The PAP Gene Family in Tomato: Comprehensive Comparative Analysis, Phylogenetic Relationships and Expression Profiles
Abstract
:1. Introduction
2. Results
2.1. Identification of the PAP Gene Family in the Tomato Genome
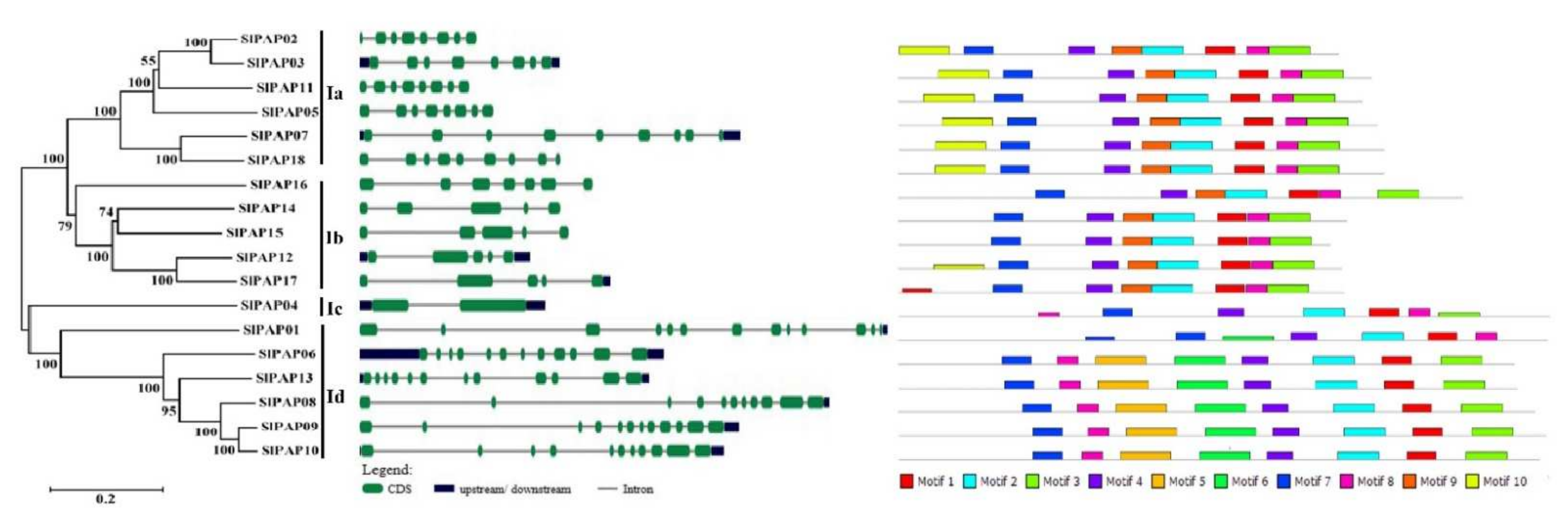
2.2. Phylogenetic Analysis of the PAP Genes in Tomato
2.3. Intron/Exon Structure and Conserved Motifs of Members of SlPAP Gene Family in Tomato
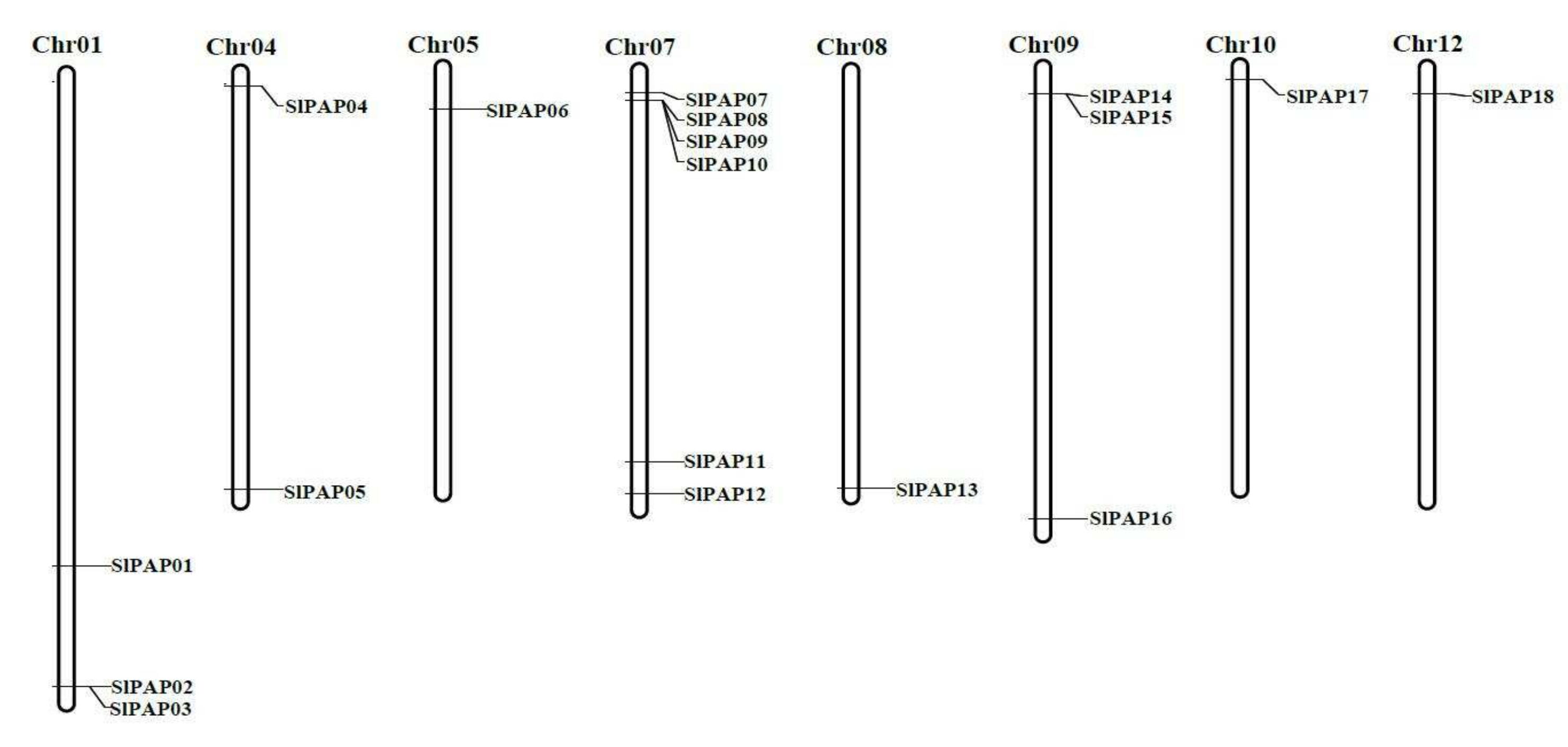
2.4. Chromosomal Distributions and Duplications of the PAP Genes in Tomato
2.5. Promoter Cis-Element Analysis
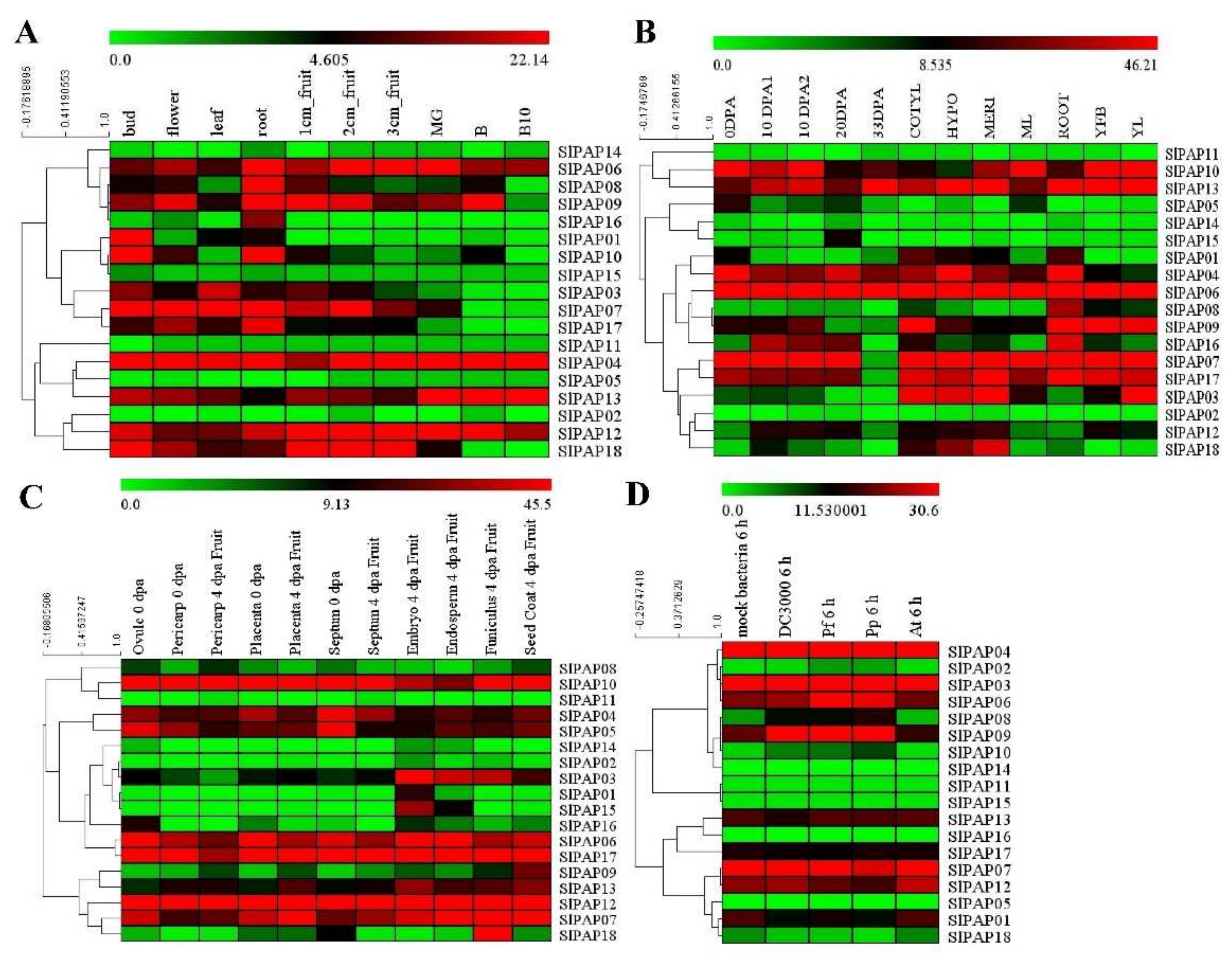
2.6. Expression Analysis of Members of SlPAP Gene Family in Different Tissues and Organs Based on RNA-Seq Data
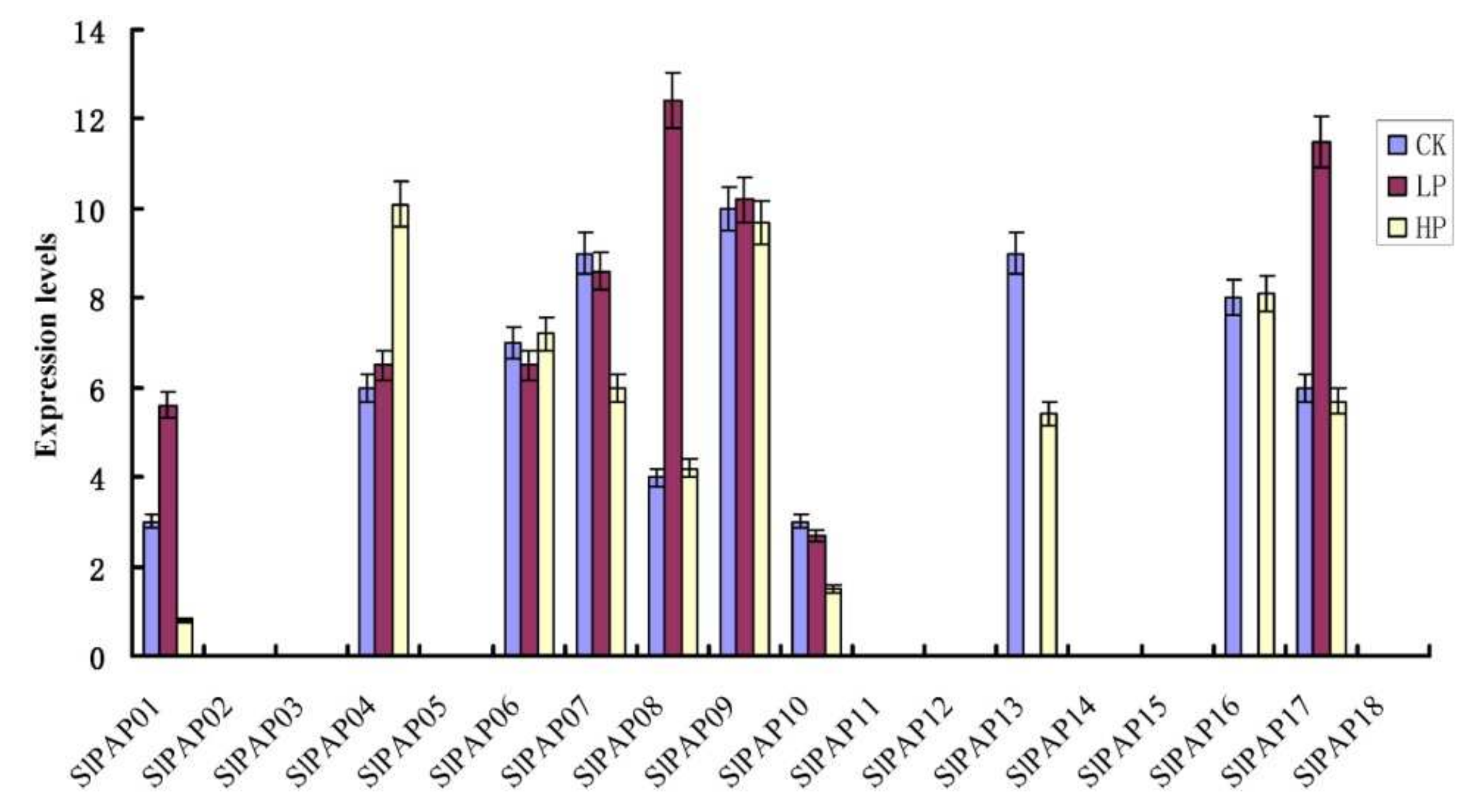
2.7. Expression Analysis of the SlPAP Genes under Phosphate Stress Treatment Condition Using qRT-PCR
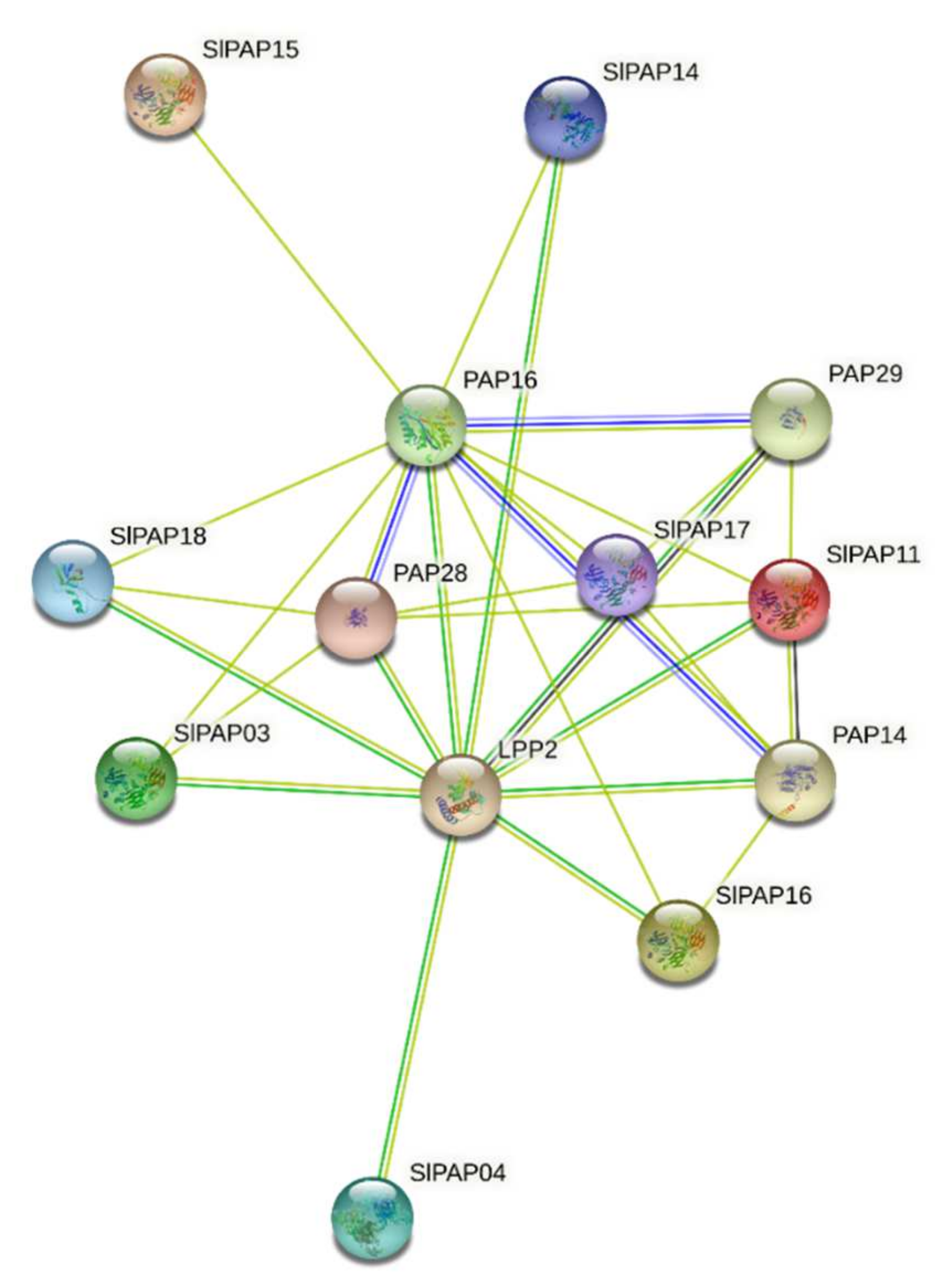
2.8. Interaction Networks of SlPAP Proteins
3. Discussion
4. Materials and Methods
4.1. Identification of SlPAP Genes in Tomato
4.2. Subcellular Localization, Conserved Motifs and Gene Structure Analyses of the SlPAP Proteins
4.3. Multiple Sequence Alignment and Phylogenetic Analysis
4.4. Chromosome Distribution of PAP Genes in Tomato
4.5. Protein–Protein Interaction Analysis
4.6. Promoter Cis-Element Analysis
4.7. Tissue-Specific Expression Analysis
4.8. Plant Materials and Growth Conditions
4.9. RNA Isolation and Quantitative Real Time-PCR (qPCR) Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Li, C.C.; Gui, S.H.; Yang, T.; Walk, T.; Wang, X.R.; Liao, H. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012, 109, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995; pp. 483–507. [Google Scholar]
- Abel, S.; Ticconi, C.A.; Delatorre, C.A. Phosphate sensing in higher plants. Physiol. Plant. 2002, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.H.; Danon, A.; Baerlein, D.A.; McDaniel, R.G. Phosphate starvation inducible metabolism in Lycopersicon esculentum: II. Characterization of the phosphate starvation inducible-excreted acid phosphatase. Plant Physiol. 1988, 87, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Muchhal, U.S.; Uthappa, M.; Kononowica, A.K.; Raghothama, K.G. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol. 1998, 116, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghothama, K.G. Phosphate acquisition. Annual review of plant physiology and plant molecular biology. Plant Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Olczak, M.; Morawiecka, B.; Watore, W. Plant purplr acid phosphatases-genes, structures and biological function. Acta BiBiochim. Pol. 2003, 50, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Li, D.P.; Zhu, H.F.; Liu, K.F.; Liu, X.; Leggewie, G.; Udvardi, M.; Wang, D.W. Purple acid phosphatases of Arabidopsis thaliana. Comparative analysis and differential regulation by phosphate deprivation. J. Biol. Chem. 2002, 277, 27772–27781. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, C.; Tian, J.; Li, K.; Shou, H. Identification of rice purple acid phosphatases related to phosphate starvation signaling. Plant Biol. 2011, 13, 7–15. [Google Scholar] [CrossRef]
- González-Muñoz, E.; Avendaño-Vázquez, A.; Montes, R.A.; Folter, S.D.; Andrés-Hernández, L.; Abreu-Goodger, C.; Sawers, R.J.H. The maize (Zea mays ssp. mays var. B73) genome encodes 33 members of the purple acid phosphatase family. Front. Plant Sci. 2015, 6, 341. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.Y.; Wang, F.; Fan, H.Q.; Fang, Y.M.; Li, M.F. Identification of tea plant purple acid phosphatase genes and their expression responses to excess iron. Int. J. Mol. Sci. 2019, 20, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Li, Z.; Qian, W.Q.; Guo, W.L.; Gao, X.; Huang, L.L.; Wang, H.; Zhu, H.F.; Wu, J.W.; Wang, D.W.; et al. The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol. 2011, 157, 1283–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, W.D.; Carson, I.; Ying, S.; Ellis, K.; Plaxton, W. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 2012, 196, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Tian, J.; Lam, H.; Lim, B.L.; Yan, X.L.; Liao, H. Biochemical and molecular characterization of PvPAP3, a novel purple acid phosphatase isolated from common bean enhancing extracellular ATP utilization. Plant Physiol. 2010, 152, 854–865. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.F.; Qian, W.Q.; Lu, X.Z.; Li, D.P.; Liu, X.; Liu, K.F.; Wang, D.W. Expression patterns of purple acid phosphatase genes in Arabidopsis organs and functional analysis of AtPAP23 predominantly transcribed in flowers. Plant Mol. Biol. 2005, 59, 581–594. [Google Scholar] [CrossRef]
- Kaida, R.; Hayashi, T.; Kaneko, T.S. Purple acid phosphatase in the walls of tobacco cells. Phytochemistry 2008, 69, 2546–2551. [Google Scholar] [CrossRef]
- Herman, V.D. Biochemical and Molecular Characterization of AtPAP25, A Novel Cell Wall-Localized Purple Acid Phosphatase Isozyme Upregulated by Phosphate-Starved Arabidopsis thaliana. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2012. [Google Scholar]
- Law, Y.S.; Zhang, R.S.; Guan, X.Q.; Cheng, S.F.; Sun, F.; Duncan, O.; Murcha, M.W.; Whelan, J.; Lim, B.L. Phosphorylation and dephosphorylation of the presequence of precursor MULTIPLE ORGANELLAR RNA EDITING FACTOR3 during import into mitochondria from Arabidopsis. Plant Physiol. 2015, 169, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.S.; Guan, X.Q.; Law, Y.S.; Sun, F.; Chen, S.; Wong, K.B.; Lim, B.L. AtPAP2 modulates the import of the small subunit of Rubisco into chloroplasts. Plant Signal. Behav. 2016, 11, e1239687. [Google Scholar] [CrossRef] [Green Version]
- Ping, T.L.; Chao, Z.; Shan, W.S.; Jia, Y.; Sheng, Z.X.; Hua, S.Y. FUSCA3 interacting with LEAFY COTYLEDON2 controls lateral root formation through regulation YUCCA4 gene expression in Arabidopsis thaliana. New Phytol. 2017, 213, 1740–1754. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Law, Y.S.; Cheng, S.F.; Lim, B.L. RNA editing of cytochrome c maturation transcripts is responsive to the energy status of leaf cells in Arabidopsis thaliana. Mitochondrion 2017, 35, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.L.; Lee, S.Y.; Yang, W.T.; Lee, S.W.; Baek, D.W.; Li, M.S.; Kim, D.H. The Burholderia pyrrocinia purple acid phosphatase PAP9 mediates phosphate acquisition in plants. J. Plant Biol. 2019, 62, 342–350. [Google Scholar] [CrossRef]
- Bozzo, G.G.; Raghothama, K.G.; Paxton, W.C. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starve tomato (Lycopericon esculentum) cell cultures. Eur. J. Biochem. 2002, 269, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.G.; Raghothama, K.G.; Plaxton, W.C. Structural and kinetic properties of a novel purplr acid phosphatase from phosophate-starved tomato (Lycopericon esculentum) cell cultures. Biochem. J. 2004, 377, 419–428. [Google Scholar] [CrossRef]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopericon esculentum) suspension cells and seedlings. Plant Cell Environ. 2006, 29, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Suen, P.K.; Zhang, S.Y.; Sun, S.S. Molecular characterization of a tomato purple acid phosphatase during seed germination and seedling growth under phosphate stress. Plant Cell Rep. 2015, 34, 981–992. [Google Scholar] [CrossRef]
- Kumar, M.; Kherawat, B.S.; Dey, P.; Saha, D.; Singh, A.; Bhatia, S.K.; Ghodake, G.S.; Kadam, A.A.; Kim, H.; Manorama; et al. Genome-wide identification and characterization of PIN-FORMED (PIN) Gene family reveals role in developmental and various stress conditions in Triticum aestivum L. Int. J. Mol. Sci. 2021, 22, 7396. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Fang, Y.X.; Zhang, Z.L.; Zheng, J.J.; Zhang, X.; Li, J.; Niu, C.Y.; Xue, D.W.; Zhang, X.Q. Genome-wide identification and expression pattern analysis of the KCS gene family in barley. Plant Growth Regul. 2021, 93, 89–103. [Google Scholar] [CrossRef]
- Pooja, M.B.; Debasish, B.K.; Kuntala, S.B.; Sarvajeet, S.G.; Niraj, A. Genome wide identification and characterization of abiotic stresss responsive IncRNAs in Capsicum annuum. Plant Physiol. Biochem. 2021, 162, 221–236. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Kabi, M.; Debnath, D.; Saha, D.; Khandual, A.; Rout, S.; Manorama; et al. Genome-wide identification and characterization of Brassinazole-resistant (BZR) Gene Family and Its expression in the Various Developmental stage and Stress Conditions in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 8743. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, H.; Liu, J.; Du, Q.; Lu, S.; Liu, C. Genome-wide identification and functional characterization of natural antisense transcripts in Salvia miltiorrhiza. Sci. Rep. 2021, 11, 4769. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.W.; Zhou, W.; Xie, L.N.; Wang, L.S.; Zhang, Y.L.; Zhang, Q.Z. Genome-wide identification and expression analysis of the AT-hook Motif Nuclear Localized gene family in soybean. BMC Genom. 2021, 22, 361. [Google Scholar] [CrossRef]
- Tomscha, J.L.; Trull, M.C.; Deikman, J.; Lynch, J.P.; Guiltinan, M.J. Phosphatase under-producer mutants have altered phosphorus relations. Plant Physiol. 2004, 135, 334–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lung, S.C.; Leung, A.; Kuang, R.; Wang, Y.; Leung, P.; Lim, B.L. Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase. Phytochemistry 2008, 69, 365–373. [Google Scholar] [CrossRef]
- Sun, F.; Carrie, C.; Law, S.; Murcha, M.W.; Zhang, R.S.; Law, Y.S.; Suen, P.K.; Whelan, J.; Lim, B.L. AtPAP2 is a tail-anchored protein in the outer membrane of chloroplasts and mitochondria. Plant Signal. Behav. 2012, 7, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.Q.; Li, J.Y.; Cheng, W.Z.; Guo, H.H.; Liu, X.M.; Gao, H.B. AtPAP2, a unique member of the PAP family, functions in the plasma membrane. Genes 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pozo, J.C.; Allona, I.; Rubio, V.; Leyva, A.; de la Peña, A.; Aragoncillo, C.; Paz-Ares, J. A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilizing/oxidative stress conditions. Plant J. 1999, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wong, F.L.; Phang, T.H.; Cheung, M.Y.; Li, W.Y.; Shao, G.; Yan, X.L.; Lam, H.M. GmPAP3, a novel purple acid phosphatase-like gene in soybean induced by NaCl stress but not phosphorus deficiency. Gene 2003, 318, 103–111. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008, 146, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Bhadouria, J.; Singh, A.P.; Mehra, P.; Verma, L.; Srivastawa, R.; Parida, S.K.; Giri, J. Identification of purple acid phosphatases in chickpea and potential roles of CaPAP7 in seed phytate accumulation. Sci. Rep. 2017, 7, 11012. [Google Scholar] [CrossRef] [Green Version]
- Kaida, R.; Satoh, Y.; Bulone, V.; Yamada, Y.; Kaku, T.; Hayashi, T.; Kaneko, T.S. Activation of β-glucan synthases by wall-bound purple acid phosphatase in tobacco cells. Plant Physiol. 2009, 150, 1822–1830. [Google Scholar] [CrossRef] [Green Version]
- Kaida, R.; Serada, S.; Norioka, N.; Norioka, S.; Neumetzler, L.; Pauly, M.; Sampedro, J.; Zarra, I.; Hayashi, T.; Kaneko, T.S. Potential role for purple acid phosphatase in the dephosphorylation of wall proteins in tobacco cells. Plant Physiol. 2010, 153, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddie, G.W.; Schenk, G.; Angel, N.Z.; Walsh, N.; Guddat, L.W.; de Jersey, J.; Cassady, A.I.; Hamilton, S.E.; Hume, D.A. Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone 2000, 27, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Cashikar, A.G.; Kumaresan, R.; Rao, N.M. Biochemical characterization and subcellular localization of the red kidney bean purple acid phosphatase. Plant Physiol. 1997, 114, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veljanovski, V.; Vanderbeld, B.; Knowles, V.L.; Snedden, W.A.; Plaxton, W.C. Biochemical and molecular characterization of AtPAP26, a vacuolar purple acid phosphatase up-regulated in phosphate-deprived Arabidopsis suspension cells and seedlings. Plant Physiol. 2006, 142, 1282–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vecchio, H.A.; Ying, S.; Park, J.; Knowles, V.L.; Kanno, S.; Tanoi, K.; She, Y.; Plaxton, W.C. The cell wall-targeted purple acid phosphatase AtPAP25 is critical for acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. Plant J. 2014, 80, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Vision, T.J.; Daniel, G.B.; Steven, D.T. The Origins of Genomic Duplications in Arabidopsis. Science 2000, 290, 2114–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.T.; Qian, W.Q.; Hurley, B.A.; She, Y.M.; Wang, D.W.; Plaxton, W.C. Biochemical and molecular characterization of AtPAP12 and AtPAP26: The predominant purple acid phosphatase isozymes secreted by phosphate-starved Arabidopsis thaliana. Plant Cell Environ. 2010, 33, 1789–1803. [Google Scholar] [CrossRef]
- Tian, J.L. Functional Analysis of Genes in Rice Purple Acid Phosphatase Ia Subgroup. Master’s Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Chou, K.C.; Shen, H.B. A new method for predicting the subcellular localization of eukaryotic proteins with both single and multiple sites: Euk-mPLoc 2.0. PLoS ONE 2010, 5, e9931. [Google Scholar] [CrossRef]
- Howe, E.; Holton, K.; Nair, S.; Schlauch, D.; Sinha, R.; Quackenbush, J. Mev: Multiexperiment viewer. In Biomedical Informatics for Cancer Research; Ochs, M.F., Ed.; Springer US: New York, NY, USA, 2010; pp. 267–277. [Google Scholar]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef]


| Gene | Gene Accession No. | Chromosome Location | Length of Protein | Predicted Size (kDa) | pI | No.of Exons/Intros | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| SlPAP01 | Solyc01g068380.2.1 | ch01:77582424..77592344 | 640 | 71.3 | 5.85 | 13/12 | extracellular |
| SlPAP02 | Solyc01g110050.1.1 | ch01:96853466..96855663 | 433 | 49.7 | 7.04 | 8/7 | extracellular |
| SlPAP03 | Solyc01g110060.2.1 | ch01:96857667..96861427 | 466 | 53.2 | 6.34 | 8/7 | extracellular |
| SlPAP04 | Solyc04g005450.2.1 | ch04:310785..314269 | 649 | 73.0 | 6.67 | 2/1 | ER, extracellular |
| SlPAP05 | Solyc04g080920.1.1 | ch04:64973575..64976085 | 472 | 54.2 | 6.41 | 8/7 | extracellular |
| SlPAP06 | Solyc05g012260.2.1 | ch05:5546798..5552510 | 607 | 68.2 | 6.41 | 13/12 | extracellular |
| SlPAP07 | Solyc07g007670.2.1 | ch07:2316679..2323829 | 478 | 54.8 | 6.60 | 10/9 | extracellular |
| SlPAP08 | Solyc07g008550.2.1 | ch07:3488895..3497719 | 627 | 71.0 | 6.53 | 11/10 | Cytomembrane, ER, extracellular |
| SlPAP09 | Solyc07g008560.2.1 | ch07:3500707..3507834 | 637 | 72.3 | 6.68 | 12/11 | ER, extracellular |
| SlPAP10 | Solyc07g008570.2.1 | ch07:3522999..3529845 | 631 | 71.7 | 6.70 | 11/10 | ER, extracellular |
| SlPAP11 | Solyc07g053070.1.1 | ch07:61511946..61514007 | 457 | 52.8 | 6.82 | 8/7 | extracellular |
| SlPAP12 | Solyc07g064500.2.1 | ch07:66617664..66620861 | 437 | 49.5 | 5.89 | 5/4 | extracellular |
| SlPAP13 | Solyc08g083250.2.1 | ch08:65763315..65768754 | 609 | 68.6 | 6.08 | 12/11 | extracellular |
| SlPAP14 | Solyc09g009600.1.1 | ch09:3016831..3020600 | 442 | 50.4 | 6.20 | 5/4 | extracellular |
| SlPAP15 | Solyc09g009610.1.1 | ch09:3023290..3027216 | 426 | 47.9 | 5.80 | 5/4 | extracellular |
| SlPAP16 | Solyc09g091910.1.1 | ch09:71122299..71126678 | 556 | 63.2 | 5.84 | 7/6 | extracellular |
| SlPAP17 | Solyc10g006300.2.1 | ch10:950380..955091 | 439 | 49.6 | 6.85 | 5/4 | extracellular |
| SlPAP18 | Solyc12g009800.1.1 | ch12:3005117..3008886 | 478 | 55.3 | 8.22 | 9/8 | extracellular |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Zhou, G.; Wan, H. The PAP Gene Family in Tomato: Comprehensive Comparative Analysis, Phylogenetic Relationships and Expression Profiles. Plants 2022, 11, 563. https://doi.org/10.3390/plants11040563
Pang X, Cheng Y, Ruan M, Ye Q, Wang R, Yao Z, Zhou G, Wan H. The PAP Gene Family in Tomato: Comprehensive Comparative Analysis, Phylogenetic Relationships and Expression Profiles. Plants. 2022; 11(4):563. https://doi.org/10.3390/plants11040563
Chicago/Turabian StylePang, Xin, Yuan Cheng, Meiying Ruan, Qingjing Ye, Rongqing Wang, Zhuping Yao, Guozhi Zhou, and Hongjian Wan. 2022. "The PAP Gene Family in Tomato: Comprehensive Comparative Analysis, Phylogenetic Relationships and Expression Profiles" Plants 11, no. 4: 563. https://doi.org/10.3390/plants11040563






