Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves
Abstract
:1. Introduction
2. Description of the Two-Dimensional Model
3. Results
3.1. The Influence of Light Intensity and H+-ATPase Activity on Parameters of Photosynthetic Assimilation of CO2
3.2. The Influence of CO2 Conductance of Plasma Membrane and Chloroplast Envelopes on Parameters of Photosynthetic Assimilation of CO2
4. Discussion
5. Materials and Methods
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foyer, C.H.; Ruban, A.V.; Nixon, P.J. Photosynthesis solutions to enhance productivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160374. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [PubMed] [Green Version]
- Tholen, D.; Zhu, X.-G. The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol. 2011, 156, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Berghuijs, H.N.; Yin, X.; Ho, Q.T.; Driever, S.M.; Retta, M.A.; Nicolaï, B.M.; Struik, P.C. Mesophyll conductance and reaction-diffusion models for CO2 transport in C3 leaves; needs, opportunities and challenges. Plant Sci. 2016, 252, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Farquhar, G.; Berry, J. Biochemical model of C3 photosynthesis. In Photosynthesis In Silico: Advances in Photosynthesis and Respiration; Laisk, A., Nedbal, L., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 29, pp. 209–230. [Google Scholar]
- Von Caemmerer, S. Steady-state models of photosynthesis. Plant Cell Environ. 2013, 36, 1617–1630. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Ichii, M.; Asanuma, K.; Kusutani, A. Optimum and sub-optimal temperature effects on stomata and photosynthesis rate of determinate soybeans. Acta Hortic. 1996, 440, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, Y.; Zhu, L.; Peng, S.; Li, Y. Temperature responses of photosynthesis and stomatal conductance in rice and wheat plants. Agric. Forest Meteorol. 2021, 300, 108322. [Google Scholar] [CrossRef]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnoli, E.; Lauteri, M. Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol. 1991, 95, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, I.A.A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and photosynthetic traits are associated with investigating sodium chloride tolerance of Brassica napus L. cultivars. Plants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [PubMed] [Green Version]
- Ivanov, B.; Khorobrykh, S. Participation of photosynthetic electron transport in production and scavenging of reactive oxygen species. Antioxid. Redox Signal. 2003, 5, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef]
- Elferjani, R.; Benomar, L.; Momayyezi, M.; Tognetti, R.; Niinemets, Ü.; Soolanayakanahally, R.Y.; Théroux-Rancourt, G.; Tosens, T.; Ripullone, F.; Bilodeau-Gauthier, S.; et al. A meta-analysis of mesophyll conductance to CO2 in relation to major abiotic stresses in poplar species. J. Exp. Bot. 2021, 72, 4384–4400. [Google Scholar]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ortuño, M.F.; Ribas-Carbo, M.; Diaz-Espejo, A.; Flórez-Sarasa, I.D.; Medrano, H. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol. 2007, 175, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Long, S.P. Photosynthetic efficiency and mesophyll conductance are unaffected in Arabidopsis thaliana aquaporin knock-out lines. J. Exp. Bot. 2020, 71, 318–329. [Google Scholar] [CrossRef]
- Sade, N.; Gallé, A.; Flexas, J.; Lerner, S.; Peleg, G.; Yaaran, A.; Moshelion, M. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 2014, 239, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bulychev, A.A.; Cherkashin, A.A.; Vredenberg, V.; Rubin, A.B.; Zykov, V.C.; Muller, S.H. Fluorescence and photosynthetic activity of chloroplasts in acid and alkaline zones of Chara corallina cells. Fisiol. Rasteniy 2001, 48, 384–391. [Google Scholar]
- Sukhova, E.M.; Sukhov, V.S. Dependence of the CO2 uptake in a plant cell on the plasma membrane H+-ATPase activity: Theoretical analysis. Biochem. Mosc. Suppl. Ser. A 2018, 12, 146–159. [Google Scholar] [CrossRef]
- Morsomme, P.; Boutry, M. The plant plasma membrane H+-ATPase: Structure, function and regulation. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1465, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sherstneva, O.N.; Vodeneev, V.A.; Surova, L.M.; Novikova, E.M.; Sukhov, V.S. Application of a mathematical model of variation potential for analysis of its influence on photosynthesis in higher plants. Biochem. Mosc. Suppl. Ser. A 2016, 10, 269–277. [Google Scholar] [CrossRef]
- Gallé, A.; Lautner, S.; Flexas, J.; Ribas-Carbo, M.; Hanson, D.; Roesgen, J.; Fromm, J. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ. 2013, 36, 542–552. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental stimuli and physiological responses: The current view on electrical signalling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.R.; Maischak, H.; Mithöfer, A.; Boland, W.; Felle, H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 2009, 149, 1593–1600. [Google Scholar] [CrossRef]
- Zimmermann, M.R.; Mithöfer, A.; Will, T.; Felle, H.H.; Furch, A.C. Herbivore-triggered electrophysiological reactions: Candidates for systemic signals in higher plants and the challenge of their identification. Plant Physiol. 2016, 170, 2407–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Aalto, T.; Juurola, E. A three-dimensional model of CO2 transport in airspaces and mesophyll cells of a silver birch leaf. Plant Cell Environ. 2002, 25, 1399–1409. [Google Scholar] [CrossRef]
- Buckley, T.N.; Farquhar, G.D. A new analytical model for whole-leaf potential electron transport rate. Plant Cell Environ. 2004, 27, 1487–1502. [Google Scholar] [CrossRef]
- Ho, Q.T.; Berghuijs, H.N.; Watté, R.; Verboven, P.; Herremans, E.; Yin, X.; Retta, M.A.; Aernouts, B.; Saeys, W.; Helfen, L.; et al. Three-dimensional microscale modelling of CO2 transport and light propagation in tomato leaves enlightens photosynthesis. Plant Cell Environ. 2016, 39, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhova, E.M.; Vodeneev, V.A.; Sukhov, V.S. Mathematical modeling of photosynthesis and analysis of plant productivity. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 2021, 15, 52–72. [Google Scholar] [CrossRef]
- Sukhova, E.; Ratnitsyna, D.; Gromova, E.; Sukhov, V. Development of two-dimensional model of photosynthesis in plant leaves and analysis of induction of spatial heterogeneity of CO2 assimilation rate under action of excess light and drought. Plants 2022, 11, 3285. [Google Scholar] [CrossRef]
- Pavlovič, A. The effect of electrical signals on photosynthesis and respiration. In Plant Electrophysiology; Volkov, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 33–62. [Google Scholar]
- Szechyńska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.K.; Das, S.; Maharatna, K.; Masi, E.; Santopolo, L.; Mancuso, S.; Vitaletti, A. Exploring strategies for classification of external stimuli using statistical features of the plant electrical response. J. R. Soc. Interface 2015, 12, 20141225. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.M.; Ferreira, A.S.; Saraiva, G.F.; Toledo, G.R. Plant “electrome” can be pushed toward a self-organized critical state by external cues: Evidences from a study with soybean seedlings subject to different environmental conditions. Plant Signal. Behav. 2017, 12, e1290040. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.K.; Malik, O.; Gupta, S. Chemical sensing employing plant electrical signal response-classification of stimuli using curve fitting coefficients as features. Biosensors 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Winter, H.; Robinson, D.G.; Heldt, H.W. Subcellular volumes and metabolite concentrations in spinach leaves. Planta 1994, 193, 530–535. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhov, V.S.; Vodeneev, V.A. Mathematical model of action potential in higher plant. In Mathematics, Computing, Education. Regular and Chaotic Dynamics; Riznichenko, G.Y., Ed.; Moskow-Izhevsk: Moscow, Russia, 2005; pp. 267–278. (In Russian) [Google Scholar]
- Sukhova, E.; Ratnitsyna, D.; Sukhov, V. Stochastic spatial heterogeneity in activities of H+-ATP-ases in electrically connected plant cells decreases threshold for cooling-induced electrical responses. Int. J. Mol. Sci. 2021, 22, 8254. [Google Scholar] [CrossRef]
- Gradmann, D. Impact of apoplast volume on ionic relations in plant cells. J. Membr. Biol. 2001, 184, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Akinchits, E.; Sukhov, V. Mathematical models of electrical activity in plants. J. Membr. Biol. 2017, 250, 407–423. [Google Scholar] [CrossRef]
- Sukhov, V.; Vodeneev, V. A mathematical model of action potential in cells of vascular plants. J. Membr. Biol. 2009, 232, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hansen, U.-P.; Gradmann, D.; Sanders, D.; Slayman, C.L. Interpretation of current-voltage relationships for ‘‘active’’ ion transport systems: I. Steady-state reaction-kinetic analysis of class-I mechanisms. J Membr. Biol. 1981, 63, 165–190. [Google Scholar] [PubMed]
- Hansen, U.-P.; Tittor, J.; Gradmann, D. Interpretation of current voltage relationships for ‘‘active’’ ion transport systems: II. Nonsteady-state reaction kinetic analysis of class-I mechanisms with one slow time-constant. J. Membr. Biol. 1983, 75, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Shimazaki, K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999, 18, 5548–5558. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Nerush, V.; Orlova, L.; Vodeneev, V. Simulation of action potential propagation in plants. J. Theor. Biol. 2011, 291, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Théroux Rancourt, G.; Éthier, G.; Pepin, S. Greater efficiency of water use in poplar clones having a delayed response of mesophyll conductance to drought. Tree Physiol. 2015, 35, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Buckley, T.N.; Lockhart, E.L.; Barbour, M.M. The response of mesophyll conductance to short- and long-term environmental conditions in chickpea genotypes. AoB Plants 2018, 11, ply073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.-J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sherstneva, O.; Sukhova, E.; Grinberg, M.; Mysyagin, S.; Vodeneev, V.; Sukhov, V. Inactivation of H+-ATPase participates in the influence of variation potential on photosynthesis and respiration in peas. Plants 2020, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Progr. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Pavlovič, A.; Slováková, L.; Pandolfi, C.; Mancuso, S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J. Exp. Bot. 2011, 62, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI assessment of long-term changes in carotenoids/chlorophyll ratio and short-term changes in de-epoxidation state of the xanthophyll cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Grams, T.E.; Koziolek, C.; Lautner, S.; Matyssek, R.; Fromm, J. Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ. 2007, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Grams, T.E.; Lautner, S.; Felle, H.H.; Matyssek, R.; Fromm, J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 2009, 32, 319–326. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Rosenthal, D.M.; Pimentel, C.; Long, S.P.; Farquhar, G.D. Modeling the temperature dependence of C3. In Photosynthesis in silico. Advances in Photosynthesis and Respiration; Laisk, A., Nedbal, L., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 29, pp. 231–246. [Google Scholar]
- Day, T.A.; Vogelmann, T.C. Alterations in photosynthesis and pigment distributions in pea leaves following UV-B exposure. Physiol. Plant. 1995, 94, 433–440. [Google Scholar] [CrossRef]
- Antal, T.K.; Kovalenko, I.B.; Rubin, A.B.; Tyystjärvi, E. Photosynthesis-related quantities for education and modeling. Photosynth. Res. 2013, 117, 1–30. [Google Scholar] [CrossRef]
- Roeske, C.A.; Chollet, R. Role of metabolites in the reversible light activation of pyruvate, orthophosphate dikinase in Zea mays mesophyll cells in Vivo. Plant Physiol. 1989, 90, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, W.H. Plant sensing and signaling in response to K+-deficiency. Mol. Plant. 2010, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]

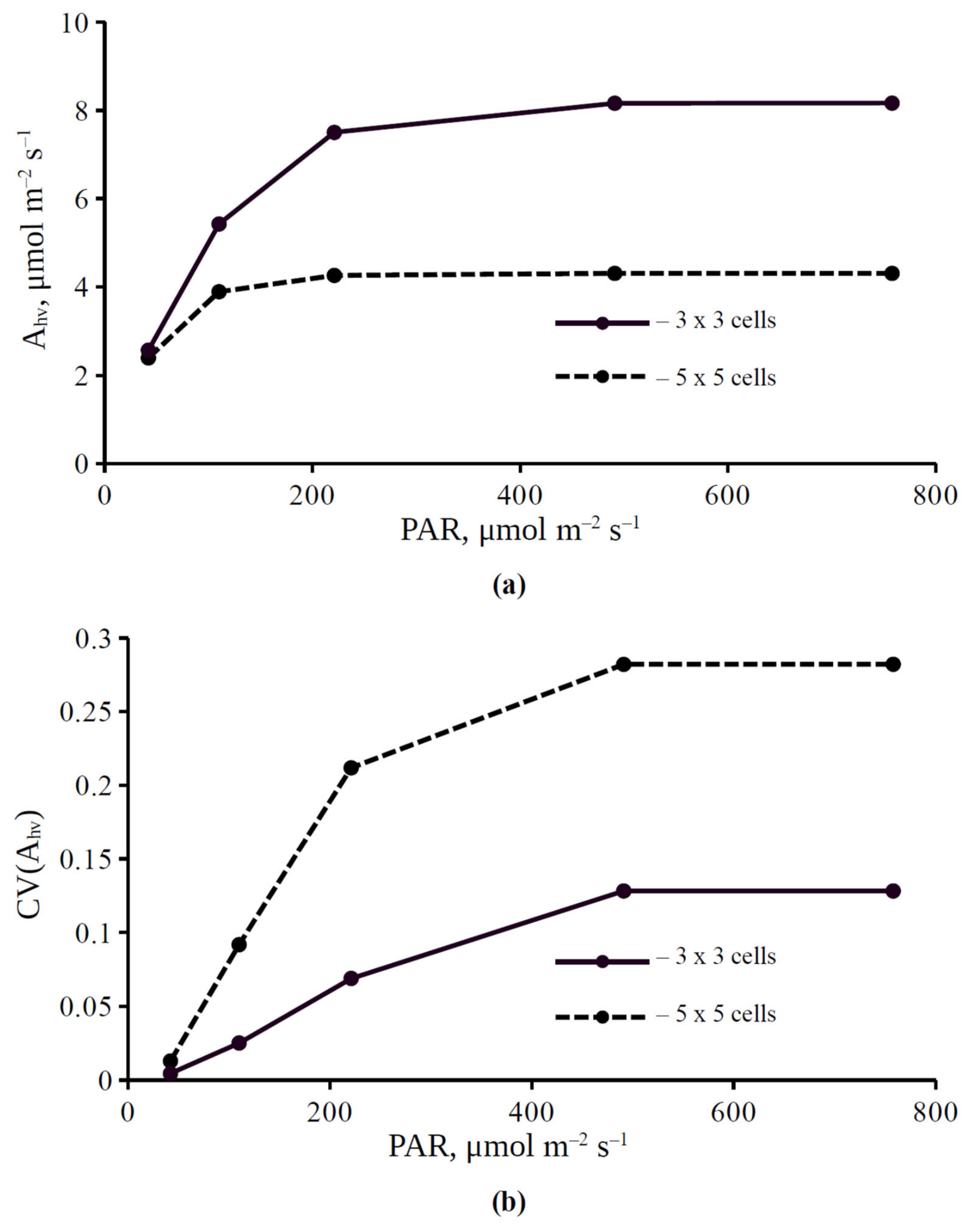

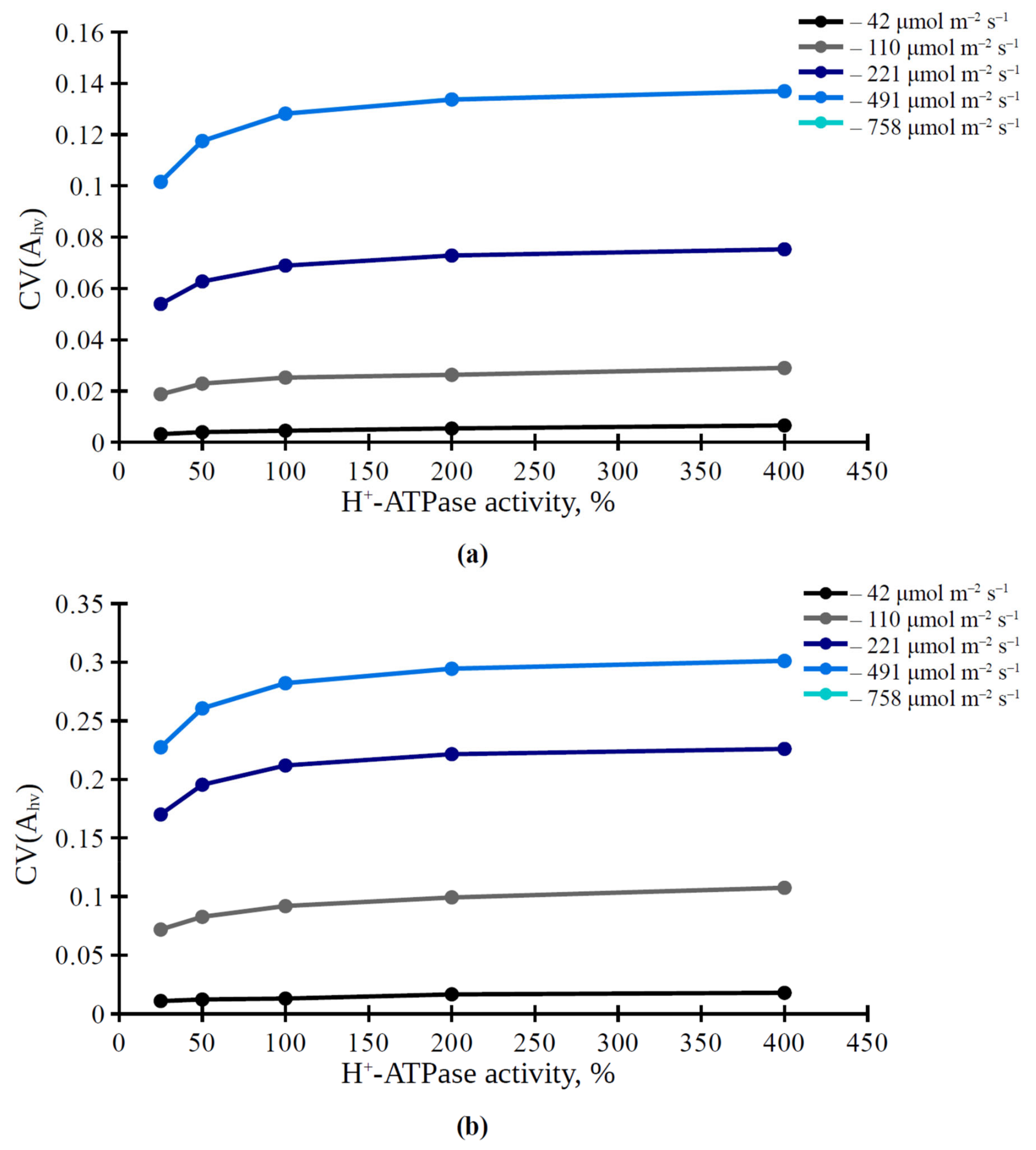
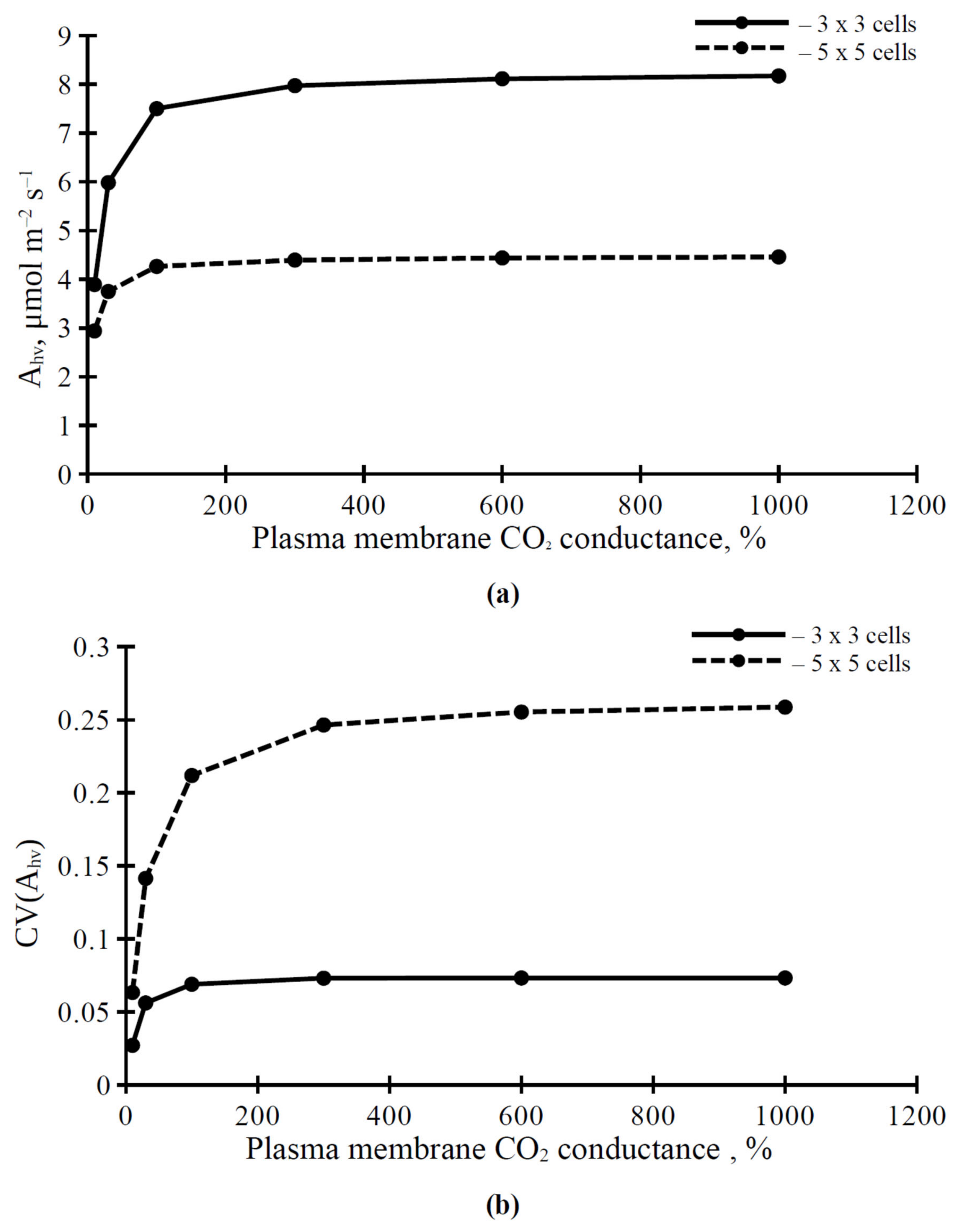
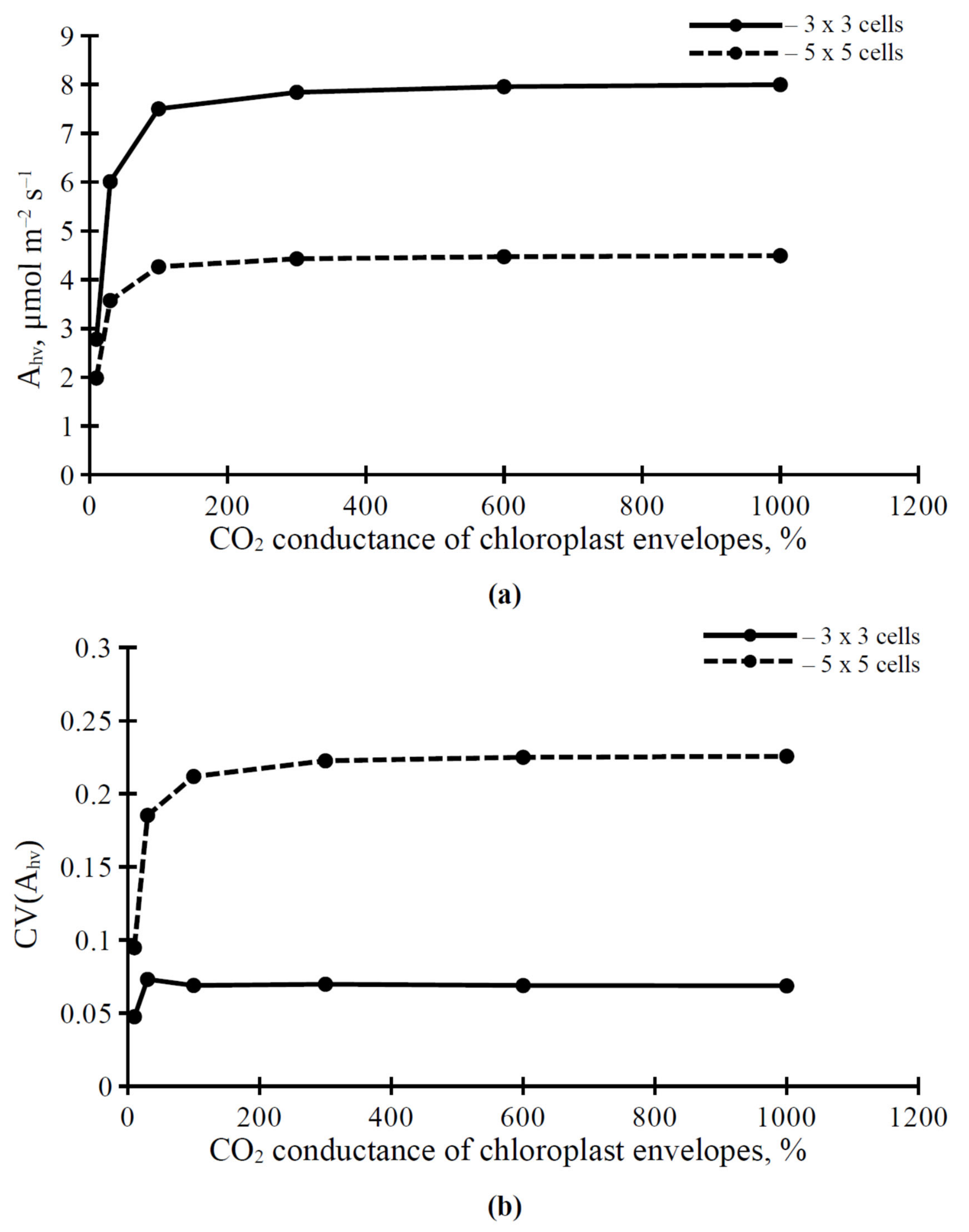
| Plasma Membrane Conductance for CO2, % | H+-ATPase Activity, % | ||||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | 400 | |
| 10 | 3.9173 | 3.8942 | 3.8903 | 3.8810 | 3.8814 |
| 30 | 6.0721 | 6.0103 | 5.9816 | 5.9794 | 5.9878 |
| 100 | 7.6391 | 7.5581 | 7.5013 | 7.4731 | 7.4587 |
| 300 | 8.1319 | 8.0246 | 7.9733 | 7.9631 | 7.9688 |
| 600 | 8.2778 | 8.1676 | 8.1147 | 8.1039 | 8.1097 |
| 1000 | 8.3554 | 8.2256 | 8.1722 | 8.1612 | 8.1669 |
| Plasma Membrane Conductance for CO2, % | H+-ATPase Activity, % | ||||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | 400 | |
| 10 | 0.021215 | 0.024805 | 0.027133 | 0.028507 | 0.029249 |
| 30 | 0.044012 | 0.051382 | 0.056118 | 0.058793 | 0.060186 |
| 100 | 0.053948 | 0.062738 | 0.068933 | 0.072829 | 0.075273 |
| 300 | 0.05696 | 0.066834 | 0.073089 | 0.076535 | 0.078442 |
| 600 | 0.057005 | 0.066942 | 0.073231 | 0.076678 | 0.078577 |
| 1000 | 0.056446 | 0.066944 | 0.07324 | 0.076687 | 0.078579 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhova, E.; Ratnitsyna, D.; Sukhov, V. Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves. Plants 2022, 11, 3435. https://doi.org/10.3390/plants11243435
Sukhova E, Ratnitsyna D, Sukhov V. Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves. Plants. 2022; 11(24):3435. https://doi.org/10.3390/plants11243435
Chicago/Turabian StyleSukhova, Ekaterina, Daria Ratnitsyna, and Vladimir Sukhov. 2022. "Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves" Plants 11, no. 24: 3435. https://doi.org/10.3390/plants11243435






