, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Effgen, S.; Meyer, R.C.; Theres, K.; Koornneef, M. Epistatic Natural Allelic Variation Reveals a Function of AGAMOUS-LIKE6 in Axillary Bud Formation in Arabidopsis. Plant Cell 2012, 24, 2364–2379. [Google Scholar] [CrossRef] [Green Version]
- Schauer, S.E.; Schlüter, P.M.; Baskar, R.; Gheyselinck, J.; Bolaños, A.; Curtis, M.D.; Grossniklaus, U. Intronic regulatory elements determine the divergent expression patterns of AGAMOUS-LIKE6 subfamily members in Arabidopsis. Plant J. 2009, 59, 987–1000. [Google Scholar] [CrossRef]
- Yoo, S.K.; Hong, S.M.; Lee, J.S.; Ahn, J.H. A genetic screen for leaf movement mutants identifies a potential role for AGAMOUS-LIKE 6 (AGL6) in circadian-clock control. Mol. Cells 2011, 31, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liang, W.; Jia, R.; Yin, C.; Zong, J.; Kong, H.; Zhang, D. The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res. 2010, 20, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-x.; Zhang, K.; Li, L.; Fei, Y.; Chen, F. Characterization of Two AGL6–Like Genes from a Chinese Endemic Woody Tree, Manglietia patungensis (Magnoliaceae) Provides Insight into Perianth Development and Evolution in Basal Angiosperms. Forests 2019, 10, 669. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, Z.; Yong, X.; Ahmad, S.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. SEP-class genes in Prunus mume and their likely role in floral organ development. BMC Plant Biol. 2017, 17, 10. [Google Scholar] [CrossRef]



| Primers | Primers Sequence |
|---|---|
| qPmAGL6-1-F | TCTCGGACCTCTGAATGTGA |
| qPmAGL6-1-R | TTCTGTCTCCAGCTTGACC |
| qPmAGL6-2-F | ACCGTCAATGTTGCTACTCC |
| qPmAGL6-2-R | TCACCAGTTCTTTCAGGCGAA |
| qPP2A-F | ATATAGCTGCTCAGTTCAACC |
| qPP2A-R | AAAAACAGTCACCACATTCTT |
| qActin-F | TCTCTATGCCAGTGGTCGTA |
| qActin-R | CCTCAGGACAACGGAATC |
| qAP1-F | CAGATCAAGGAGAGGGAA |
| qAP1-R | TTGATACAGACCACCCAT |
| qSOC-F | CTCCAATATGCAAGATACCA |
| qSOC-R | TATGCCTTCTCCCAAGAGTT |
| qLFY-F | CCCAAGAAGGGTTATCTGA |
| qLFY-R | AAACGGATGCTCCCTCTG |
| qCO-F | CCATTAACCATAACGCATAC |
| qCO-R | GTCAGGTTGTTGCTCTACT |
| qFT-F | TGGAACAACCTTTGGCAATG |
| qFT-R | GTCTTCTTCCTCCGCAGC |
| qCYC-F | AAGGCTTTGAGTTTCCTGAGGTG |
| qCYC-R | TGAGCAGCCAGTCTAACGTTTTAC |
| qFLC-F | CGAAGCTGATAATATGGAGATGT |
| qFLC-R | AGATATACAACGTGCACCCTTCA |
| PmAGL6-1F | ATGGGGAGAAGGAAAGTGGTG |
| PmAGL6-1R | TCAAAGAACCCATCCCTG |
| PmAGL6-2F | ATGGGGAGAAGGAAAGTGGTG |
| PmAGL6-2R | TTAAAGAAGCCAACCCCGC |
| Transgenic Line/WT | Days Needed for Flowering | Number of Rosette Leaves | Transgenic Line/WT | Days Needed for Flowering | Number of Rosette Leaves |
|---|---|---|---|---|---|
| WT | 42.20 ± 1.66a | 10.30 ± 1.37a | B1 | 31.80 ± 0.51bc | 7.50 ± 0.40c |
| A1 | 26.80 ± 0.80d | 7.40 ± 0.68c | B2 | 33.40 ± 1.27b | 7.90 ± 1.38bc |
| A2 | 30.20 ± 0.74c | 7.80 ± 0.49bc | B3 | 31.60 ± 0.52bc | 8.20 ± 0.36bc |
| A3 | 32.10 ± 1.74bc | 8.40 ± 1.68bc | B4 | 30.40 ± 0.69bc | 7.20 ± 0.73bc |
| A4 | 29.20 ± 0.75c | 70.60 ± 0.98bc | B5 | 33.01 ± 1.17b | 7.40 ± 0.48c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Song, J.; Han, X.; Yu, Y.; Wu, Q.; Qi, S.; Xu, Z. Functional Divergence Analysis of AGL6 Genes in Prunus mume. Plants 2023, 12, 158. https://doi.org/10.3390/plants12010158
Wang L, Song J, Han X, Yu Y, Wu Q, Qi S, Xu Z. Functional Divergence Analysis of AGL6 Genes in Prunus mume. Plants. 2023; 12(1):158. https://doi.org/10.3390/plants12010158
Chicago/Turabian StyleWang, Lei, Jinhai Song, Xu Han, Yunyan Yu, Qikui Wu, Shuai Qi, and Zongda Xu. 2023. "Functional Divergence Analysis of AGL6 Genes in Prunus mume" Plants 12, no. 1: 158. https://doi.org/10.3390/plants12010158





