Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology
Abstract
:1. Introduction
2. Heavy Metals in the Environment
3. Process of Phytoremediation
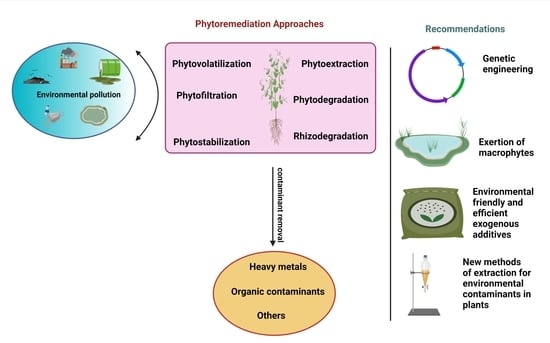
4. Phytoremediation Approaches
4.1. Phytoextraction
4.2. Rhizofiltration
4.3. Rhizodegradation
4.4. Phytostabilization
4.5. Phytodegradation
4.6. Phytovolatilization
4.7. Phytodesalination
5. The Progression of Genetic Engineering
6. Factors Affecting the Metal Uptake
7. Plant Assortment Benchmarks for Phytoremediation
8. Biochemcial Aspect of Phytoremediation
- Lipid peroxidation;
- Biological macromolecule deterioration;
- Membrane dismantling;
- Ion leakage;
- DNA strand cleavage.
9. Exertion of Aquatic Macrophytes in Phytoremediation
9.1. Eichhornia crassipis (Water hyacinth)
9.2. Azolla caroliniana (Mosquito fern)
9.3. Pistia stratiotes (Water lettuce)
9.4. Lemnoideae (Duckweeds)
9.5. Ludwigia stolonifera
9.6. Salvinia (Butterfly fern)
9.7. Hydrilla verticillate (Hydrilla)
9.8. Schoenoplectus californicus (Giant bulrush)
10. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peligro, F.R.; Pavlovic, I.; Rojas, R.; Barriga, C. Removal of heavy metals from simulated wastewater by in situ formation of layered double hydroxides. Chem. Eng. J. 2016, 306, 1035–1040. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Al-Alawy, A.F.; Al-Ameri, M.K. Treatment of Simulated Oily Wastewater by Ultrafiltration and Nanofiltration Processes. Iraqi J. Chem. Pet. Eng. 2017, 18, 71–85. [Google Scholar]
- Huang, H.; Zhang, D.; Zhao, Z.; Zhang, P.; Gao, F. Comparison investigation on phosphate recovery from sludge anaerobic supernatant using the electrocoagulation process and chemical precipitation. J. Clean. Prod. 2017, 141, 429–438. [Google Scholar] [CrossRef]
- Levchuk, I.; Màrquez, J.J.R.; Sillanpää, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Lone, M.I.; He, Z.-L.; Stoffella, P.J.; Yang, X.-E. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- He, S.; He, Z.; Yang, X.; Baligar, V.C. Mechanisms of Nickel Uptake and Hyperaccumulation by Plants and Implications for Soil Remediation. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 117–189. [Google Scholar]
- Pusz, A.; Wiśniewska, M.; Rogalski, D. Assessment of the Accumulation Ability of Festuca rubra L. and Alyssum saxatile L. Tested on Soils Contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources 2021, 10, 46. [Google Scholar] [CrossRef]
- Nedjimi, B. Germination characteristics of Peganum harmala L. (Nitrariaceae) subjected to heavy metals: Implications for the use in polluted dryland restoration. Int. J. Environ. Sci. Technol. 2019, 17, 2113–2122. [Google Scholar] [CrossRef]
- Macnair, M.R. The hyperaccumulation of metals by plants. Adv. Bot. Res. 2003, 40, 63–105. [Google Scholar]
- Baker, A.J.; Whiting, S.N. In search of the Holy Grail—A further step in understanding metal hyperaccumulation? N. Phytol. 2002, 155, 1–4. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; Breedon, T.; McGareth, S.P. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 2000, 23, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Spielmann, J.; Ahmadi, H.; Scheepers, M.; Weber, M.; Nitsche, S.; Carnol, M.; Bosman, B.; Kroymann, J.; Motte, P.; Clemens, S.; et al. The two copies of the zinc and cadmium ZIP6 transporter of Arabidopsis halleri have distinct effects on cadmium tolerance. Plant Cell Environ. 2020, 43, 2143–2157. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef]
- Verma, A.; Roy, A.; Bharadvaja, N. Remediation of heavy metals using nanophytoremediation. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 273–296. [Google Scholar]
- Bani, A.; Pavlova, D.K.; Echevarria, G.; Mullaj, A.; Reeves, R.D.; Morel, J.-L.; Sulejman, S. Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans. Bot. Serb. 2010, 34, 3–14. [Google Scholar]
- Tognacchini, A.; Rosenkranz, T.; van der Ent, A.; Machinet, G.E.; Echevarria, G.; Puschenreiter, M. Nickel phytomining from industrial wastes: Growing nickel hyperaccumulator plants on galvanic sludges. J. Environ. Manag. 2019, 254, 109798. [Google Scholar] [CrossRef]
- Rai, P.K. Technical Note: Phytoremediation of Hg and Cd from Industrial Effluents using an Aquatic Free Floating Macrophyte Azolla pinnata. Int. J. Phytoremediat. 2008, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Potential of water fern (Azolla pinnata R. Br.) in phytoremediation of integrated industrial effluent of SIIDCUL, Haridwar, India: Removal of physicochemical and heavy metal pollutants. Int. J. Phytoremediat. 2020, 22, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Ibañéz, S.G.; Oller, A.L.W.; Paisio, C.E.; Alderete, L.G.S.; Gonzalez, P.S.; Medina, M.I.; Agostini, E. The challenges of remediating metals using phytotechnologies. In Heavy Metals in the Environment: Microorganisms and Bioremediation; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2018; pp. 173–191. [Google Scholar]
- Sharma, R.; Bhardwaj, R.; Gautam, V.; Bali, S.; Kaur, R.; Kaur, P.; Sharma, M.; Kumar, V.; Sharma, A.; Thukral, A.K. Phytoremediation in Waste management: Hyperaccumulation diversity and techniques. In Plants Under Metal and Metalloid Stress; Springer: Berlin/Heidelberg, Germany, 2018; pp. 277–302. [Google Scholar]
- Singh, D.; Tiwari, A.; Gupta, R. Phytoremediation of lead from wastewater using aquatic plants. Int. J. Biomed. Res. 2011, 2, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, J.-Y.; Kim, K.-W. Phytoremediation of soil contaminated with heavy metals using Brassica napus. Geosyst. Eng. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2019, 39, 266–281. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Singh, J.K. Bioremediation of heavy metals from contaminated sites using potential species: A review. Indian J. Environ. Prot. 2017, 37, 65. [Google Scholar]
- Koptsik, G.N. Problems and prospects concerning the phytoremediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Sahay, S.; Inam, A.; Iqbal, S. Risk analysis by bioaccumulation of Cr, Cu, Ni, Pb and Cd from wastewater-irrigated soil to Brassica species. Int. J. Environ. Sci. Technol. 2020, 17, 2889–2906. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Correction to: Phytoremediation of toxic metals present in soil and water environment: A critical review. Environ. Sci. Pollut. Res. 2020, 27, 44861–44862. [Google Scholar] [CrossRef]
- Chandra, S.; Gusain, Y.S.; Bhatt, A. Metal hyperaccumulator plants and environmental pollution. In Microbial Biotechnology in Environmental Monitoring and Cleanup; IGI Global: Hershey, PA, USA, 2018; pp. 305–317. [Google Scholar]
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation Potential of Hemp (Cannabis sativa L.): Identification and Characterization of Heavy Metals Responsive Genes. CLEAN Soil Air Water 2015, 44, 195–201. [Google Scholar] [CrossRef]
- Alufasi, R.; Zeman, S.; Bagar, T.; Chingwaru, W. Cannabis sativa L. and its potential applications in environmental bioremediation. A review. Hmelj. Bilt. 2020, 27, 161–172. [Google Scholar]
- Sumiahadi, A.; Acar, R. A review of phytoremediation technology: Heavy metals uptake by plants. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012023. [Google Scholar] [CrossRef]
- Kubota, M.; Nishi, K. Salicylic acid accumulates in the roots and hypocotyl after inoculation of cucumber leaves with Colletotrichum lagenarium. J. Plant Physiol. 2006, 163, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Shehata, H.S.; Galal, T.M.; Safety, F. Trace metal concentration in planted cucumber (Cucumis sativus L.) from contaminated soils and its associated health risks. J. Consum. Prot. Food Saf. 2020, 15, 205–217. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2009, 164, 1059–1063. [Google Scholar] [CrossRef]
- Ibezim-Ezeani, M.; Ihunwo, O. Assessment of Pb, Cd, Cr and Ni in Water and Water Hyacinth (Eichhornia crassipes) Plant from Woji Creek, Rivers State, Nigeria. J. Appl. Sci. Environ. Manag. 2020, 24, 719–727. [Google Scholar] [CrossRef]
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis. CLEAN Soil Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Chehregani, A.; Malayeri, B.E. Removal of heavy metals by native accumulator plants. Int. J. Agric. Biol. 2007, 9, 462–465. [Google Scholar]
- Mohsenzadeh, F.; Mohammadzadeh, R. Phytoremediation Ability of the New Heavy Metal Accumulator Plants. Environ. Eng. Geosci. 2018, 24, 441–450. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.; Poonia, P. Phytomining: A review. Miner. Eng. 2009, 22, 1007–1019. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Yadav, S.K.; Juwarkar, A.A.; Kumar, G.P.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and phyto-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.G.; Caro, M.D.C.G.; Barrera, M.D.C.L.; García, M.T.; Barbin, D.; Mateos, P. Metal Accumulation by Jatropha curcas L. Adult Plants Grown on Heavy Metal-Contaminated Soil. Plants 2020, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- Laribe, F.; Agamuthu, P. Assessment of phytoremediation potentials of Lantana camara in Pb impacted soil with organic waste additives. Ecol. Eng. 2015, 83, 513–520. [Google Scholar] [CrossRef]
- Saini, V.K.; Suthar, S.; Karmveer, C.; Kumar, K. Valorization of Toxic Weed Lantana camara L. Biomass for Adsorptive Removal of Lead. J. Chem. 2017, 2017, 5612594. [Google Scholar] [CrossRef] [Green Version]
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of lavender (Lavandula vera L.) for phytoremediation of soils contaminated with heavy metals. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 9, 522–529. [Google Scholar]
- Checcucci, A.; Bazzicalupo, M.; Mengoni, A. Exploiting nitrogen-fixing rhizobial symbionts genetic resources for improving phytoremediation of contaminated soils. In Enhancing Cleanup of Environmental Pollutants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 275–288. [Google Scholar]
- Gunduz, S.; Uygur, F.N.; Kahramanoğlu, I. Heavy metal phytoremediation potentials of Lepidum sativum L., Lactuca sativa L., Spinacia oleracea L. and Raphanus sativus L. Herald J. Agric. Food Sci. Res. 2012, 1, 1–5. [Google Scholar]
- Bhatti, S.S.; Bhat, S.A.; Singh, J. Aquatic Plants as Effective Phytoremediators of Heavy Metals. In Contaminants and Clean Technologies; CRC Press: Boca Raton, FL, USA, 2020; p. 189. [Google Scholar]
- Carrasco-Gil, S.; Siebner, H.; LeDuc, D.L.; Webb, S.M.; Millán, R.; Andrews, J.C.; Hernández, L.E. Mercury Localization and Speciation in Plants Grown Hydroponically or in a Natural Environment. Environ. Sci. Technol. 2013, 47, 3082–3090. [Google Scholar] [CrossRef]
- Kumari, S.; Amit; Jamwal, R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Kocoń, A.; Jurga, B. The evaluation of growth and phytoextraction potential of Miscanthus × giganteus and Sida hermaphrodita on soil contaminated simultaneously with Cd, Cu, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2017, 24, 4990–5000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konkolewska, A.; Piechalak, A.; Ciszewska, L.; Antos-Krzemińska, N.; Skrzypczak, T.; Hanć, A.; Sitko, K.; Małkowski, E.; Barałkiewicz, D.; Małecka, A. Combined use of companion planting and PGPR for the assisted phytoextraction of trace metals (Zn, Pb, Cd). Environ. Sci. Pollut. Res. 2020, 27, 13809–13825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, N.; van der Ent, A.; Mulligan, D.R.; Nguyen, A. Zinc and lead accumulation characteristics and in vivo distribution of Zn2+ in the hyperaccumulator Noccaea caerulescens elucidated with fluorescent probes and laser confocal microscopy. Environ. Exp. Bot. 2018, 147, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yao, H.; Wong, M.H.; Ye, Z. Dynamic changes in radial oxygen loss and iron plaque formation and their effects on Cd and as accumulation in rice (Oryza sativa L.). Environ. Geochem. Health 2013, 35, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, J. Ameliorative effects of Lanthanum (III) on Copper (II) stressed rice (Oryza sativa) and its molecular mechanism revealed by transcriptome profiling. Plant Physiol. Biochem. 2020, 152, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, A.; Usha, R.; Swamy, P. Phytoremediation—An overview review. J. Ind. Pollut. Control 2010, 26, 83–88. [Google Scholar]
- Mitra, A.; Chatterjee, S.; Voronina, A.V.; Walther, C.; Gupta, D.K. Lead toxicity in plants: A review. In Lead in Plant and the Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 99–116. [Google Scholar]
- Arshad, M. Lead Phytoextraction by Scented Pelargonium cultivars: Soil-Plant Interactions and Tool Development for Understanding Lead Hyperaccumulation. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, July 2009. [Google Scholar]
- Manzoor, M.; Gul, I.; Manzoor, A.; Kamboh, U.R.; Hina, K.; Kallerhoff, J.; Arshad, M. Lead availability and phytoextraction in the rhizosphere of Pelargonium species. Environ. Sci. Pollut. Res. 2020, 27, 39753–39762. [Google Scholar] [CrossRef]
- Srivastava, N. Phytomicrobiome: Synergistic Relationship in Bioremediation of Soil for Sustainable Agriculture. In Phytomicrobiome Interactions and Sustainable Agriculture; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 150–163. [Google Scholar]
- Hu, P.-J.; Qiu, R.-L.; Senthilkumar, P.; Jiang, D.; Chen, Z.-W.; Tang, Y.-T.; Liu, F.-J. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ. Exp. Bot. 2009, 66, 317–325. [Google Scholar] [CrossRef]
- Silambarasan, T.S.; Balakumaran, M.D.; Suresh, S.; Balasubramanian, V.; Sanjivkumar, M.; Sendilkumar, B.; Dhandapani, R. Bioremediation of Tannery Effluent Contaminated Soil: A Green Approach. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 283–300. [Google Scholar]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Ahmed, D.A.E.-A.; Gheda, S.F.; Ismail, G.A. Efficacy of two seaweeds dry mass in bioremediation of heavy metal polluted soil and growth of radish (Raphanus sativus L.) plant. Environ. Sci. Pollut. Res. 2021, 28, 12831–12846. [Google Scholar] [CrossRef]
- Angelova, V.R.; Ivanova, R.V.; Todorov, G.M.; Ivanov, K.I. Potential of Salvia sclarea L. for phytoremediation of soils contaminated with heavy metals. Int. J. Agric. Biosyst. Eng. 2016, 10, 780–790. [Google Scholar]
- Abhilash, P.C.; Tripathi, V.; Edrisi, S.A.; Dubey, R.K.; Bakshi, M.; Dubey, P.K.; Singh, H.B.; Ebbs, S.D. Sustainability of crop production from polluted lands. Energy Ecol. Environ. 2016, 1, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.K.; Jena, R.K.; Hota, S.; Kumar, A.; Ray, P.; Fagodiya, R.K.; Malav, L.; Yadav, K.K.; Gupta, D.; Khan, S.; et al. Recent development in bioremediation of soil pollutants through biochar for environmental sustainability. In Biochar Applications in Agriculture and Environment Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–140. [Google Scholar]
- Boechat, C.L.; Carlos, F.S.; do Nascimento, C.W.; de Quadros, P.D.; de Sa, E.L.S.; Camargo, F.A.O. Bioaugmentation-assisted phytoremediation of As, Cd, and Pb using Sorghum bicolor in a contaminated soil of an abandoned gold ore processing plant. Rev. Bras. Ciênc. Solo. 2020, 44, e0200081. [Google Scholar] [CrossRef]
- Salazar, M.J.; Pignata, M.L. Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. J. Geochem. Explor. 2014, 137, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Asante-Badu, B.; Kgorutla, L.E.; Li, S.S.; Danso, P.O.; Xue, Z.; Qiang, G. Phytoremediation of organic and inorganic compounds in a natural and an agricultural environment: A review. Appl. Ecol. Environ. Res. 2020, 18, 6875–6904. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Pazcel, E.M.M.; Wannaz, E.D.; Pignata, M.L.; Salazar, M.J. Tagetes minuta L. Variability in Terms of Lead Phytoextraction from Polluted Soils: Is Historical Exposure a Determining Factor? Environ. Process. 2018, 5, 243–259. [Google Scholar] [CrossRef]
- Jacobs, A.; De Brabandere, L.; Drouet, T.; Sterckeman, T.; Noret, N. Phytoextraction of Cd and Zn with Noccaea caerulescens for urban soil remediation: Influence of nitrogen fertilization and planting density. Ecol. Eng. 2018, 116, 178–187. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Yang, J. Two potential multi-metal hyperaccumulators found in four mining sites in Hunan Province, China. Catena 2017, 148, 67–73. [Google Scholar] [CrossRef]
- Watson, A.Y.; Bates, R.R.; Kennedy, D. Assessment of human exposure to air pollution: Methods, measurements, and models. In Air Pollution, the Automobile, and Public Health; National Academies Press: Washington, DC, USA, 1988. [Google Scholar]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef] [PubMed]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, S.; Düring, R.-A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. SOIL 2015, 1, 23–33. [Google Scholar] [CrossRef] [Green Version]
- García-Delgado, M.; Rodríguez-Cruz, M.S.; Lorenzo, L.; Arienzo, M.; Sánchez-Martín, M.J. Seasonal and time variability of heavy metal content and of its chemical forms in sewage sludges from different wastewater treatment plants. Sci. Total Environ. 2007, 382, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Beharti, A. Phytoremediation: As a degradation of heavy metals. Int. J. Eng. Technol. Res. 2014, 2, 137–139. [Google Scholar]
- Peuke, A.D.; Rennenberg, H. Phytoremediation: Molecular biology, requirements for application, environmental protection, public attention and feasibility. EMBO Rep. 2005, 6, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef]
- Raskin, I.; Ensley, B.D. Phytoremediation of Toxic Metals; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Leguizamo, M.A.O.; Gómez, W.D.F.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef]
- Abioye, O.; Ijah, U.; Aransiola, S. Phytoremediation of soil contaminants by the biodiesel plant Jatropha curcas. In Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–137. [Google Scholar]
- Thakur, S.; Singh, L.; Ab Wahid, Z.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.Á. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Ernst, W.; Verkleij, J.; Schat, H. Metal tolerance in plants. Acta Bot. Neerl. 1992, 41, 229–248. [Google Scholar] [CrossRef]
- Gupta, D.K.; Vandenhove, H.; Inouhe, M. Role of phytochelatins in heavy metal stress and detoxification mechanisms in plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 73–94. [Google Scholar]
- Eapen, S.; D’Souza, S. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol. Adv. 2005, 23, 97–114. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Jakovljevic, T.; Radojcic-Redovnikovic, I.; Laslo, A. Phytoremediation of heavy metals: Applications and experiences in Croatia abstract. Zastita Mater. 2016, 57, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors Affecting Phytoextraction: A Review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.J.; Brooks, R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Robinson, B.; Leblanc, M.; Petit, D.; Brooks, R.R.; Kirkman, J.H.; Gregg, P.E.H. The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 1998, 203, 47–56. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, H. Sedum alfredii: A new lead accumulating ecotype. J. Integr. Plant Biol. 2002, 44, 1365. [Google Scholar]
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The Use of Plants to Remove Heavy Metals from Soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, D.M.; Hou, W. Hyperaccumulation of Lead by Roots, Hypocotyls, and Shoots of Brassica juncea. Biol. Plant. 2000, 43, 603–606. [Google Scholar] [CrossRef]
- Kertulis-Tartar, G.; Ma, L.Q.; Tu, C.; Chirenje, T. Phytoremediation of an arsenic-contaminated site using Pteris vittata L.: A two-year study. Int. J. Phytoremediat. 2006, 8, 311–322. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Dietz, A.C.; Schnoor, J.L. Advances in phytoremediation. Environ. Health Perspect. 2001, 109 (Suppl. 1), 163–168. [Google Scholar]
- Chaney, R.L.; Baklanov, I.A. Phytoremediation and phytomining: Status and promise. Adv. Bot. Res. 2017, 83, 189–221. [Google Scholar]
- Masu, S.; Cojocariu, L.; Grecu, E.; Morariu, F.; Bordean, D.-M.; Horablaga, M.; Nita, L.; Nita, S. Lolium Perenne—A Phytoremediation Option in Case of Total Petroleum Hydrocarbons Polluted Soils. Rev. Chim. 2018, 69, 1110–1114. [Google Scholar] [CrossRef]
- Holan, Z.R.; Volesky, B. Biosorption of lead and nickel by biomass of marine algae. Biotechnol. Bioeng. 1994, 43, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and Mechanisms of Microbial Remediation of Heavy Metals in Soil: A Critical Review. Appl. Sci. 2018, 8, 1336. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef]
- He, H.J.; Xiang, Z.H.; Chen, X.J.; Chen, H.; Huang, H.; Wen, M.; Yang, C.P. Biosorption of Cd(II) from synthetic wastewater using dry biofilms from biotrickling filters. Int. J. Environ. Sci. Technol. 2018, 15, 1491–1500. [Google Scholar] [CrossRef]
- Vymazal, J. The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol. Eng. 2002, 18, 633–646. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp.: Macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- Dwivedi, S. Bioremediation of heavy metal by algae: Current and future perspective. J. Adv. Lab. Res. Biol. 2012, 3, 195–199. [Google Scholar]
- Hirvaniya, N.R.; Khatnani, T.D.; Rawat, S. Bioremediation of heavy metals from wastewater treatment plants by microorganisms. In Microbial Ecology of Wastewater Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 411–434. [Google Scholar]
- Sánchez, A.; Ballester, A.; Blazquez, M.L.; Gonzalez, F.; Munoz, J.; Hammaini, A. Biosorption of copper and zinc by Cymodocea nodosa. FEMS Microbiol. Rev. 1999, 23, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Boutahar, L.; Espinosa, F.; Sempere-Valverde, J.; Selfati, M.; Bazairi, H. Trace element bioaccumulation in the seagrass Cymodocea nodosa from a polluted coastal lagoon: Biomonitoring implications. Mar. Pollut. Bull. 2021, 166, 112209. [Google Scholar] [CrossRef] [PubMed]
- Fourest, E.; Volesky, B. Alginate Properties and Heavy Metal Biosorption by Marine Algae. Appl. Biochem. Biotechnol. 1997, 67, 215–226. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Jiang, Q.; Yang, Y. Concentrations of various elements in seaweed and seawater from Shen′ao Bay, Nan′ao Island, Guangdong coast, China: Environmental monitoring and the bioremediation potential of the seaweed. Sci. Total Environ. 2018, 659, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.; Volesky, B.; Vieira, R. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, S.C.S.; Hernandez-Vargas, G.; Iqbal, H.M.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.R.; Hernández-Carteño, J.; Estévez, M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp. J. Mar. Sci. Eng. 2020, 8, 899. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Verma, N.; Sharma, R. Bioremediation of toxic heavy metals: A patent review. Recent Pat. Biotechnol. 2017, 11, 171–187. [Google Scholar] [CrossRef]
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zayed, A.M.; Qian, J.; de Souza, M.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: II. Water Hyacinth. J. Environ. Qual. 1999, 28, 339–344. [Google Scholar] [CrossRef]
- Verma, P.; Rawat, S. Rhizoremediation of Heavy Metal-and Xenobiotic-Contaminated Soil: An Eco-Friendly Approach. In Removal of Emerging Contaminants Through Microbial Processes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–113. [Google Scholar]
- Hooda, V. Phytoremediation of toxic metals from soil and waste water. J. Environ. Biol. 2007, 28, 367. [Google Scholar] [PubMed]
- Dhanwal, P.; Kumar, A.; Dudeja, S.; Chhokar, V.; Beniwal, V. Recent advances in phytoremediation technology. In Advances in Environmental Biotechnology; Springer: Singapore, 2017; pp. 227–241. [Google Scholar]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Nat. Biotechnol. 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhu, G.; Liu, Y.; Wu, B.; Ng, W.J.; Appan, A.; Tan, S.K. Phytoextraction, phytotransformation and rhizodegradation of ibuprofen associated with Typha angustifolia in a horizontal subsurface flow constructed wetland. Water Res. 2016, 102, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. Assisted phytoremediation for restoring soil fertility in contaminated and degraded land. Ital. J. Agron. 2018, 13 (Suppl. 1), 34–44. [Google Scholar]
- Kaimi, E.; Mukaidani, T.; Miyoshi, S.; Tamaki, M. Ryegrass enhancement of biodegradation in diesel-contaminated soil. Environ. Exp. Bot. 2006, 55, 110–119. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.-P.; Gawroński, S.; Schröder, P.; Vangronsveld, J. Successes and limitations of phytotechnologies at field scale: Outcomes, assessment and outlook from COST Action 859. J. Soils Sediments 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.-Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [Green Version]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sediments 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.D.; Berti, W.R. Phytoextraction and phytostabilization: Technical, economic and regulatory considerations of the soil-lead issue. In Phytoremediation of Contaminated Soil and Water; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Masarovičová, E.; Kráľová, K.; Kummerová, M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010, 32, 823–829. [Google Scholar] [CrossRef]
- Pandey, J.; Verma, R.K.; Singh, S. Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: A review. Int. J. Phytoremediat. 2019, 21, 405–418. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Bandiera, M.; Cortivo, C.D.; Barion, G.; Mosca, G.; Vamerali, T. Phytoremediation Opportunities with Alimurgic Species in Metal-Contaminated Environments. Sustainability 2016, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Pandey, S. Status of phytoremediation in world scenario. Int. J. Environ. Bioremediat. Biodegrad. 2014, 2, 178–191. [Google Scholar]
- Deng, Z.; Cao, L. Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere 2017, 168, 1100–1106. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Castro, S.; Davis, L.C.; Erickson, L.E. Phytotransformation of benzotriazoles. Int. J. Phytoremediat. 2003, 5, 245–265. [Google Scholar] [CrossRef]
- Doty, S.L.; Shang, T.Q.; Wilson, A.M.; Moore, A.L.; Newman, L.A.; Strand, S.E.; Gordon, M.P. Metabolism of the soil and groundwater contaminants, ethylene dibromide and trichloroethylene, by the tropical leguminous tree, Leuceana leucocephala. Water Res. 2003, 37, 441–449. [Google Scholar] [CrossRef]
- Miller, R.R. Phytoremediation—Technology Overview; Groundwater Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on sources and effect of heavy metal in soil: Its bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 2018, 1–22. [Google Scholar]
- Limmer, M.A.; Burken, J.G. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.; Vithanage, M. Phytoremediation in Constructed Wetlands. In Phytoremediation, Management of Environmental Contaminants; Springer International Publishing: Cham, Switzerland, 2015; pp. 243–263. [Google Scholar]
- Ahmadpour, P.; Ahmadpour, F.; Mahmud, T.M.M.; Abdu, A.; Soleimani, M.; Tayefeh, F.H. Phytoremediation of heavy metals: A green technology. Afr. J. Biotechnol. 2012, 11, 14036–14043. [Google Scholar]
- Guignardi, Z.; Schiavon, M. Biochemistry of plant selenium uptake and metabolism. In Selenium in Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–34. [Google Scholar]
- Van Huysen, T.; Abdel-Ghany, S.; Hale, K.L.; LeDuc, D.; Terry, N.; Pilon-Smits, E.A.H. Overexpression of cystathionine-?-synthase enhances selenium volatilization in Brassica juncea. Planta 2003, 218, 71–78. [Google Scholar] [CrossRef]
- Doucette, W.; Klein, H.; Chard, J.; Dupont, R.; Plaehn, W.; Bugbee, B. Volatilization of Trichloroethylene from Trees and Soil: Measurement and Scaling Approaches. Environ. Sci. Technol. 2013, 47, 5813–5820. [Google Scholar] [CrossRef]
- Rugh, C.L.; Gragson, G.M.; Meagher, R.B.; Merkle, S.A. Toxic Mercury Reduction and Remediation Using Transgenic Plants with a Modified Bacterial Gene. HortScience 1998, 33, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Dushenkov, V.; Kumar, P.B.A.N.; Motto, H.; Raskin, I. Rhizofiltration: The Use of Plants to Remove Heavy Metals from Aqueous Streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar] [CrossRef]
- Dubchak, S.; Bondar, O. Bioremediation and phytoremediation: Best approach for rehabilitation of soils for future use. In Remediation Measures for Radioactively Contaminated Areas; Springer: Berlin/Heidelberg, Germany, 2019; pp. 201–221. [Google Scholar]
- Arya, S.; Devi, S.; Angrish, R.; Singal, I.; Rani, K. Soil reclamation through phytoextraction and phytovolatilization. In Volatiles and Food Security; Springer: Berlin/Heidelberg, Germany, 2017; pp. 25–43. [Google Scholar]
- McCutcheon, S.; Schnoor, J. Overview of phytotransformation and control of wastes. In Phytoremediation: Transformation and Control of Contaminants; Wiley & Sons: New York, NY, USA, 2003; pp. 1–58. [Google Scholar]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes Present New Opportunities in Phytoremediation of Heavy Metals and Saline Soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schoftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of petroleum hydrocarbon-contaminated soils: Improvement opportunities and field applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of saline land by halophytes for Indian soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Zorrig, W.; Rabhi, M.; Ferchichi, S.; Smaoui, A.; Abdelly, C. Phytodesalination: A solution for salt-affected soils in arid and semi-arid regions. J. Arid Land Stud. 2012, 22, 299–302. [Google Scholar]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Marques, A.P.; Rangel, A.O.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-Up Technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Muszynska, E. Recent strategies of increasing metal tolerance and phytoremediation potential using genetic transformation of plants. Plant Biotechnol. Rep. 2018, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Sarma, H. Metal Hyperaccumulation in Plants: A Review Focusing on Phytoremediation Technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Kinnersley, A.M. The role of phytochelates in plant growth and productivity. Plant Growth Regul. 1993, 12, 207–218. [Google Scholar] [CrossRef]
- Liao, S.; Chang, W. Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan. Photogramm. Eng. Remote Sens. 2004, 54, 177–185. [Google Scholar]
- Hasan, M.; Uddin, N.; Ara-Sharmeen, I.; Alharby, H.F.; Alzahrani, Y.; Hakeem, K.R.; Zhang, L. Assisting Phytoremediation of Heavy Metals Using Chemical Amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babau, A.; Micle, V.; Damian, G.; Sur, I. Preliminary investigations regarding the potential of Robinia pseudoacacia L. (leguminosae) in the phytoremediation of sterile dumps. J. Environ. Prot. Ecol. 2020, 21, 46–55. [Google Scholar]
- Prasad, M.N.V.; Freitas, H.M.D.O. Metal hyperaccumulation in plants—Biodiversity prospecting for phytoremediation technology. Electron. J. Biotechnol. 2003, 6, 285–321. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2016, 256, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Basta, N.; Gradwohl, R. Remediation of heavy metal-contaminated soil using rock phosphate. Better Crops 1998, 82, 29–31. [Google Scholar]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Anton, A.; Mathe-Gaspar, G. Factors affecting heavy metal uptake in plant selection for phytoremediation. Z. Naturforsch. 2005, 60, 244–246. [Google Scholar]
- Plant, J.A.; Raiswell, R. Principles of environmental geochemistry. In Applied Environmental Geochemistry; Academic Press: London, UK, 1983; pp. 1–39. [Google Scholar]
- Brümmer, G.; Herms, U. Influence of soil reaction and organic matter on the solubility of heavy metals in soils. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 1983; pp. 233–243. [Google Scholar]
- Gerritse, R.; van Driel, W. The Relationship between Adsorption of Trace Metals, Organic Matter, and pH in Temperate Soils; Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Phytoremediation in the tropics-influence of heavy crude oil on root morphological characteristics of graminoids. Environ. Pollut. 2005, 138, 86–91. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Leckie, J.O. Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Coll. Interf. Sci. 1981, 79, 209–221. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.; Raskin, I. Phytoremediation. Ann. Rev. Plant Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Van Ginneken, L.; Meers, E.; Guisson, R.; Ruttens, A.; Elst, K.; Tack, F.M.G.; Vangronsveld, J.; Diels, L.; Dejonghe, W. Phytoremediation for Heavy Metal-Contaminated Soils Combined with Bioenergy Production. J. Environ. Eng. Landsc. Manag. 2007, 15, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Seuntjens, P.; Nowack, B.; Schulin, R. Root-zone modeling of heavy metal uptake and leaching in the presence of organic ligands. Plant Soil 2004, 265, 61–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Xu, W.; Liao, Q.; Zhang, H.; Hao, S.; Chen, S. Pyrolysis of various phytoremediation residues for biochars: Chemical forms and environmental risk of Cd in biochar. Bioresour. Technol. 2019, 299, 122581. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J. Perspectives and utilization technologies of chicory (Cichorium intybus L.): A review. Afr. J. Biotechnol. 2011, 10, 1966–1977. [Google Scholar]
- Sinha, S.; Mishra, R.K.; Sinam, G.; Mallick, S.; Gupta, A.K. Comparative Evaluation of Metal Phytoremediation Potential of Trees, Grasses, and Flowering Plants from Tannery-Wastewater-Contaminated Soil in Relation with Physicochemical Properties. Soil Sediment Contam. Int. J. 2013, 22, 958–983. [Google Scholar] [CrossRef]
- Tordoff, G.; Baker, A.; Willis, A. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Compton, H.R.; Prince, G.R.; Fredericks, S.C.; Gussman, C.D. Phytoremediation of dissolved phase organic compounds: Optimal site considerations relative to field case studies. Remediat. J. 2003, 13, 21–37. [Google Scholar] [CrossRef]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of Heavy Metals: An Eco-Friendly and Sustainable Approach. In Bioremediation and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 215–231. [Google Scholar]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Wu, J.J. Landscape Ecology, Cross-Disciplinarity, and Sustainability Science; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cunningham, S.D.; Ow, D.W. Promises and Prospects of Phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef]
- Hong-Bo, S.; Li-Ye, C.; Cheng-Jiang, R.; Hua, L.; Dong-Gang, G.; Wei-Xiang, L. Understanding molecular mechanisms for improving phytoremediation of heavy metal-contaminated soils. Crit. Rev. Biotechnol. 2009, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-accumulation Metals in Plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Willscher, S.; Jablonski, L.; Fona, Z.; Rahmi, R.; Witting, J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy 2017, 168, 153–158. [Google Scholar] [CrossRef]
- Wei, S.; Pan, S. Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J. Soils Sediments 2010, 10, 886–894. [Google Scholar] [CrossRef]
- Ha, N.T.; Sakakibara, M.; Sano, S. Accumulation of Indium and other heavy metals by Eleocharis acicularis: An option for phytoremediation and phytomining. Bioresour. Technol. 2011, 102, 2228–2234. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Rosen, B.P. Perspectives for genetic engineering for the phytoremediation of arsenic-contaminated environments: From imagination to reality? Curr. Opin. Biotechnol. 2009, 20, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Selvam, A.; Wong, J. Phytochelatin systhesis and cadmium uptake of Brassica napus. Environ. Technol. 2008, 29, 765–773. [Google Scholar] [CrossRef]
- Singh, O.V.; Labana, S.; Pandey, G.; Budhiraja, R.; Jain, R.K. Phytoremediation: An overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 2003, 61, 405–412. [Google Scholar] [CrossRef]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, C.K.; Fox, T.C.; Garvin, D.F.; Kochian, L. The Role of Iron-Deficiency Stress Responses in Stimulating Heavy-Metal Transport in Plants1. Plant Physiol. 1998, 116, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, S.; Sirohi, P.; Sharma, D.; Singh, N.K. Micronutrient movement and signalling in plants from a biofortification perspective. In Plant Micronutrients; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–171. [Google Scholar]
- Hutchinson, G. A Treatise on Limnology Volume III: Limnological Botany; John Wiley & Sons: Hoboke, NJ, USA, 1975. [Google Scholar]
- Galal, T.M.; Shehata, H.S. Evaluation of the invasive macrophyte Myriophyllum spicatum L. as a bioaccumulator for heavy metals in some watercourses of Egypt. Ecol. Indic. 2014, 41, 209–214. [Google Scholar] [CrossRef]
- Outridge, P.; Noller, B. Accumulation of toxic trace elements by freshwater vascular plants. Rev. Environ. Contam. Toxicol. 1991, 121, 1–63. [Google Scholar]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: A comparative assessment. Environ. Sci. Pollut. Res. 2020, 27, 12138–12151. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Al-Sodany, Y.M.; Al-Yasi, H.M. Phytostabilization as a phytoremediation strategy for mitigating water pollutants by the floating macrophyte Ludwigia stolonifera (Guill. & Perr.) PH Raven. Int. J. Phytoremediat. 2020, 22, 373–382. [Google Scholar]
- Ebel, M.; Evangelou, M.; Schaeffer, A. Cyanide phytoremediation by water hyacinths (Eichhornia crassipes). Chemosphere 2007, 66, 816–823. [Google Scholar] [CrossRef]
- Rai, P.K.; Tripathi, B.D. Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from G.B. Pant Sagar of Singrauli Industrial region, India. Environ. Monit. Assess. 2009, 148, 75–84. [Google Scholar] [CrossRef]
- Ibrahim, N.; El Afandi, G. Phytoremediation uptake model of heavy metals (Pb, Cd and Zn) in soil using Nerium oleander. Heliyon 2020, 6, e04445. [Google Scholar] [CrossRef]
- Pantola, R.C.; Alam, A. Potential of Brassicaceae Burnett (Mustard family; Angiosperms) in Phytoremediation of Heavy Metals. Int. J. Sci. Res. Environ. Sci. 2014, 2, 120–138. [Google Scholar] [CrossRef]
- Selvapathy, P.; Sreedhar, P. Heavy metals removal by water hyacinth. J. Indian Public Health Eng. 1991, 3, 11–17. [Google Scholar]
- Sen, A.K.; Mondal, N.G. Salvinia natans—as the scavenger of Hg (II). Water Air Soil Pollut. 1987, 34, 439–446. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Biores. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, R.; Fahruddin, F.; Samawi, M.F. Phytoremediation relationship of lead (Pb) by Eichhornia crassipes on pH, BOD and COD in groundwater. J. Phys. Conf. Ser. 2019, 1341, 022020. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Noor, I.M.; Karim, H.I.A. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef] [Green Version]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Mishra, S.; Maiti, A. The efficiency of Eichhornia crassipes in the removal of organic and inorganic pollutants from wastewater: A review. Environ. Sci. Pollut. Res. 2017, 24, 7921–7937. [Google Scholar] [CrossRef]
- Arora, A.; Saxena, S.; Sharma, D.K. Tolerance and phytoaccumulation of Chromium by three Azolla species. World J. Microbiol. Biotechnol. 2005, 22, 97–100. [Google Scholar] [CrossRef]
- Hanafy, R.R.; Eweda, W.E.; Zayed, M.; Khalil, H.M. Potentiality of Using a Pinnata to Bioremediate Different Heavy Metals from Polluted Draining Water. Arab Univ. J. Agric. Sci. 2018, 26, 359–372. [Google Scholar] [CrossRef]
- Odjegba, V.J.; Fasidi, I.O. Accumulation of Trace Elements by Pistia stratiotes: Implications for phytoremediation. Ecotoxicology 2004, 13, 637–646. [Google Scholar] [CrossRef]
- Zimmels, Y.; Kirzhner, F.; Malkovskaja, A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environ. Manag. 2006, 81, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Saralegui, A.B.; Cirelli, A.F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 2004, 57, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Saleh, H.M.; Aglan, R.F.; Mahmoud, H.H. Ludwigia stolonifera for remediation of toxic metals from simulated wastewater. Chem. Ecol. 2018, 35, 164–178. [Google Scholar] [CrossRef]
- Saleh, H.M.; Mahmoud, H.H.; Aglan, R.F.; Bayoumi, T.A. Biological Treatment of Wastewater Contaminated with Cu(II), Fe(II) And Mn(II) Using Ludwigia stolonifera Aquatic Plant. Environ. Eng. Manag. J. 2019, 18, 1327–1336. [Google Scholar] [CrossRef]
- Sánchez-Galván, G.; Monroy, O.; Gómez, J.; Olguín, E.J. Assessment of the Hyperaccumulating Lead Capacity of Salvinia minima Using Bioadsorption and Intracellular Accumulation Factors. Water Air Soil Pollut. 2008, 194, 77–90. [Google Scholar] [CrossRef]
- Santos, J.d.S.; Pontes, M.D.S.; Grillo, R.; Fiorucci, A.R.; de Arruda, G.J.; Santiago, E.F. Physiological mechanisms and phytoremediation potential of the macrophyte Salvinia biloba towards a commercial formulation and an analytical standard of glyphosate. Chemosphere 2020, 259, 127417. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, B.; Wang, L.-A.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A review on phytoremediation of mercury contaminated soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef]
- Arreghini, S.; de Cabo, L.; de Iorio, A.F. Phytoremediation of two types of sediment contaminated with Zn by Schoenoplectus americanus. Int. J. Phytoremediat. 2006, 8, 223–232. [Google Scholar] [CrossRef]
- Murray-Gulde, C.L.; Huddleston, G.M.; Garber, K.V.; Rodgers, J.H. Contributions of Schoenoplectus californicus in a Constructed Wetland System Receiving Copper Contaminated Wastewater. Water Air Soil Pollut. 2005, 163, 355–378. [Google Scholar] [CrossRef]
- Miglioranza, K.S.; de Moreno, J.E.; Moreno, V.C.J. Organochlorine pesticides sequestered in the aquatic macrophyte Schoenoplectus californicus (CA Meyer) Sojak from a shallow lake in Argentina. Water Res. 2004, 38, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Kara, Y. Bioaccumulation of Copper from Contaminated Wastewater by Using Lemna minor. Bull. Environ. Contam. Toxicol. 2004, 72, 467–471. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Mkandawire, M.; Dudel, E. Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci. Total Environ. 2005, 336, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Mahrous, A.; Elshahat, R.; Kassem, M. Accumulation of Iron, Zinc and Lead by Azolla pinnata and Lemna minor and activity in contaminated water. Egypt. J. Chem. 2021, 64, 5017–5030. [Google Scholar] [CrossRef]
- Delgado, M.D.M.; Bigeriego, M.; Guardiola, E. Uptake of Zn, Cr and Cd by water hyacinths. Water Res. 1993, 27, 269–272. [Google Scholar] [CrossRef]
- Romero-Hernández, J.A.; Amaya-Chavez, A.; Balderas-Hernandez, P.; Roa-Morales, G.; Gonzalez-Rivas, N.; Balderas-Plata, M.A. Tolerance and hyperaccumulation of a mixture of heavy metals (Cu, Pb, Hg, and Zn) by four aquatic macrophytes. Int. J. Phytoremediat. 2017, 19, 239–245. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Kalu, C.M.; Rauwane, M.E.; Ntushelo, K. Microbial Spectra, Physiological Response and Bioremediation Potential of Phragmites australis for Agricultural Production. Front. Sustain. Food. Syst. 2021, 5, 696196. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef]
- Wang, K.-S.; Huang, L.-C.; Lee, H.-S.; Chen, P.-Y.; Chang, S.-H. Phytoextraction of cadmium by Ipomoea aquatica (water spinach) in hydroponic solution: Effects of cadmium speciation. Chemosphere 2008, 72, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, A.-J.; Zhao, F.-J.; Xu, G.-Z.; Duan, G.-L.; Zhu, Y.-G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, M.; Zarkami, R.; Sadeghi, R. Uptake and accumulation of heavy metals by water body and Azolla filiculoides in the Anzali wetland. Appl. Water Sci. 2021, 11, 91. [Google Scholar] [CrossRef]
- Cardwell, A.; Hawker, D.; Greenway, M. Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere 2002, 48, 653–663. [Google Scholar] [CrossRef]
- Rana, V.; Maiti, S.K. Municipal wastewater treatment potential and metal accumulation strategies of Colocasia esculenta (L.) Schott and Typha latifolia L. in a constructed wetland. Environ. Monit. Assess. 2018, 190, 328. [Google Scholar] [CrossRef] [PubMed]
- Choo, T.; Lee, C.; Low, K.; Hishamuddin, O. Accumulation of chromium (VI) from aqueous solutions using water lilies (Nymphaea spontanea). Chemosphere 2005, 62, 961–967. [Google Scholar] [CrossRef]
- Saha, L.; Tiwari, J.; Bauddh, K.; Ma, Y. Recent Developments in Microbe–Plant-Based Bioremediation for Tackling Heavy Metal-Polluted Soils. Front. Microbiol. 2021, 12, 731723. [Google Scholar] [CrossRef]
- Parikh, P.; Unadkat, K. Potential of Free Floating Macrophytes for Bioremediation of Heavy Metals-A Conceptual Review. In Strategies and Tools for Pollutant Mitigation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 309–336. [Google Scholar]
- Demırezen, D.; Aksoy, A. Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere 2004, 56, 685–696. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. 2019, 27, 4905–4916. [Google Scholar] [CrossRef]
- Elayan, N.M.S. Phytoremediation of Arsenic and Lead from Contaminated Waters by the Emergent Aquatic Macrophyte Althernanthera [sic] Philoxeroides [sic](alligatorweed); Southern University: Baton Rouge, LA, USA, 1999. [Google Scholar]
- Malaviya, P.; Singh, A.; Anderson, T.A. Aquatic phytoremediation strategies for chromium removal. Rev. Environ. Sci. Biotechnol. 2020, 19, 897–944. [Google Scholar] [CrossRef]
- Gupta, M.; Chandra, P. Bioaccumulation and toxicity of mercury in rooted-submerged macrophyte Vallisneria spiralis. Environ. Pollut. 1998, 103, 327–332. [Google Scholar] [CrossRef]
- Solanki, P.; Dotaniya, M.L.; Khanna, N.; Meena, S.S.; Rabha, A.K.; Rawat, A.; Dotaniya, C.K.; Srivastava, R.K. Recent Advances in Bioremediation for Clean-Up of Inorganic Pollutant-Contaminated Soils. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 299–310. [Google Scholar]
- Sudarshan, P.; Mahesh, M.K.; Ramachandra, T.V. Dynamics of Metal Pollution in Sediment and Macrophytes of Varthur Lake, Bangalore. Bull. Environ. Contam. Toxicol. 2020, 104, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Polechońska, L.; Klink, A. Trace metal bioindication and phytoremediation potentialities of Phalaris arundinacea L. (reed canary grass). J. Geochem. Explor. 2014, 146, 27–33. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A.; Dambiec, M.; Rudecki, A. Evaluation of Ceratophyllum demersum as the accumulative bioindicator for trace metals. Ecol. Indic. 2018, 93, 274–281. [Google Scholar] [CrossRef]
- Suñe, N.; Sánchez, G.; Caffaratti, S.; Maine, M. Cadmium and chromium removal kinetics from solution by two aquatic macrophytes. Environ. Pollut. 2007, 145, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Eid, E.M.; Dakhil, M.A.; Hassan, L.M. Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int. J. Phytoremediat. 2018, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.P.; Boaventura, R.A. Uptake and release kinetics of copper by the aquatic moss Fontinalis antipyretica. Water Res. 1998, 32, 1305–1313. [Google Scholar] [CrossRef]
- Zotina, T.; Dementyev, D.; Alexandrova, Y. Long-term trends and speciation of artificial radionuclides in two submerged macrophytes of the Yenisei River: A comparative study of Potamogeton lucens and Fontinalis antipyretica. J. Environ. Radioact. 2020, 227, 106461. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Delgado-González, C.R.; Madariaga-Nacarrete, A.; Fernandz-Cortes, J.M.; Islas-Pelcastre, M.; Oza, G.; Iqbal, H.M.N.; Sharma, A. Advances and Applications of Water Phytoremediation: A Potential Biotechnological Approach for the Treatment of Heavy Metals from Contaminated Water. Environ. Res. Public Health 2021, 18, 5215. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.; Basibuyuk, M.; Forster, C. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Polechońska, L.; Klink, A. Validation of Hydrocharis morsus-ranae as a possible bioindicator of trace element pollution in freshwaters using Ceratophyllum demersum as a reference species. Environ. Pollut. 2020, 269, 116145. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.M.; Ganjo, D.G.A. Native aquatic plants for phytoremediation of metals in outdoor experiments: Implications of metal accumulation mechanisms, Soran City-Erbil, Iraq. Int. J. Phytoremediat. 2021, 23, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Molisani, M.; Rocha, R.; Machado, W.; Barreto, R.C.; Lacerda, L.D. Mercury contents in aquatic macrophytes from two reservoirs in the Paraíba do Sul: Guandú river system, SE Brazil. Braz. J. Biol. 2006, 66, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ao, Y.; Yang, X.; Li, T. Treating eutrophic water for nutrient reduction using an aquatic macrophyte (Ipomoea aquatica Forsskal) in a deep flow technique system. Agric. Water Manag. 2008, 95, 607–615. [Google Scholar] [CrossRef]
- Verma, V.; Tewari, S.; Rai, J. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Phytoaccumulation of zinc by the aquatic plant, Lemna gibba L. Bioresour. Technol. 2009, 100, 6137–6140. [Google Scholar] [CrossRef]
- Babic, M.; Radić, S.; Cvjetko, P.; Roje, V.; Pevalek-Kozlina, B.; Pavlica, M. Antioxidative response of Lemna minor plants exposed to thallium(I)-acetate. Aquat. Bot. 2009, 91, 166–172. [Google Scholar] [CrossRef]
- Kanoun-Boulé, M.; Vicente, J.A.; Nabais, C.; Prasad, M.; Freitas, H. Ecophysiological tolerance of duckweeds exposed to copper. Aquat. Toxicol. 2009, 91, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Uysal, Y.; Taner, F. Effect of pH, temperature, and lead concentration on the bioremoval of lead from water using Lemna minor. Int. J. Phytoremediat. 2009, 11, 591–608. [Google Scholar] [CrossRef]
- Skinner, K.; Wright, N.; Porter-Goff, E. Mercury uptake and accumulation by four species of aquatic plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B. Salvinia: An aquatic fern with potential use in phytoremediation. Environ. We Int. J. Sci. Technol. 2009, 4, 23–27. [Google Scholar]
- Aravind, P.; Prasad, M.; Malec, P.; Waloszek, A.; Strzałka, K. Zinc protects Ceratophyllum demersum L. (free-floating hydrophyte) against reactive oxygen species induced by cadmium. J. Trace Elements Med. Biol. 2009, 23, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Monferrán, M.V.; Sanchez, A.J.A.; Pignata, M.L.; Wuderlin, D.A. Copper-induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environ. Pollut. 2009, 157, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Sivaci, E.R.; Sivaci, A.; Sökmen, M. Biosorption of cadmium by Myriophyllum spicatum L. and Myriophyllum triphyllum orchard. Chemosphere 2004, 56, 1043–1048. [Google Scholar] [CrossRef]
- Mueller, U.; Sachs, J. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Liu, Q.; Chen, M.; Liu, X.; Wang, Y.; Sun, Y.; Zhang, L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]



| Hyperaccumulator | Heavy Metal | Reference |
|---|---|---|
| Arabidopsis halleri | Zn | [17,18] |
| Achillea millefolium | Hg | [19,20] |
| Alyssum murale | Ni | [21,22] |
| Azolla pinnata | Cd | [23,24] |
| Thalaspi caerulescene | Zn | [25] |
| Brassica juncea L. | Cu, Zn, Pb | [26,27] |
| Brassica napus L. | Cu, Zn, Pb | [28,29] |
| Brassica oleracea, Raphanus sativus | Zn, Cd, Ni, Cu | [30] |
| Brassica nigra | Pb | [31,32] |
| Betula occidentalis | Pb | [31,33] |
| Cardaminopsis halleri | Zn, Pb, Cd, Cu | [34] |
| Cannabis sativa L. | Cd | [35,36] |
| Cicer aeritinum L. | Cd, Pb, Cr, Cu | [37] |
| Cucumis sativus L. | Pb | [38,39] |
| Eichhornia crassipes L. | Cr, Zn | [40,41] |
| Eleocharis acicularis | As | [42,43] |
| Euphorbia cheiradenia | Pb, Zn, Cu, Ni | [44,45] |
| Haumaniastrum katangense | Cu | [45,46] |
| Helianthus annuus | Pb, Cd | [47] |
| Jaltropa curcas L. | Cu, Mn, Cr, As, Zn, Hg | [48,49] |
| Lantana camara L. | Pb | [50,51] |
| Lavadula vera L. | Pb | [52] |
| Lens culunaris Medic. | Pb | [53] |
| Lepidium sativum L. | As, Cd, Pb | [54,55] |
| Lactuca sativa L. | Cu, Mn, Zn, Ni, Cd, | [37] |
| Marrubium vulgare | Hg | [56,57] |
| Miscanthus x giganteus | Cu, Ni, Pb, Zn | [58] |
| Medicago sativa | Pb | [31,59] |
| Noccaea Caerulescens | Pb | [60] |
| Oryza sativa L. | Cu, Cd | [61,62] |
| Minuartia verna, Agrostis tenius | Pb | [63,64] |
| Pelargonium | Pb | [65,66] |
| Pisum sativum L. | Pb, Cu, Zn, Ni, As, Cr | [67] |
| Potentila griffithii | Zn | [68,69] |
| Pteris vittata | Hg | [19,70] |
| Rapanus sativus L. | Cd, Fe, Pb, Cu | [54,71] |
| Salvia sclarea L. | Pb, Cd, Zn | [69,72] |
| Spinacia oleracea L. | Cu, Ni, Zn, Pb, Cr | [73,74] |
| Sorghum bicolor L. | Cd, Cu, Zn | [72,75] |
| Sorghum halepense L. | Pb | [76,77] |
| Trifolium alexandrinum | Zn, Pb, Cu, Cd | [78,79] |
| Tagetes minuta | As, Pb | [76,80] |
| Thlaspi caerulescens | Cd | [31,81] |
| Viola principis | Pb | [82] |
| Alga | Heavy Metal | Reference |
|---|---|---|
| Ascophyllum nodosum | Ni, Pb | [119,120] |
| Cladophora fascicularis | Pb (II) | [121,122] |
| Cladophora glomerata | Zn, Cu | [123,124] |
| Cladophora glomerata, Oedogonium rivulare | Cu, Pb, Cd, Co | [125,126] |
| Cymodocea nodosa | Cu, Zn | [127,128] |
| Fucus vesiculosis, Laminaria japonica | Zn | [129,130] |
| Oscillatoria quadripunctulata, | Cu, Pb | [30] |
| Sargassum filipendula | Cu | [131,132] |
| Sargassum natans | Pb | [119,133] |
| Spirogyra hyaline | Cd, Hg, Pb, As | [134,135] |
| Common Name | Scientific Name | Trace Elements | References |
|---|---|---|---|
| Duckweed | Lemna gibba L. | As, U, Zn | [256,257] |
| Lesser duckweed | Lemna minor L. | As, Zn, Cu, Hg | [258,259] |
| Water hyacinth | Eichornia crassipes | As, Fe, Cu, Zn, Pb, Cd, Cr, Ni, Hg | [257,259,260] |
| Common reed | Phragmites australis | Cr, Cu, Ni, Pb, S, V, Cd, | [260,261] |
| Water spinach | Ipomoea aquatic | As, Cd, Pb, Hg, Cu, Zn | [262,263] |
| Water fern | Azolla filiculoides, azolla pinnata | As, Hg, Cd | [264,265] |
| Elephant ear | Colocasia esculenta | Cd, Pb, Cu, Zn | [55,266] |
| Water lily | Nymphaea violacea, Nymphaea aurora | Cd, Pb, Cu, Zn | [23,267,268] |
| Water pepper | Polygonum hydropiper | As | [266,267] |
| Marshwort | Nymphoides germinate | Cd, Cu, Pb, Zn | [264,268] |
| Lesser bulrush | Typha latifolia | Cd, Pb, Cr, Ni, Zn, Cu | [269,270] |
| Brazillian waterweed | Veronica aquatic | As, Cr | [271,272] |
| Tape grass/eel grass | Vallisneria spiralis | Hg | [273,274] |
| Alligator weed | Althernanthera philoxeroides | As, Pb | [271,275] |
| Reed canary grass | Phalaris arundinacea L. | Pb, Zn, Cu, Cd | [276,277] |
| Water lettuce | Pistia stratiotes | As, Cr, Pb, Ag, Cd, Cu, Hg, Ni, Zn | [278,279] |
| Willow moss | Fontinalis antipyretica | Cu, Zn | [280,281] |
| Needle spikerush | Eleocharis acicularis | As, Ag, Pb, Cu, Cd, Zn, Ni, Mg | [282,283] |
| Rigid hornwort | Ceretophyllum demersum | As, Pb, Zn, Cu | [284,285] |
| Watercresses | Nasturtium officinale | Cu, Zn, Ni | [78,286] |
| Plants | Heavy Metals | Accumulation (Dry Weight Basis) | Reference |
|---|---|---|---|
| Eichhornia crassipes | Hg | 119ng Hg g−1 | [287] |
| Cd | 3992 µg Cd g−1 | [237] | |
| Cu | 314 µg Cu g−1 | [288] | |
| Cr | 2.31 mg Cr g−1 | [289] | |
| Cd | 1.98 mg Cd g−1 | [289] | |
| Ni | 1.68 mg Ni g−1 | [289] | |
| Elodea densa | Hg | 177 µg Hg g−1 | [287] |
| Lemna gibba | Ur | 897 µg Ur g−1 | [290] |
| As | 1022 µg As g−1 | [290] | |
| Lemna minor | Zn | 4.23–25.81 mg Zn g−1 | [291] |
| Ti | 221 µg Ti g−1 | [292] | |
| Cu | 400 µg Cu g−1 | [293] | |
| Pb | 8.62 mg Pb g−1 | [294] | |
| Pistia stratiotes | Hg | 83 µg Hg g−1 | [295] |
| Cr | 2.50 mg Cr g−1 | [289] | |
| Cd | 2.13 mg Cd g−1 | [289] | |
| Ni | 1.95 mg Ni g−1 | [289] | |
| Salvinia natans | Cr | 7.40 mg Cr g−1 | [296] |
| Ceratophyllum demersum | As | 525 µg As g−1 | [237] |
| Cd | 1293 µg Cd g−1 | [237] | |
| Zn | 57 µg Zn g−1 | [297] | |
| Potamogeton pusillus | Cu | 162 µg Cu g−1 | [298] |
| Vallisneria spiralis | Cr | 2.85 mg Cr g−1 | [289] |
| Cd | 2.62 mg Cd g−1 | [289] | |
| Ni | 2.14 mg Ni g−1 | [289] | |
| Hg | 158 µg Hg g−1 | [232] | |
| Myriphyllum triphyllum | Cd | 17 µg Cd g−1 | [299] |
| Sagittaria montevidensis | Hg | 62 mg Hg g−1 | [287] |
| Wolffia globose | As | 1000 µg As g−1 | [300] |
| Spirodela polyrhiza | As | 7.65 n mol As g−1 | [282] |
| Mentha sp. | Fe | 378 µg Fe g−1 | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. https://doi.org/10.3390/plants11091255
Sabreena, Hassan S, Bhat SA, Kumar V, Ganai BA, Ameen F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants. 2022; 11(9):1255. https://doi.org/10.3390/plants11091255
Chicago/Turabian StyleSabreena, Shahnawaz Hassan, Sartaj Ahmad Bhat, Vineet Kumar, Bashir Ahmad Ganai, and Fuad Ameen. 2022. "Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology" Plants 11, no. 9: 1255. https://doi.org/10.3390/plants11091255