Presence of the Herbaceous Marsh Species Schoenoplectus americanus Enhances Surface Elevation Gain in Transitional Coastal Wetland Communities Exposed to Elevated CO2 and Sediment Deposition Events
Abstract
:1. Introduction
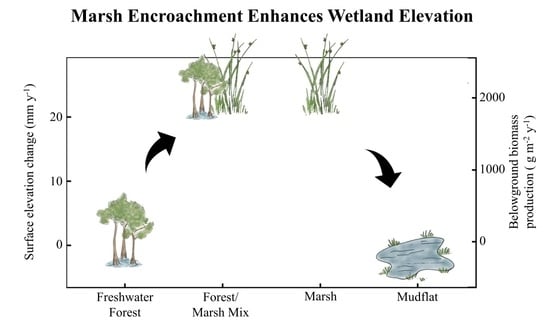
2. Results
3. Discussion
4. Methods
4.1. Experimental Design
4.2. Mesocosm Design
4.3. Experimental Conditions
4.4. Data Collection
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepard, C.C.; Crain, C.M.; Beck, M.W. The Protective Role of Coastal Marshes: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e27374. [Google Scholar] [CrossRef]
- Rezaie, A.M.; Loerzel, J.; Ferreira, C.M. Valuing Natural Habitats for Enhancing Coastal Resilience: Wetlands Reduce Property Damage from Storm Surge and Sea Level Rise. PLoS ONE 2020, 15, e0226275. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global Carbon Sequestration in Tidal, Saline Wetland Soils. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Crooks, S.; Sutton-Grier, A.E.; Troxler, T.G.; Herold, N.; Bernal, B.; Schile-Beers, L.; Wirth, T. Coastal Wetland Management as a Contribution to the US National Greenhouse Gas Inventory. Nat. Clim. Chang. 2018, 8, 1109–1112. [Google Scholar] [CrossRef]
- Barbier, E.B. Wetlands as Natural Assets. Hydrol. Sci. J. 2011, 56, 1360–1373. [Google Scholar] [CrossRef]
- Carruthers, T.J.B.; Kiskaddon, E.P.; Baustian, M.M.; Darnell, K.M.; Moss, L.C.; Perry, C.L.; Stagg, C. Tradeoffs in Habitat Value to Maximize Natural Resource Benefits from Coastal Restoration in a Rapidly Eroding Wetland: Is Monitoring Land Area Sufficient? Restor. Ecol. 2022, 30. [Google Scholar] [CrossRef]
- Redfield, A.C. Ontogeny of a Salt Marsh Estuary. Science 1965, 147, 50–55. [Google Scholar] [CrossRef]
- Treat, C.C.; Kleinen, T.; Broothaerts, N.; Dalton, A.S.; Dommain, R.; Douglas, T.A.; Drexler, J.Z.; Finkelstein, S.A.; Grosse, G.; Hope, G.; et al. Widespread Global Peatland Establishment and Persistence over the Last 130,000 y. Proc. Natl. Acad. Sci. USA 2019, 116, 4822–4827. [Google Scholar] [CrossRef] [Green Version]
- Nyman, J.; DeLaune, R.; Roberts, H.; Patrick, W. Relationship between Vegetation and Soil Formation in a Rapidly Submerging Coastal Marsh. Mar. Ecol. Prog. Ser. 1993, 96, 269–279. [Google Scholar] [CrossRef]
- Morris, J.T.; Barber, D.C.; Callaway, J.C.; Chambers, R.; Hagen, S.C.; Hopkinson, C.S.; Johnson, B.J.; Megonigal, P.; Neubauer, S.C.; Troxler, T.; et al. Contributions of Organic and Inorganic Matter to Sediment Volume and Accretion in Tidal Wetlands at Steady State. Earths Future 2016, 4, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Cahoon, D.R.; McKee, K.L.; Morris, J.T. How Plants Influence Resilience of Salt Marsh and Mangrove Wetlands to Sea-Level Rise. Estuaries Coasts 2021, 44, 883–898. [Google Scholar] [CrossRef]
- Krauss, K.W.; Cormier, N.; Osland, M.J.; Kirwan, M.L.; Stagg, C.L.; Nestlerode, J.A.; Russell, M.J.; From, A.S.; Spivak, A.C.; Dantin, D.D.; et al. Created Mangrove Wetlands Store Belowground Carbon and Surface Elevation Change Enables Them to Adjust to Sea-Level Rise. Sci. Rep. 2017, 7, 1030. [Google Scholar] [CrossRef] [Green Version]
- Rogers, K.; Kelleway, J.J.; Saintilan, N.; Megonigal, J.P.; Adams, J.B.; Holmquist, J.R.; Lu, M.; Schile-Beers, L.; Zawadzki, A.; Mazumder, D.; et al. Wetland Carbon Storage Controlled by Millennial-Scale Variation in Relative Sea-Level Rise. Nature 2019, 567, 91–95. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Sanders, C.J.; Tang, J. Tidal Wetland Resilience to Sea Level Rise Increases Their Carbon Sequestration Capacity in United States. Nat. Commun. 2019, 10, 5434. [Google Scholar] [CrossRef]
- Morris, J.T.; Sundareshwar, P.V.; Nietch, C.T.; Kjerfve, B.; Cahoon, D.R. Responses of Coastal Wetlands to Rising Sea Level. Ecology 2002, 83, 2869–2877. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Guntenspergen, G.R. Feedbacks between Inundation, Root Production, and Shoot Growth in a Rapidly Submerging Brackish Marsh. J. Ecol. 2012, 100, 764–770. [Google Scholar] [CrossRef]
- Stagg, C.L.; Osland, M.J.; Moon, J.A.; Hall, C.T.; Feher, L.C.; Jones, W.R.; Couvillion, B.R.; Hartley, S.B.; Vervaeke, W.C. Quantifying Hydrologic Controls on Local- and Landscape-Scale Indicators of Coastal Wetland Loss. Ann. Bot. 2020, 125, 365–376. [Google Scholar] [CrossRef]
- Conner, W.H.; Doyle, T.W.; Krauss, K.W. Ecology of Tidal Freshwater Forested Wetlands of the Southeastern United States; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Brinson, M.M.; Christian, R.R.; Blum, L.K. Multiple States in the Sea-Level Induced Transition from Terrestrial Forest to Estuary. Estuaries 1995, 18, 648–659. [Google Scholar] [CrossRef]
- Krauss, K.W.; Noe, G.B.; Duberstein, J.A.; Conner, W.H.; Stagg, C.L.; Cormier, N.; Jones, M.C.; Bernhardt, C.E.; Graeme Lockaby, B.; From, A.S.; et al. The Role of the Upper Tidal Estuary in Wetland Blue Carbon Storage and Flux. Glob. Biogeochem. Cycles 2018, 32, 817–839. [Google Scholar] [CrossRef]
- Ensign, S.H.; Hupp, C.R.; Noe, G.B.; Krauss, K.W.; Stagg, C.L. Sediment Accretion in Tidal Freshwater Forests and Oligohaline Marshes of the Waccamaw and Savannah Rivers, USA. Estuaries Coasts 2014, 37, 1107–1119. [Google Scholar] [CrossRef]
- IPCC. 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM_final.pdf (accessed on 4 April 2022).
- Cherry, J.A.; McKee, K.L.; Grace, J.B. Elevated CO2 Enhances Biological Contributions to Elevation Change in Coastal Wetlands by Offsetting Stressors Associated with Sea-Level Rise. J. Ecol. 2009, 97, 67–77. [Google Scholar] [CrossRef]
- Langley, J.A.; McKee, K.L.; Cahoon, D.R.; Cherry, J.A.; Megonigal, J.P. Elevated CO2 Stimulates Marsh Elevation Gain, Counterbalancing Sea-Level Rise. Proc. Natl. Acad. Sci. USA 2009, 106, 6182–6186. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.; Drake, B. Short-Term Decomposition of Litter Produced by Plants Grown in Ambient and Elevated Atmospheric CO2 Concentrations. Glob. Chang. Biol. 1997, 3, 29–35. [Google Scholar] [CrossRef]
- Pendall, E.; Bridgham, S.; Hanson, P.J.; Hungate, B.; Kicklighter, D.W.; Johnson, D.W.; Law, B.E.; Luo, Y.; Megonigal, J.P.; Olsrud, M.; et al. Below-ground Process Responses to Elevated CO2 and Temperature: A Discussion of Observations, Measurement Methods, and Models. New Phytol. 2004, 162, 311–322. [Google Scholar] [CrossRef]
- Wolf, A.A.; Drake, B.G.; Erickson, J.E.; Megonigal, J.P. An Oxygen-Mediated Positive Feedback between Elevated Carbon Dioxide and Soil Organic Matter Decomposition in a Simulated Anaerobic Wetland. Glob. Chang. Biol. 2007, 13, 2036–2044. [Google Scholar] [CrossRef]
- Kossin, J.; Hall, T.; Knutson, T.R.; Kunkel, K.; Trapp, R.; Waliser, D.; Wehner, M. Extreme Storms; Climate Science Special Report: A Sustained Assessment Activity of the U.S. Global Change Research Program; U.S. Global Change Research Program: Washington, DC, USA, 2017; pp. 375–404. [Google Scholar]
- Osland, M.J.; Feher, L.C.; Anderson, G.H.; Vervaeke, W.C.; Krauss, K.W.; Whelan, K.R.T.; Balentine, K.M.; Tiling-Range, G.; Smith, T.J.; Cahoon, D.R. A Tropical Cyclone-Induced Ecological Regime Shift: Mangrove Forest Conversion to Mudflat in Everglades National Park (Florida, USA). Wetlands 2020, 40, 1445–1458. [Google Scholar] [CrossRef]
- Stagg, C.L.; Osland, M.J.; Moon, J.A.; Feher, L.C.; Laurenzano, C.; Lane, T.C.; Jones, W.R.; Hartley, S.B. Extreme Precipitation and Flooding Contribute to Sudden Vegetation Dieback in a Coastal Salt Marsh. Plants 2021, 10, 1841. [Google Scholar] [CrossRef]
- Feher, L.C.; Osland, M.J.; Anderson, G.H.; Vervaeke, W.C.; Krauss, K.W.; Whelan, K.R.T.; Balentine, K.M.; Tiling-Range, G.; Smith, T.J.; Cahoon, D.R. The Long-Term Effects of Hurricanes Wilma and Irma on Soil Elevation Change in Everglades Mangrove Forests. Ecosystems 2020, 23, 917–931. [Google Scholar] [CrossRef]
- Turner, R.E.; Baustian, J.J.; Swenson, E.M.; Spicer, J.S. Wetland Sedimentation from Hurricanes Katrina and Rita. Science 2006, 314, 449–452. [Google Scholar] [CrossRef]
- Tweel, A.W.; Turner, R.E. Landscape-Scale Analysis of Wetland Sediment Deposition from Four Tropical Cyclone Events. PLoS ONE 2012, 7, e50528. [Google Scholar] [CrossRef]
- McKee, K.L.; Mendelssohn, I.A.; Hester, M.W. Hurricane Sedimentation in a Subtropical Salt Marsh-Mangrove Community Is Unaffected by Vegetation Type. Estuar. Coast. Shelf Sci. 2020, 239, 106733. [Google Scholar] [CrossRef]
- McKee, K.L.; Cherry, J.A. Hurricane Katrina Sediment Slowed Elevation Loss in Subsiding Brackish Marshes of the Mississippi River Delta. Wetlands 2009, 29, 2–15. [Google Scholar] [CrossRef]
- Baustian, J.J.; Mendelssohn, I.A. Hurricane-Induced Sedimentation Improves Marsh Resilience and Vegetation Vigor under High Rates of Relative Sea Level Rise. Wetlands 2015, 35, 795–802. [Google Scholar] [CrossRef]
- Stagg, C.L.; Laurenzano, C.; Krauss, K.W.; Vervaeke, W.; McKee, K.L. Above- and Belowground Biomass Production, Decomposition, and Wetland Elevation Change in Transitional Coastal Wetland Communities Exposed to Elevated CO2 and Sediment Deposition: A Mesocosm Study from 2012 to 2014. U.S. Geological Survey Data Release 2022. Available online: https://doi.org/10.5066/P90JCZWU (accessed on 28 February 2022). [CrossRef]
- Reed, D.J. The Response of Coastal Marshes to Sea-Level Rise: Survival or Submergence? Earth Surf. Process. Landf. 1995, 20, 39–48. [Google Scholar] [CrossRef]
- Turner, R.E.; Swenson, E.M.; Milan, C.S. Organic and Inorganic Contributions to Vertical Accretion in Salt Marsh Sediments. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 583–595. ISBN 978-0-306-47534-4. [Google Scholar]
- Nyman, J.A.; Walters, R.J.; Delaune, R.D.; Patrick, W.H. Marsh Vertical Accretion via Vegetative Growth. Estuar. Coast. Shelf Sci. 2006, 69, 370–380. [Google Scholar] [CrossRef]
- McKee, K.L. Biophysical Controls on Accretion and Elevation Change in Caribbean Mangrove Ecosystems. Estuar. Coast. Shelf Sci. 2011, 91, 475–483. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Blum, L.K. Enhanced Decomposition Offsets Enhanced Productivity and Soil Carbon Accumulation in Coastal Wetlands Responding to Climate Change. Biogeosciences 2011, 8, 987–993. [Google Scholar] [CrossRef]
- Allen, J.R.; Cornwell, J.C.; Baldwin, A.H. Contributions of Organic and Mineral Matter to Vertical Accretion in Tidal Wetlands across a Chesapeake Bay Subestuary. J. Mar. Sci. Eng. 2021, 9, 751. [Google Scholar] [CrossRef]
- Stagg, C.L.; Schoolmaster, D.R.; Piazza, S.C.; Snedden, G.; Steyer, G.D.; Fischenich, C.J.; McComas, R.W. A Landscape-Scale Assessment of Above- and Belowground Primary Production in Coastal Wetlands: Implications for Climate Change-Induced Community Shifts. Estuaries Coasts 2017, 40, 856–879. [Google Scholar] [CrossRef]
- Stagg, C.L.; Schoolmaster, D.R.; Krauss, K.W.; Cormier, N.; Conner, W.H. Causal Mechanisms of Soil Organic Matter Decomposition: Deconstructing Salinity and Flooding Impacts in Coastal Wetlands. Ecology 2017, 98, 2003–2018. [Google Scholar] [CrossRef]
- Osland, M.J.; Gabler, C.A.; Grace, J.B.; Day, R.H.; McCoy, M.L.; McLeod, J.L.; From, A.S.; Enwright, N.M.; Feher, L.C.; Stagg, C.L.; et al. Climate and Plant Controls on Soil Organic Matter in Coastal Wetlands. Glob. Chang. Biol. 2018, 24, 5361–5379. [Google Scholar] [CrossRef]
- Baustian, M.M.; Stagg, C.L.; Perry, C.L.; Moss, L.C.; Carruthers, T.J.B. Long-Term Carbon Sinks in Marsh Soils of Coastal Louisiana Are at Risk to Wetland Loss. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005832. [Google Scholar] [CrossRef]
- Cormier, N.; Krauss, K.W.; Conner, W.H. Periodicity in Stem Growth and Litterfall in Tidal Freshwater Forested Wetlands: Influence of Salinity and Drought on Nitrogen Recycling. Estuaries Coasts 2013, 36, 533–546. [Google Scholar] [CrossRef]
- Tilman, D. Tests of Resource Competition Theory Using Four Species of Lake Michigan Algae. Ecology 1981, 62, 802–815. [Google Scholar] [CrossRef]
- Schoolmaster, D.R., Jr.; Stagg, C.L. Resource Competition Model Predicts Zonation and Increasing Nutrient Use Efficiency along a Wetland Salinity Gradient. Ecology 2018, 99, 670–680. [Google Scholar] [CrossRef]
- Bloom, A.A.; Exbrayat, J.-F.; van der Velde, I.R.; Feng, L.; Williams, M. The Decadal State of the Terrestrial Carbon Cycle: Global Retrievals of Terrestrial Carbon Allocation, Pools, and Residence Times. Proc. Natl. Acad. Sci. USA 2016, 113, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, J.; Xia, J.; He, N.; Li, M.; Niu, S. Soil and Vegetation Carbon Turnover Times from Tropical to Boreal Forests. Funct. Ecol. 2018, 32, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Stagg, C.L.; Krauss, K.W.; Cahoon, D.R.; Cormier, N.; Conner, W.H.; Swarzenski, C.M. Processes Contributing to Resilience of Coastal Wetlands to Sea-Level Rise. Ecosystems 2016, 19, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- From, A.S.; Krauss, K.W.; Noe, G.B.; Cormier, N.; Stagg, C.L.; Moss, R.F.; Whitbeck, J.L. Belowground Productivity Varies by Assessment Technique, Vegetation Type, and Nutrient Availability in Tidal Freshwater Forested Wetlands Transitioning to Marsh. PLoS ONE 2021, 16, e0253554. [Google Scholar] [CrossRef]
- McKee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean Mangroves Adjust to Rising Sea Level through Biotic Controls on Change in Soil Elevation. Glob. Ecol. Biogeogr. 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Baustian, J.J.; Mendelssohn, I.A.; Hester, M.W. Vegetation’s Importance in Regulating Surface Elevation in a Coastal Salt Marsh Facing Elevated Rates of Sea Level Rise. Glob. Chang. Biol. 2012, 18, 3377–3382. [Google Scholar] [CrossRef]
- Craft, C.B. Tidal Freshwater Forest Accretion Does Not Keep Pace with Sea Level Rise. Glob. Chang. Biol. 2012, 18, 3615–3623. [Google Scholar] [CrossRef]
- Langley, J.A.; Megonigal, J.P. Ecosystem Response to Elevated CO2 Levels Limited by Nitrogen-Induced Plant Species Shift. Nature 2010, 466, 96–99. [Google Scholar] [CrossRef]
- Jones, J.A.; Cherry, J.A.; McKee, K.L. Species and Tissue Type Regulate Long-Term Decomposition of Brackish Marsh Plants Grown under Elevated CO2 Conditions. Estuar. Coast. Shelf Sci. 2016, 169, 38–45. [Google Scholar] [CrossRef]
- Rejmánková, E.; Houdková, K. Wetland Plant Decomposition under Different Nutrient Conditions: What Is More Important, Litter Quality or Site Quality? Biogeochemistry 2006, 80, 245–262. [Google Scholar] [CrossRef]
- Norby, R.J.; Cotrufo, M.F.; Ineson, P.; O’Neill, E.G.; Canadell, J.G. Elevated CO2, Litter Chemistry, and Decomposition: A Synthesis. Oecologia 2001, 127, 153–165. [Google Scholar] [CrossRef]
- Mendelssohn, I.A.; Sorrell, B.K.; Brix, H.; Schierup, H.-H.; Lorenzen, B.; Maltby, E. Controls on Soil Cellulose Decomposition along a Salinity Gradient in a Phragmites Australis Wetland in Denmark. Aquat. Bot. 1999, 64, 381–398. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, L.; Andrews, J.A.; White, L.; Matamala, R.; Schäfer, K.V.R.; Schlesinger, W.H. Elevated CO2 Differentiates Ecosystem Carbon Processes: Deconvolution Analysis of Duke Forest FACE Data. Ecol. Monogr. 2001, 71, 357–376. [Google Scholar] [CrossRef] [Green Version]
- Megonigal, J.P.; Schlesinger, W.H. Enhanced CH4 Emission from a Wetland Soil Exposed to Elevated CO2. Biogeochemistry 1997, 37, 77–88. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced Root Exudation Induces Microbial Feedbacks to N Cycling in a Pine Forest under Long-Term CO2 Fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Girkin, N.T.; Turner, B.L.; Ostle, N.; Craigon, J.; Sjögersten, S. Root Exudate Analogues Accelerate CO2 and CH4 Production in Tropical Peat. Soil Biol. Biochem. 2018, 117, 48–55. [Google Scholar] [CrossRef]
- Mueller, P.; Schile-Beers, L.M.; Mozdzer, T.J.; Chmura, G.L.; Dinter, T.; Kuzyakov, Y.; de Groot, A.V.; Esselink, P.; Smit, C.; D’Alpaos, A.; et al. Global-Change Effects on Early-Stage Decomposition Processes in Tidal Wetlands – Implications from a Global Survey Using Standardized Litter. Biogeosciences 2018, 15, 3189–3202. [Google Scholar] [CrossRef] [Green Version]
- Drake, B.G. Rising Sea Level, Temperature, and Precipitation Impact Plant and Ecosystem Responses to Elevated CO2 on a Chesapeake Bay Wetland: Review of a 28-Year Study. Glob. Chang. Biol. 2014, 20, 3329–3343. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional Responses of Plants to Elevated Atmospheric CO2 – Do Photosynthetic and Productivity Data from FACE Experiments Support Early Predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.L.; Rooth, J.E. Where Temperate Meets Tropical: Multi-Factorial Effects of Elevated CO2, Nitrogen Enrichment, and Competition on a Mangrove-Salt Marsh Community. Glob. Chang. Biol. 2008, 14, 971–984. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; Mc MURTRIE, R.E.; Oren, R.; Parton, W.J.; et al. Progressive Nitrogen Limitation of Ecosystem Responses to Rising Atmospheric Carbon Dioxide. BioScience 2004, 54, 731. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.E.; Megonigal, J.P.; Peresta, G.; Drake, B.G. Salinity and Sea Level Mediate Elevated CO2 Effects on C3–C4 Plant Interactions and Tissue Nitrogen in a Chesapeake Bay Tidal Wetland. Glob. Chang. Biol. 2007, 13, 202–215. [Google Scholar] [CrossRef]
- Noyce, G.L.; Kirwan, M.L.; Rich, R.L.; Megonigal, J.P. Asynchronous Nitrogen Supply and Demand Produce Nonlinear Plant Allocation Responses to Warming and Elevated CO2. Proc. Natl. Acad. Sci. USA 2019, 116, 21623–21628. [Google Scholar] [CrossRef] [Green Version]
- Vann, C.D.; Megonigal, J.P. Productivity Responses of Acer Rubrum and Taxodium Distichum Seedlings to Elevated CO2 and Flooding. Environ. Pollut. 2002, 116, S31–S36. [Google Scholar] [CrossRef]
- Jones, S.F.; Stagg, C.L.; Krauss, K.W.; Hester, M.W. Flooding Alters Plant-Mediated Carbon Cycling Independently of Elevated Atmospheric CO2 Concentrations. J. Geophys. Res. Biogeosci. 2018, 123, 1976–1987. [Google Scholar] [CrossRef]
- Slocum, M.G.; Mendelssohn, I.A.; Kuhn, N.L. Effects of Sediment Slurry Enrichment on Salt Marsh Rehabilitation: Plant and Soil Responses over Seven Years. Estuaries 2005, 28, 519–528. [Google Scholar] [CrossRef]
- Stagg, C.L.; Mendelssohn, I.A. Restoring Ecological Function to a Submerged Salt Marsh. Restor. Ecol. 2010, 18, 10–17. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Rivera-Monroy, V.H.; Chambers, R.M.; Zhao, X.; Lamb-Wotton, L.; Gorsky, A.; Gaiser, E.E.; Troxler, T.G.; Kominoski, J.S.; Hiatt, M. Hurricanes Fertilize Mangrove Forests in the Gulf of Mexico (Florida Everglades, USA). Proc. Natl. Acad. Sci. USA 2020, 117, 4831–4841. [Google Scholar] [CrossRef]
- Tweel, A.W.; Turner, R.E. Contribution of Tropical Cyclones to the Sediment Budget for Coastal Wetlands in Louisiana, USA. Landsc. Ecol. 2014, 29, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- La Peyre, M.K.; Gossman, B.; Piazza, B.P. Short- and Long-Term Response of Deteriorating Brackish Marshes and Open-Water Ponds to Sediment Enhancement by Thin-Layer Dredge Disposal. Estuaries Coasts 2009, 32, 390–402. [Google Scholar] [CrossRef]
- Baustian, J.J.; Mendelssohn, I.A. Sea Level Rise Impacts to Coastal Marshes May Be Ameliorated by Natural Sedimentation Events. Wetlands 2018, 38, 689–701. [Google Scholar] [CrossRef]
- Graham, S.A.; Mendelssohn, I.A. Functional Assessment of Differential Sediment Slurry Applications in a Deteriorating Brackish Marsh. Ecol. Eng. 2013, 51, 264–274. [Google Scholar] [CrossRef]
- USDA Plants Database. Available online: https://plants.usda.gov/home (accessed on 12 April 2022).
- Berg, R. Tropical Cyclone Report: Hurricane Isaac (AL09012) 21 August–1 September 2012; National Hurricane Center: University Park, Miami-Dade County, FL, USA, 2013; pp. 1–78. [Google Scholar]
- Neill, C. Comparison of Soil Coring and Ingrowth Methods for Measuring Belowground Production. Ecology 1992, 73, 1918–1921. [Google Scholar] [CrossRef]
- Maltby, E. Use of Cotton Strip Assay in Wetland and Upland Environments-an International Perspective. In Cotton Strip Assay: An Index of Decomposition in Soils; Harrison, A.F., Latter, P.M., Walton, D.W.H., Institute of Terrestrial Ecology, Eds.; ITE Symposium; Institute of Terrestrial Ecology: Grange-over-Sands, Cumbria, UK, 1988; ISBN 978-1-870393-06-5. [Google Scholar]
- Slocum, M.G.; Roberts, J.; Mendelssohn, I.A. Artist Canvas as a New Standard for the Cotton-Strip Assay. J. Plant Nutr. Soil Sci. 2009, 172, 71–74. [Google Scholar] [CrossRef]
- Hill, M.O.; Latter, P.M.; Bancroft, G. Standardization of Rotting Rates by a Linearizing Transformation. In Cotton Strip Assay: An Index of Decomposition in Soils; Harrison, A.F., Latter, P.M., Walton, D.W.H., Institute of Terrestrial Ecology, Eds.; ITE Symposium; Institute of Terrestrial Ecology: Grange-over-Sands, Cumbria, UK, 1988; pp. 21–24. ISBN 978-1-870393-06-5. [Google Scholar]
- Cahoon, D.R.; Lynch, J.C.; Perez, B.C.; Segura, B.; Holland, R.D.; Stelly, C.; Stephenson, G.; Hensel, P. High-Precision Measurements of Wetland Sediment Elevation: II. The Rod Surface Elevation Table. J. Sediment. Res. 2002, 72, 734–739. [Google Scholar] [CrossRef]
- R Core Team (2020)—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 1 September 2020).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. Available online: http://www.jstatsoft.org/v82/i13/ (accessed on 1 September 2020).
- Lenth, R.V.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 1 September 2020).
- Stagg, C.L.; Mendelssohn, I.A. Controls on Resilience and Stability in a Sediment-Subsidized Salt Marsh. Ecol. Appl. 2011, 21, 1731–1744. [Google Scholar] [CrossRef]
- National Wetlands Inventory|U.S. Fish & Wildlife Service. Available online: https://www.fws.gov/program/national-wetlands-inventory (accessed on 13 April 2022).







| Treatment | Aboveground Biomass Production | |
|---|---|---|
| F-Value | p-Value | |
| Species | 139.416 | <0.0001 *** |
| Community | 83.826 | <0.0001 *** |
| CO2 | 1.216 | 0.276 |
| Sediment | 3.612 | 0.063 |
| Species × Community | 213.463 | <0.0001 *** |
| Species × CO2 | 1.202 | 0.278 |
| Species × Sediment | 1.355 | 0.25 |
| Community × CO2 | 1.107 | 0.355 |
| Community × Sediment | 0.736 | 0.536 |
| CO2 × Sediment | 2.196 | 0.145 |
| Species × Community × CO2 | 1.156 | 0.336 |
| Species × Community × Sediment | 1.957 | 0.133 |
| Species × CO2 × Sediment | 3.365 | 0.073 |
| Community × CO2 × Sediment | 2.585 | 0.064 |
| Species × Community × CO2 × Sediment | 2.768 | 0.052 |
| Treatment | Belowground Biomass Production | Decomposition | Surface Elevation Change | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Community | 15.012 | <0.0001 *** | 96.465 | <0.0001 *** | 108.633 | <0.0001 *** |
| CO2 | 1.445 | 0.232 | 0.276 | 0.652 | 5.433 | 0.145 |
| Sediment | 3.848 | 0.052 | 30.22 | <0.0001 *** | 12.505 | 0.001 ** |
| Community × CO2 | 0.447 | 0.72 | 5.759 | 0.001 ** | 1.34 | 0.273 |
| Community × Sediment | 1.762 | 0.158 | 24.68 | <0.0001 *** | 0.787 | 0.507 |
| CO2 × Sediment | 1.295 | 0.257 | 0.267 | 0.605 | 0.986 | 0.326 |
| Community × CO2 × Sediment | 1.503 | 0.218 | 1.244 | 0.292 | 0.283 | 0.837 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagg, C.L.; Laurenzano, C.; Vervaeke, W.C.; Krauss, K.W.; McKee, K.L. Presence of the Herbaceous Marsh Species Schoenoplectus americanus Enhances Surface Elevation Gain in Transitional Coastal Wetland Communities Exposed to Elevated CO2 and Sediment Deposition Events. Plants 2022, 11, 1259. https://doi.org/10.3390/plants11091259
Stagg CL, Laurenzano C, Vervaeke WC, Krauss KW, McKee KL. Presence of the Herbaceous Marsh Species Schoenoplectus americanus Enhances Surface Elevation Gain in Transitional Coastal Wetland Communities Exposed to Elevated CO2 and Sediment Deposition Events. Plants. 2022; 11(9):1259. https://doi.org/10.3390/plants11091259
Chicago/Turabian StyleStagg, Camille LaFosse, Claudia Laurenzano, William C. Vervaeke, Ken W. Krauss, and Karen L. McKee. 2022. "Presence of the Herbaceous Marsh Species Schoenoplectus americanus Enhances Surface Elevation Gain in Transitional Coastal Wetland Communities Exposed to Elevated CO2 and Sediment Deposition Events" Plants 11, no. 9: 1259. https://doi.org/10.3390/plants11091259