Transgene Was Silenced in Hybrids between Transgenic Herbicide-Resistant Crops and Their Wild Relatives Utilizing Alien Chromosomes
Abstract
:1. Introductions
2. Results
2.1. Transmission and Expression of Transgenes in Backcross Progenies
2.2. The mRNA Expression of the PAT Gene in Resistant-Gene-Silencing Plants Was Significantly Lower Than That in Resistant-Gene-Expressing Plants
2.3. PAT Possesses the Same Insertion Site in Backcross Progenies with That in Brassica napus
2.4. Methylation at the Promoter Was an Important Factor Leading to Gene Silencing
2.5. 5-azaC Suppresses the Occurrence of Gene Silencing
2.6. Backcross Progenies Showed Similar Fitness with Wild Brassica juncea
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Biochemical Reagents and Instruments
4.3. Plasmid and Competent State
4.4. Analysis Software
4.5. Selection for Tolerance to Herbicide and Fitness-Associated Traits in the Backcross Progeny
4.6. Amplification of DNA Flanking Sequences of Glufosinate-Resistant Transgenic Brassica juncea Transformants (Tail PCR)
4.7. 5-azaC Treatment Testing
4.8. RNA Extraction
4.9. cDNA Synthesis
4.10. qPCR Testing
4.11. Methylation Site Detection
4.12. Fitness Analysis
4.13. Data Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, T.; Chen, G.M.; Bu, C.P.; Liu, F.X.; Liu, L.; Zhao, X.X. Transgene introgression from Brassica napus to different varieties of Brassica juncea. Plant Breed. 2018, 137, 171–180. [Google Scholar] [CrossRef]
- Hauser, T.P.; Jørgensen, R.B.; Østergård, H. Fitness of backcross and F2 hybrids between weedy Brassica rapa and oilseed rape (B. napus). Heredity 1998, 81, 436–443. [Google Scholar] [CrossRef]
- Jenczewski, E.; Ronfort, J.; Chèvre, A.M. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environ. Biosaf. Res. 2003, 2, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Snow, A.A. Gene flow from genetically modified rice and its environmental consequences. Bio. Sci. 2005, 55, 669–678. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Prentice, H.C.; Hancock, J.F. Gene flow and introgression from domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 1999, 30, 539–563. [Google Scholar] [CrossRef]
- Chèvre, A.M.; Brun, H.; Eber, F.; Letanneur, J.C.; Vallee, P.; Ermel, M.; Glais, I.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Stabilization of resistance to Leptosphaeria maculans in Brassica napus–B. juncea recombinant lines and its introgression into spring-type Brassica napus. Plant Disease 2008, 92, 1208–1214. [Google Scholar] [CrossRef]
- Sun, X.Q.; Qu, Y.Q.; Li, M.M.; Song, X.L.; Hang, Y.Y. Genetic diversity, genetic structure and migration routes of wild Brassica juncea in China assessed by SSR markers. Genet. Resour. Crop Evol. 2018, 65, 1581–1590. [Google Scholar] [CrossRef]
- Frello, S.; Hansen, K.R.; Jensen, J.; Jørgensen, B.R. Inheritance of rapeseed (Brassica napus)-specific RAPD markers and a transgene in the cross B. juncea × (B. juncea × B. napus). Theor. Appl. Genet. 1995, 91, 236–241. [Google Scholar] [CrossRef]
- Bing, D.J.; Downey, R.K.; Rakow, G.F.W. Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breed. 1996, 115, 470–473. [Google Scholar] [CrossRef]
- Jørgensen, R.B.; Andersen, B.; Hauser, T.P.; Landbo, L.; Østergård, H. Introgression of crop genes from oilseed rape (Brassica napus) to related wild species—An avenue for the escape of engineered genes. Acta Hortic. 1998, 459, 211–217. [Google Scholar] [CrossRef]
- Song, X.L.; Huangfu, C.H.; Qiang, S. Gene flow from transgenic glufosinate-or glyphosate-tolerant oilseed rape to wild rape. Chin. J. Plant Ecol. 2007, 31, 729–737. [Google Scholar]
- Liu, Y.B.; Wei, W.; Ma, K.P.; Darmency, H. Backcrosses to Brassica napus of hybrids between B. juncea and B. napus as a source of herbicide-resistant volunteer-like feral populations. Plant Sci. 2010, 179, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Stewart, C.N.; Li, J.S., Jr.; Wei, W. One species to another: Sympatric Bt transgene gene flow from Brassica napus alters the reproductive strategy of wild relative Brassica juncea under herbivore treatment. Ann. Bot. 2018, 122, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Yan, J.; Zhang, Y.C.; Li, H.W.; Zheng, A.H.; Zhang, Q.L.; Wang, J.; Bian, Q.; Shao, Z.C.; Wang, Y.; et al. Gene Flow Risks From Transgenic Herbicide-Tolerant Crops to Their Wild Relatives Can Be Mitigated by Utilizing Alien Chromosomes. Front. Plant Sci. 2021, 12, 670209. [Google Scholar] [CrossRef] [PubMed]
- Gueritaine, G.; Sester, M.; Eber, F.; Chevre, A.M.; Darmency, H. Fitness of backcross six of hybrids between transgenic oilseed rape (Brassica napus) and wild radish (Raphanus raphanistrum). Mol. Ecol. 2002, 11, 1419–1426. [Google Scholar] [CrossRef]
- Lu, C.M.; Kato, M.F. Destiny of a transgene escape from Brassica napus into Brassica rapa. Theor. Appl. Genet. 2002, 105, 78–84. [Google Scholar] [CrossRef]
- Che`vre, A.M.; Adamczyk, K.; Eber, F.; Huteau, V.; Coriton, O.; Letanneur, J.C.; Laredo, C.; Jenczewski, E. Modelling gene flow between oilseed rape and wild radish. I. Evol. Chromosome Structure. Theor. Appl. Genet. 2007, 114, 209–221. [Google Scholar] [CrossRef]
- Devos, Y.; Schrijver, A.D.; Reheul, D. Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives. Env. Monit. Assess. 2009, 149, 303–322. [Google Scholar] [CrossRef]
- Jørgensen, R.B.; Hauser, T.; D’Hertefeldt, T.; Andersen, N.S.; Hooftma, D. The variability of processes involved in transgene dispersal-case studies from Brassica and related genera. Environ. Sci. Pollut. Res. Int. 2009, 16, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tomiuk, J.; Hauser, T.P.; Jørgensen, R.B. A- or C-chromosomes, does it matter for the transfer of transgenes from Brassica napus. Theor. Appl. Genet. 2000, 100, 750–754. [Google Scholar] [CrossRef]
- Metz, P.L.J.; Jacobsen, E.; Nap, J.P.; Pereira, A.; Stiekema, W.J. The impact on biosafety of the phosphinothricin-tolerance transgene in inter-specific B. rapa × B. napus hybrids and their successive backcrosses. Theor. Appl. Genet. 1997, 95, 442–450. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Zuo, J.; Huangfu, C.; Qiang, S. Potential gene flow of two herbicide-tolerant transgenes from oilseed rape to wild B. juncea var. gracilis. Theor. Appl. Genet. 2010, 120, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Lawrence, J.R.; Warwick, S.I.; Mason, P.; Braun, L.; Halfhill, M.D.; Neal, C. Inheritance of GFP-Bt transgenes from Brassica napus in backcrosses with three wild B. rapa accessions. Environ. Biosaf. Res. 2004, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kørner, C.J.; Pitzalis, N.; Peña, E.J.; Erhardt, M.; Vazquez, F.; Heinlein, M. Crosstalk between PTGS and TGS pathways in natural antiviral immunity and disease recovery. Nat. Plants. 2018, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Chen, Z. Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 2007, 19, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Havecker, E.R.; Wallbridge, L.M.; Hardcastle, T.J.; Bush, M.S.; Kelly, K.A.; Dunn, R.M.; Schwach, F.; Doonan, J.H.; Baulcombe, D.C. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell 2010, 22, 321–334. [Google Scholar] [CrossRef]
- Taochy, C.; Yu, A.; Bouché, N.; Bouteiller, N.; Elmayan, T.; Dressel, U.; Carroll, J.B.; Vaucheret, H. Post-transcriptional gene silencing triggers dispensable DNA methylation in gene body in Arabidopsis. Nucleic Acids Res. 2019, 47, 9104–9114. [Google Scholar] [CrossRef]
- Long, H.; Chen, C.L.; Wang, B.; Feng, Y.N. rDNA genetic imbalance and nucleolar chromatin restructuring is induced by distant hybridization between Raphanus sativus and Brassica alboglabra. PLoS ONE 2015, 10, e0117198. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Stewart, C.N., Jr.; Zheng, M.; Guan, Z.J.; Tang, Z.X.; Wei, W.; Ma, K.P. Stable Bacillus thuringiensis transgene introgression from Brassica napus to wild mustard B. juncea. Plant Sci. 2014, 227, 45. [Google Scholar] [CrossRef]
- Meyer, P.; Heidmann, I.; Niedenhof, I. Differences in DNA-methylation are associated with a paramutation phenomenon in transgenic petunia. Plant J. 1993, 4, 89–100. [Google Scholar] [CrossRef]
- Elomaa, P.; Helariutta, Y.; Griesbach, R.J.; Kotilainen, M.; Seppänen, P.; Teeri, T.H. Transgene inactivation in Petunia hybrida is influenced by the properties of the foreign gene. Mol. Gen. Genet. 1995, 248, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Lang, Z.B.; Zhu, J.K. Dynamics and function of DNA methylation in plant. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.L.A.; Kpodar, P.; DeLong, C.M.O. The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol. Biol. 1990, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Assaad, F.F.; Tucker, K.L.; Singer, E.R. Epigenetic repeat induced gene silenceing(RIGS) IN Arabiodopsis. Plant Mol. Biol. 1993, 22, 1067–1085. [Google Scholar] [CrossRef]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068. [Google Scholar] [CrossRef]
- Zilberman, D. The evolving functions of DNA methylation. Curr.Opin. Plant Biol. 2008, 11, 554–559. [Google Scholar]
- Linn, F.; Heidmann, L.; Saedler, H. Epigenetic changes in the expression of the maize Al gene in Petunia hybrida: Role of numbers of integrated gene copies and state of methylation. Mol. Gen Genet. 1990, 222, 329–336. [Google Scholar] [CrossRef]
- Panwar, V.; Mccallum, B.; Bakkeren, G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013, 73, 521. [Google Scholar] [CrossRef] [PubMed]
- Fagard, M.; Vaucheret, H. TransGene silencing in plants: How many mechanisms? Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cui, J.; Wang, L.; Teng, N.; Jin, B. Genome-wide dna mutations in arabidopsis plants after multigenerational exposure to high temperatures. Genome Biol. 2021, 221, 160. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Park, E.Y.; Park, Y.D. Transgene instability due to promoter hypermethylation and deletion in transgenic Nicotiana benthamiana. Hortic. Environ. Biotechnol. 2014, 55, 42–49. [Google Scholar] [CrossRef]
- Houdt, H.; Montagu, M. Posttranscriptional silencing of a neomycin phosphotransferase II transgene correlates with the accumulation of unproductive RNAs and with increased cytosine methylation of 3′ flanking regions. Plant J. 1997, 12, 379–392. [Google Scholar] [CrossRef]
- Zilberman, D.; Gehring, M.; Tran, R.K.; Ballinger, T.; Henikoff, S. Genome-wide analysis of a. thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007, 39, 61–69. [Google Scholar] [CrossRef]
- English, J.J.; Mueller, E.; Baulcombe, D.C. Suppression of Virus Accumulation in Transgenic Plants Exhibiting Silencing of Nuclear Genes. Plant Cell 1996, 8, 179–188. [Google Scholar] [CrossRef]
- Gao, W.; Li, S.; Li, Z.; Huang, Y.; Deng, C.; Lu, L. Detection of genome DNA methylation change in spinach induced by 5-azaC. Mol. Cell. Probes. 2014, 28, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Vanyushin, B.F. DNA methylation and epigenetics. Russ. J. Genet. 2006, 42, 985–997. [Google Scholar] [CrossRef]
- Kinoshita, T.; Miura, A.; Choi, Y.; Kinoshita, Y.; Kakutani, T. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 2004, 303, 521–523. [Google Scholar] [CrossRef]
- Zhang, X.; Jacobsen, S.E. Genetic analyses of DNA methyltransferases in Arabidopsis thaliana. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.M. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 2001, 292, 2077–2080. [Google Scholar] [CrossRef]
- Cao, X.; Jacobsen, S.E. Role of the Arabidopsis DRM methyltransferases in denovo DNA methylation and gene silencing. Curr. Biol. 2002, 12, 1138–1144. [Google Scholar] [CrossRef]
- Cao, X.; Jacobsen, S.E. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA 2002, 99, 16491–16498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 2009, 43, 143–166. [Google Scholar] [CrossRef]
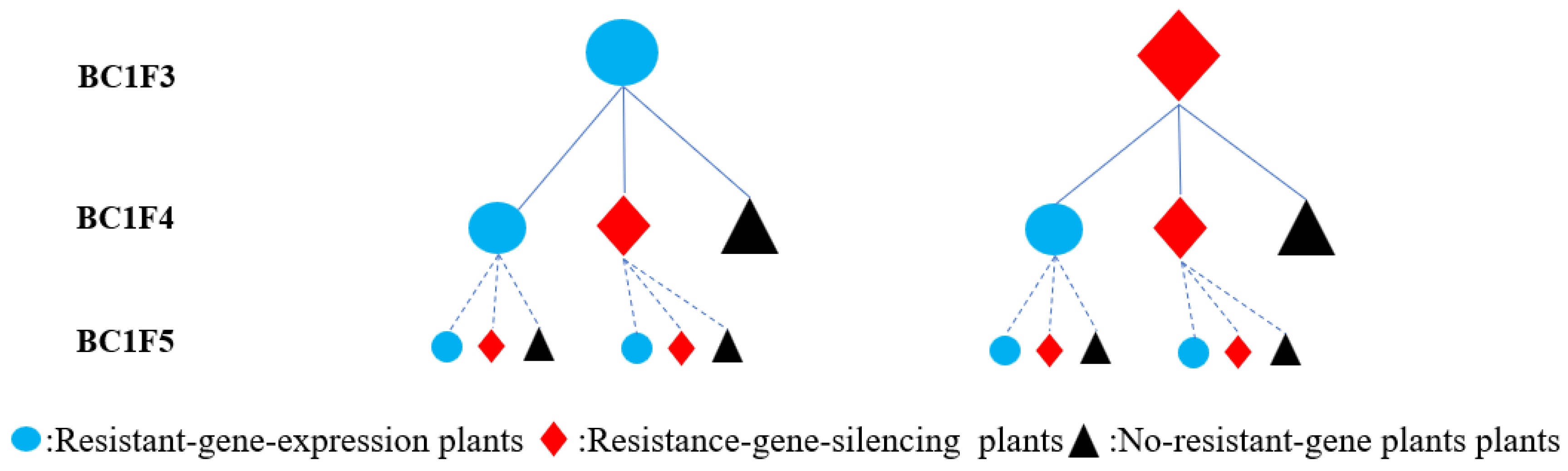




| Experiment Material | Phenotype of Parental Generation | Total Plant Number | R Plant Number | S Plant Number | N Plant Number | Percentage of Plants of R (%) | Percentage of Plants of S (%) | Percentage of Plants Carrying PAT (%) | Theoretical Segregation Ratio Ratio of Plant with PAT: without PAT | Χ2 Test of Plant Number with PAT: without PAT |
|---|---|---|---|---|---|---|---|---|---|---|
| BC1mF3 | BC1mF2 | 500 | 253 | 169 | 78 | 0.506 | 0.338 | 0.844 | 9:1 | 5.02 * |
| BC1pF3 | BC1pF2 | 500 | 231 | 206 | 63 | 0.462 | 0.413 | 0.875 | 0.85 | |
| BC1mF4 | BC1mF3 (R) | 500 | 224 | 220 | 56 | 0.448 | 0.44 | 0.888 | 1:17 | 7.18 * |
| BC1mF3 (S) | 500 | 224 | 216 | 60 | 0.448 | 0.432 | 0.88 | 9.36 * | ||
| BC1pF4 | BC1pF3 (R) | 500 | 220 | 212 | 68 | 0.44 | 0.424 | 0.864 | 14.58 * | |
| BC1pF3 (S) | 500 | 216 | 216 | 68 | 0.432 | 0.432 | 0.864 | 14.58 * | ||
| BC1mF5 | BC1mF4 (RR) | 500 | 249 | 227 | 24 | 0.498 | 0.454 | 0.952 | 1:33 | 5.87 * |
| BC1mF4 (RS) | 500 | 244 | 240 | 16 | 0.488 | 0.48 | 0.968 | 0.11 | ||
| BC1mF4 (SR) | 500 | 255 | 212 | 33 | 0.51 | 0.424 | 0.934 | 22.76 * | ||
| BC1mF4 (SS) | 500 | 237 | 236 | 27 | 0.474 | 0.472 | 0.946 | 10.28 * | ||
| BC1pF5 | BC1mF4 (RR) | 500 | 239 | 245 | 16 | 0.478 | 0.49 | 0.968 | 0.11 | |
| BC1mF4 (RS) | 500 | 242 | 234 | 24 | 0.484 | 0.468 | 0.952 | 5.87 * | ||
| BC1mF4 (SR) | 500 | 242 | 226 | 32 | 0.484 | 0.452 | 0.936 | 20.34 * | ||
| BC1mF4 (SS) | 500 | 231 | 233 | 36 | 0.462 | 0.466 | 0.928 | 30.83 * |
| Population | Resistant-Gene-Silencing | Resistant-Gene-Expressing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Methylation Sites of Promoter Region | No. | Methylation Sites of PAT Region | No. | Methylation Sites of Promoter Region | No. | Methylation Sites of PAT Region | No. | ||
| BC1mF3 | BC1mF2 | 36 243 289 322 344 440 | 6 | 648 783 825 870 989 | 5 | - | 0 | 594 612 648 825 | 4 |
| BC1pF3 | BC1pF2 | 175 243 322 326 344 440 | 6 | 612 675 825 870 1068 | 5 | - | 0 | 604 612 783 825 | 4 |
| BC1mF4 | BC1mF3 (R) | 116 168 243 251 322 344 440 | 7 | 686 989 1068 | 3 | - | 0 | 594 612 648 783 825 989 | 6 |
| BC1mF3 (S) | 71 109 243 322 344 440 | 6 | 612 825 870 982 989 1068 | 6 | - | 0 | 594 604 612 648 825 989 | 6 | |
| BC1pF4 | BC1pF3 (R) | 71 86 168 243 251 289 322 344 440 | 6 | 686 982 989 1068 | 4 | - | 0 | 594 612 648 783 989 | 5 |
| BC1pF3 (S) | 109 116 168 175 243 251 289 344 440 | 9 | 612 594 686 1068 | 4 | - | 0 | 594 612 648 783 989 | 5 | |
| BC1mF5 | BC1mF4 (RR) | 71 109 168 243 289 322 344 440 | 8 | 594 612686 825 1068 | 4 | - | 0 | 594 604 612 648 825 989 | 6 |
| BC1mF4 (RS) | 36 71 109 168 243 289 322 344 440 | 9 | 686 771 825 1068 | 4 | - | 0 | 604 612 648 783 | 4 | |
| BC1mF4 (SR) | 36 71 86 243 251 289 322 344 440 | 9 | 686 982 989 | 3 | - | 0 | 594 612 648 825 989 | 5 | |
| BC1mF4 (SS) | 36 175 243 322 344 440 | 6 | 6821 825 870 | 3 | - | 0 | 604 612 648 783 594 | 5 | |
| BC1pF5 | BC1mF4 (RR) | 109 168 243 289 322 344 440 | 7 | 612 686 989 1068 | 4 | - | 0 | 594 604 612 648 989 | 5 |
| BC1mF4 (RS) | 36 71 109 289 322 344 440 | 7 | 612 825 870 892 | 4 | - | 0 | 604 783 989 | 3 | |
| BC1mF4 (SR) | 71 86 116 243 251 289 322 344 440 | 9 | 612 989 1068 | 3 | - | 0 | 612 783 989 | 3 | |
| BC1mF4 (SS) | 36 71 243 322 344 440 | 6 | 612 686 989 1068 | 4 | - | 0 | 612 825 989 | 3 | |
| Backcross Progenies | Number of Plants Carrying Resistance Genes | Number of Plants without Resistance Genes | Number of Surviving Plants | Number of Dead Plants | Proportion of Resistant-Gene-Expressing Plants (%) |
|---|---|---|---|---|---|
| BC1mF4 (CK) | 180 | 20 | 97 | 103 | 53.89 b |
| BC1pF4 (CK) | 174 | 26 | 89 | 111 | 51.15 b |
| BC1mF4 (5-azaC) | 177 | 23 | 132 | 68 | 74.58 a |
| BC1pF4 (5-azaC) | 170 | 30 | 117 | 83 | 68.82 a |
| BC1F3 | A | B | C | D | E | F | G | H | Fitness |
|---|---|---|---|---|---|---|---|---|---|
| BC1mF3 (R) | 0.83 | 0.94 | 0.92 | 0.85 | 0.92 | 0.81 | 0.89 | 0.83 | 0.87 |
| BC1mF3 (S) | 0.88 | 0.83 | 0.91 | 0.89 | 0.86 | 0.79 | 0.86 | 0.89 | 0.86 |
| BC1pF3 (R) | 0.80 | 0.92 | 0.81 | 0.79 | 0.93 | 0.81 | 0.93 | 0.85 | 0.86 |
| BC1pF3 (S) | 0.87 | 0.83 | 0.84 | 0.91 | 0.89 | 0.80 | 0.84 | 0.83 | 0.85 |
| Bjn | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| BC1F4 | A | B | C | D | E | F | G | H | Fitness |
|---|---|---|---|---|---|---|---|---|---|
| BC1mF4 (RR) | 0.93 | 0.8 | 0.88 | 0.84 | 0.92 | 0.98 | 0.92 | 0.94 | 0.90 |
| BC1mF4 (RS) | 0.9 | 0.93 | 0.89 | 0.9 | 0.91 | 0.95 | 0.94 | 0.94 | 0.92 |
| BC1mF4 (SR) | 0.89 | 0.95 | 0.89 | 0.89 | 0.98 | 0.92 | 0.99 | 0.99 | 0.94 |
| BC1mF4 (SS) | 0.93 | 0.96 | 0.98 | 0.98 | 0.95 | 1.04 | 0.95 | 0.93 | 0.97 |
| BC1pF4 (RR) | 0.91 | 0.9 | 0.92 | 0.94 | 0.94 | 0.93 | 0.95 | 0.96 | 0.93 |
| BC1pF4 (RS) | 0.88 | 0.83 | 0.8 | 0.86 | 0.88 | 0.78 | 0.93 | 0.98 | 0.87 |
| BC1pF4 (SR) | 0.98 | 1.04 | 0.99 | 1.01 | 0.96 | 1.06 | 1.04 | 1.06 | 1.02 |
| BC1pF4 (SS) | 0.97 | 0.81 | 0.82 | 0.82 | 0.82 | 0.85 | 0.83 | 0.82 | 0.84 |
| Bjn | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| BC1F5 | A | B | C | D | E | F | G | H | Fitness |
|---|---|---|---|---|---|---|---|---|---|
| BC1mF5 (RRR) | 0.93 | 1.00 | 1.00 | 0.97 | 0.90 | 1.00 | 0.96 | 0.93 | 0.96 |
| BC1mF5 (RRS) | 0.91 | 0.98 | 0.97 | 0.99 | 0.93 | 0.92 | 0.94 | 0.98 | 0.95 |
| BC1mF5 (RSR) | 0.92 | 1.01 | 0.97 | 0.96 | 0.98 | 0.96 | 0.92 | 0.91 | 0.95 |
| BC1mF5 (RSS) | 0.97 | 0.97 | 0.91 | 0.98 | 0.98 | 0.92 | 0.94 | 0.94 | 0.95 |
| BC1mF5 (SRR) | 0.92 | 0.97 | 0.91 | 0.91 | 0.91 | 0.89 | 0.92 | 0.99 | 0.93 |
| BC1mF5 (SRS) | 1.00 | 0.99 | 0.89 | 0.92 | 0.97 | 0.93 | 0.90 | 0.97 | 0.95 |
| BC1mF5 (SSR) | 1.00 | 0.97 | 0.94 | 0.99 | 0.92 | 0.97 | 1.01 | 0.96 | 0.97 |
| BC1mF5 (SSS) | 0.96 | 0.97 | 0.90 | 0.92 | 0.91 | 0.97 | 0.94 | 0.96 | 0.94 |
| BC1pF5 (RRR) | 0.95 | 0.93 | 0.91 | 0.96 | 0.92 | 0.99 | 0.99 | 0.98 | 0.95 |
| BC1pF5 (RRS) | 0.89 | 0.98 | 1.00 | 0.93 | 0.94 | 0.94 | 0.95 | 0.92 | 0.94 |
| BC1pF5 (RSR) | 1.01 | 0.92 | 0.96 | 0.93 | 0.91 | 0.96 | 0.95 | 0.98 | 0.95 |
| BC1pF5 (RSS) | 0.96 | 0.92 | 0.99 | 0.96 | 1.01 | 0.95 | 0.94 | 0.92 | 0.96 |
| BC1pF5 (SRR) | 0.95 | 0.92 | 0.93 | 0.97 | 0.90 | 0.98 | 0.91 | 1.01 | 0.95 |
| BC1pF5 (SRS) | 0.98 | 0.94 | 0.92 | 0.96 | 0.96 | 0.90 | 0.94 | 0.99 | 0.95 |
| BC1pF5 (SSR) | 0.89 | 0.95 | 0.95 | 0.97 | 0.93 | 0.91 | 0.90 | 0.93 | 0.93 |
| BC1pF5 (SSS) | 0.98 | 0.92 | 1.00 | 0.90 | 1.01 | 0.98 | 1.01 | 0.93 | 0.97 |
| Bjn | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Huang, L.; Zhang, Y.; Qiang, S.; Song, X. Transgene Was Silenced in Hybrids between Transgenic Herbicide-Resistant Crops and Their Wild Relatives Utilizing Alien Chromosomes. Plants 2022, 11, 3187. https://doi.org/10.3390/plants11233187
Shao Z, Huang L, Zhang Y, Qiang S, Song X. Transgene Was Silenced in Hybrids between Transgenic Herbicide-Resistant Crops and Their Wild Relatives Utilizing Alien Chromosomes. Plants. 2022; 11(23):3187. https://doi.org/10.3390/plants11233187
Chicago/Turabian StyleShao, Zicheng, Lei Huang, Yuchi Zhang, Sheng Qiang, and Xiaoling Song. 2022. "Transgene Was Silenced in Hybrids between Transgenic Herbicide-Resistant Crops and Their Wild Relatives Utilizing Alien Chromosomes" Plants 11, no. 23: 3187. https://doi.org/10.3390/plants11233187





