Relationships between Agronomic Traits of Moringa Accessions and In Vitro Gas Production Characteristics of a Test Feed Incubated with or without Moringa Plant Leaf Extracts
Abstract
:1. Introduction
2. Results
2.1. Agronomic Performances of Moringa Accessions during the Establishment Year
2.2. In Vitro Total Gas, Methane Production and Organic Matter Digestibility
2.3. Total Phenolic and Total Flavonoid Contents
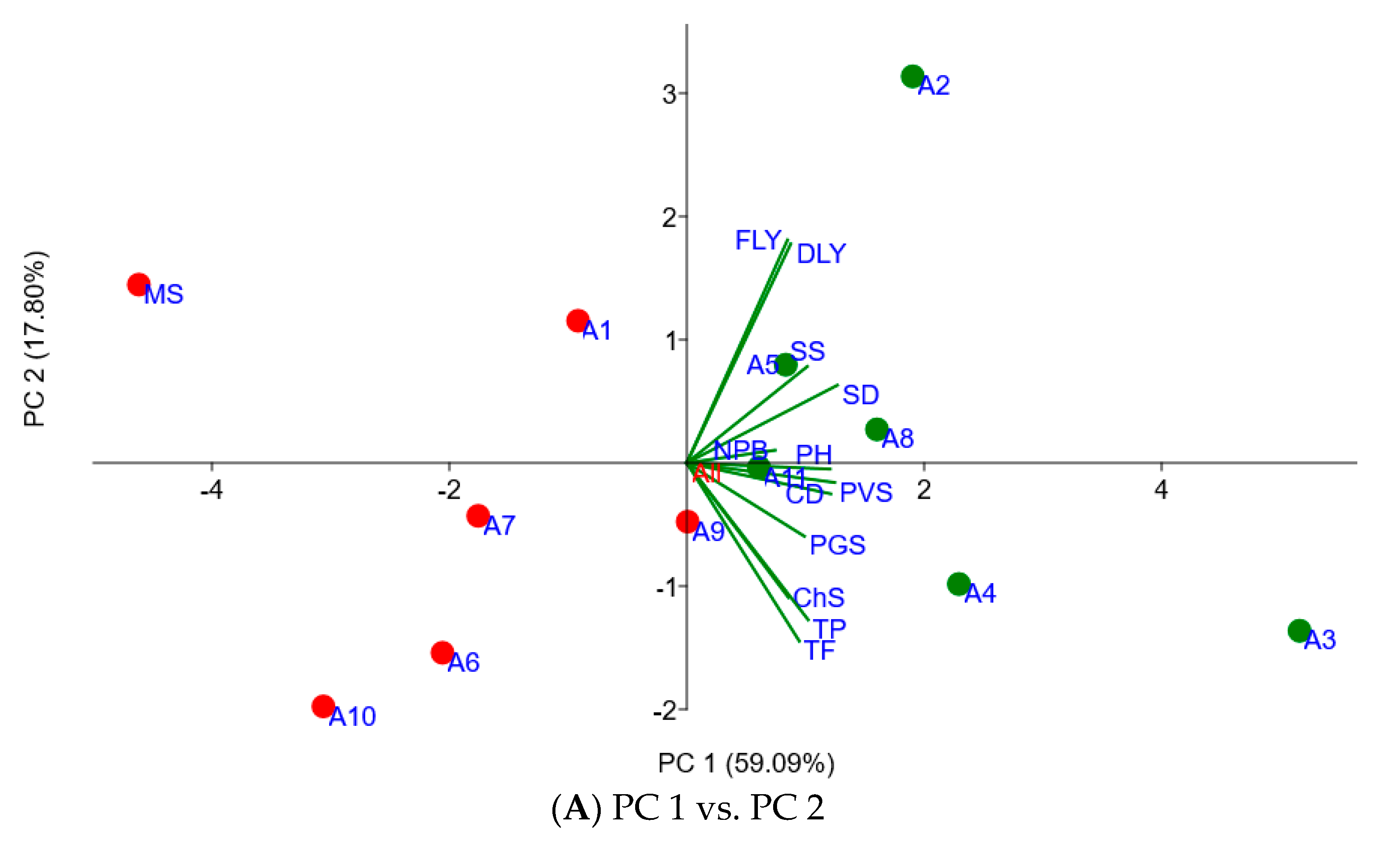
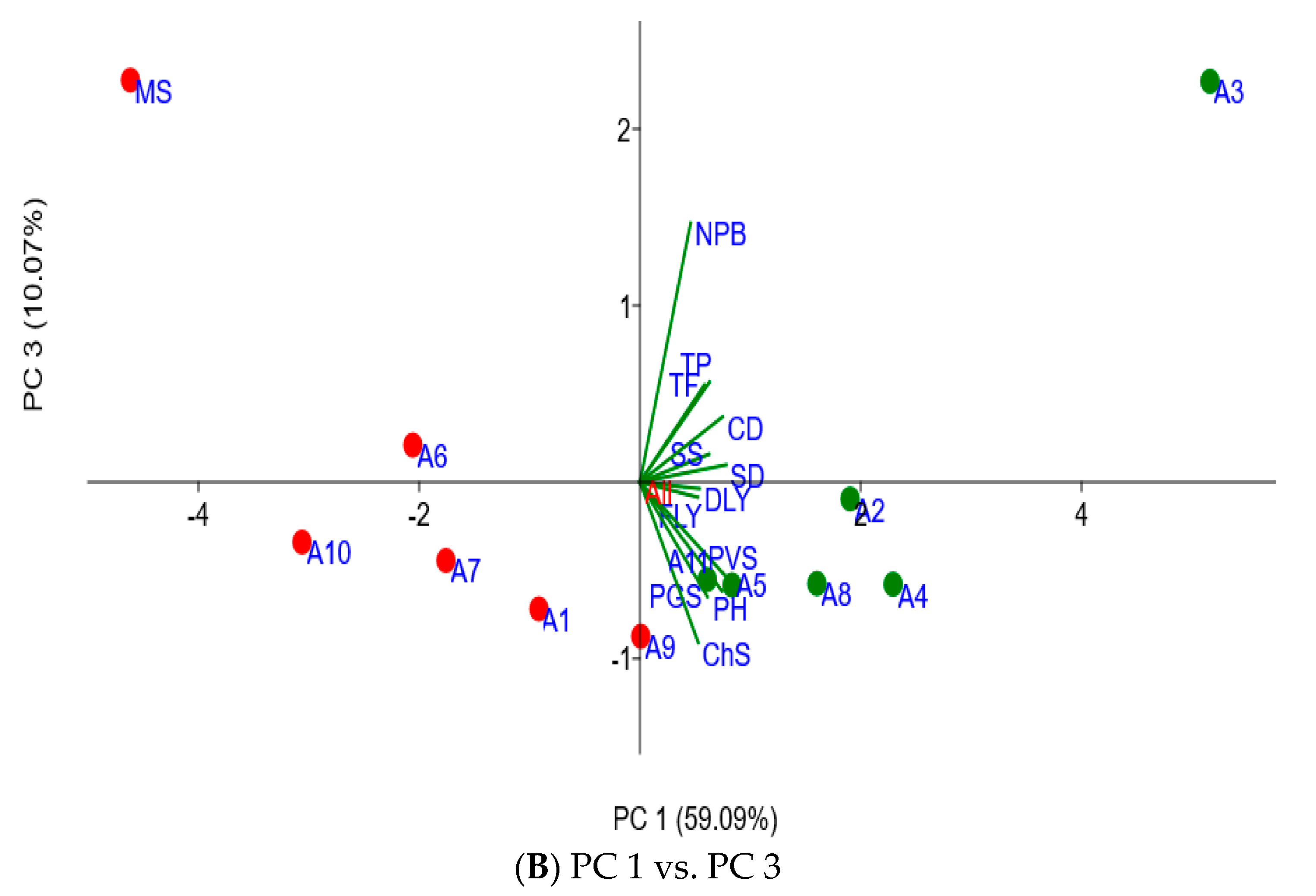
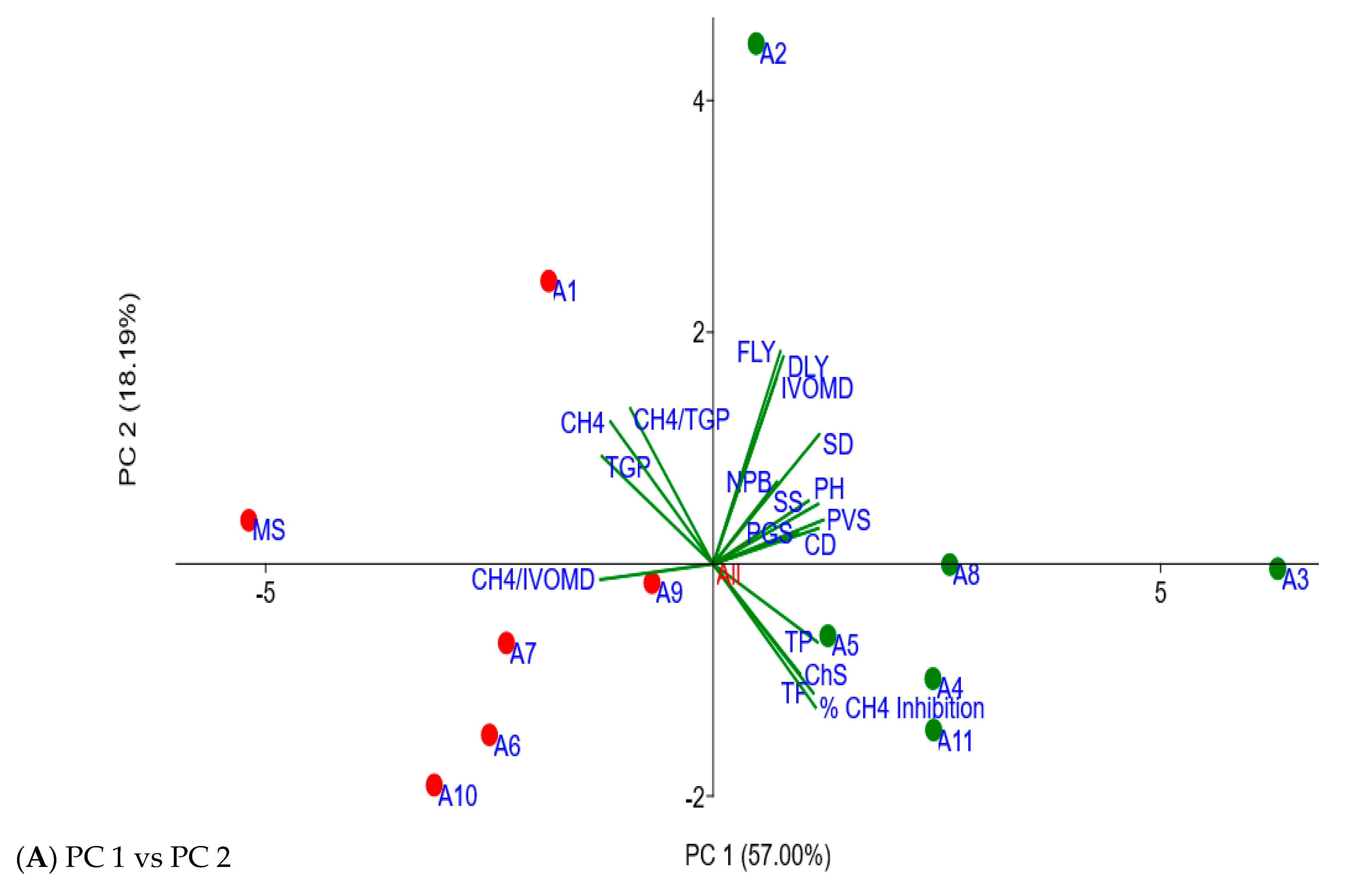
2.4. Associating Agronomic Traits of Moringa Accessions with In Vitro Fermentation Characteristics
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Agronomic Data Measurement
4.2. Plant Extraction and Determination of Total Phenolic and Total Flavonoid Contents
4.3. Medium Preparation and In Vitro Incubation
4.4. Determination of In Vitro Total Gas, Methane and Organic Matter Digestibility
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, F.; Ohene-Yankyera, K.; Aidoo, R.; Wongnaa, C.A. Economic benefits of livestock management in Ghana. Agric. Food Econ. 2021, 9, 17. [Google Scholar] [CrossRef]
- Abubakar, M.; Iqbal, A.; Kabir, A.; Manzoor, S. Introductory Chapter: Ruminants—The Husbandry, Economic, and Health Aspects. Ruminants-The Husbandry, Economic and Health Aspects; IntechOpen: London, UK, 2018. [Google Scholar]
- Khanal, P.; Dhakal, R.; Khanal, T.; Pandey, D.; Devkota, N.R.; Nielsen, M.O. Sustainable Livestock Production in Nepal: A Focus on Animal Nutrition Strategies. Agriculture 2022, 12, 679. [Google Scholar] [CrossRef]
- Black, J.L.; Davison, T.M.; Box, I. Methane emissions from ruminants in Australia: Mitigation potential and applicability of mitigation strategies. Animals 2021, 11, 951. [Google Scholar] [CrossRef]
- Hristov, A.; Oh, J.; Firkins, J.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.; Adesogan, A.; Yang, W.; Lee, C. Mitigation of methane and nitrous oxide emissions from animal operations: A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legg, S. IPCC, 2021: Climate change 2021-the physical science basis. Interact 2021, 49, 44–45. Available online: https://search.informit.org/doi/10.3316/informit.315096509383738 (accessed on 25 January 2022).
- Salaheldeen, M.; Aroua, M.; Mariod, A.; Cheng, S.F.; Abdelrahman, M.A.; Atabani, A. Physicochemical characterization and thermal behavior of biodiesel and biodiesel–diesel blends derived from crude Moringa peregrina seed oil. Energy Convers. Manag. 2015, 92, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.K.; Jhade, D.; Chouksey, R. Moringa oleifera Lam a study of ethnobotany, nutrients and pharmacological profile. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2158–2165. Available online: https://www.rjpbcs.com/pdf/2016_7/[274].pdf (accessed on 20 December 2021).
- Palada, M.; Ebert, A.; Yang, R.; Chang, L.; Chang, J.; Wu, D. Progress in research and development of Moringa at the world vegetable center. In Proceedings of the I International Symposium on Moringa (Acta Horticalturalae 1158), Manila, Philippines, 15–18 November 2015; pp. 425–434. [Google Scholar] [CrossRef]
- Radovich, T. Farm and Forestry Production and Marketing Profile for Moringa (Moringa oleifera): Specialty Crops for Pacific Island Agroforestry; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011; Available online: http://moringatrees.org/moringa-doc/production_and_marketing_moringa_farm_and_forestry.pdf (accessed on 13 November 2021).
- Rodrigues, N.P.; Salva, T.d.J.G.; Bragagnolo, N. Influence of coffee genotype on bioactive compounds and the in vitro capacity to scavenge reactive oxygen and nitrogen species. J. Agric. Food Chem. 2015, 63, 4815–4826. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Belkheir, A.K.; Gaid, M.; Liu, B.; Hänsch, R.; Beerhues, L. Benzophenone synthase and chalcone synthase accumulate in the mesophyll of Hypericum perforatum leaves at different developmental stages. Front. Plant Sci. 2016, 7, 921. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Fadl, S.; Osman, A.; Al-Zohairy, A.; Dahab, A.A.; Abo El Kheir, Z.A. Assessment of total phenolic, flavonoid content, antioxidant potential and hplc profile of three moringa species leaf extracts. Sci. J. Flowers Ornam. Plants 2020, 7, 53–70. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Szepesi, Á. Plant metabolites and regulation under environmental stress. Plants 2021, 10, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.; Correia, P.M.; Mehra, R.; Kumar, H. Recent advances in Drumstick (Moringa oleifera) leaves bioactive compounds: Composition, health benefits, bioaccessibility, and dietary applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.; Galaverna, R.S.; Angolini, C.F.; Nunes, V.V.; De Almeida, L.F.; Ruiz, A.L.; De Carvalho, J.E.; Duarte, R.M.; Duarte, M.C.; Eberlin, M.N. Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three Myrcia species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [Green Version]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.; Singh, R.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hettiarachchy, N.S.; Horax, R.; Kannan, A.; Praisoody, A.; Muhundan, A. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J. Med. Plants Res. 2011, 5, 6672–6680. [Google Scholar] [CrossRef]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 15. [Google Scholar] [CrossRef] [Green Version]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef] [Green Version]
- Giuburunca, M.; Criste, A.; Cocan, D.; Constantinescu, R.; Răducu, C.; Mireșan, V. Effects of plant secondary metabolites on methane production and fermentation parameters in in vitro ruminal cultures. Sci. Pap. Anim. Sci. Biotechnol. 2014, 47, 78–82. [Google Scholar]
- Samuel, D.; Daba, R.; Terefe, M.; Senbeto, M. Evaluation of two Moringa species for adaptability and growth performance under Bako conditions. J. Nat. Sci. Res. 2016, 6, 76–82. Available online: https://www.researchgate.net/publication/331732866 (accessed on 12 February 2020).
- Patricio, H.; Palada, M. Adaptability and horticultural characterization of different moringa accessions in Central Philippines. In Proceedings of the I International Symposium on Moringa, Malina, Philippines, 15–18 November 2015; pp. 45–54. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Rorie, R.L.; Purcell, L.C.; Mozaffari, M.; Karcher, D.E.; King, C.A.; Marsh, M.C.; Longer, D.E. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agron. J. 2011, 103, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Diagnosis of nitrogen nutrition in rice leaves influenced by potassium levels. Front. Plant Sci. 2020, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, O.; Constantinidou, E.; Tyystjärvi, E. Chlorophyll does not reflect green light–how to correct a misconception. J. Biol. Educ. 2020, 54, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Shi, P.; Omasa, K. Estimating rice chlorophyll content and leaf nitrogen concentration with a digital still color camera under natural light. Plant Methods 2014, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chlorosis in Trees and Shrubs: Symptoms, Causes and Treatment. For Quality Tree Service in Northeast Ohio. Independent Tree, Healthy Trees and Beutiful Landscapes. 2020. Available online: https://www.independenttree.com/chlorosis/# (accessed on 18 September 2022).
- Gang, C.; Suping, W.; Xiang, H.; Juan, H.; Lei, D.; Lihong, Z.; Lixia, Y. Environmental factors affecting growth and development of Banlangen (Radix Isatidis) in China. Afr. J. Plant Sci. 2015, 9, 421–426. [Google Scholar] [CrossRef]
- Coco, M. Five Factors Affecting Plant Growth. ViparSpectra. 2021. Available online: https://www.viparspectra.com/blogs/home-grower/5-factors-affecting-plant-growth (accessed on 15 September 2022).
- Kumar, P.; Singh, B.N.; Dwivedi, P. Plant growth regulators, plant adaptability and plant productivity: Areview on abscisic acid (Aba) signaling in plants under emerging environmental stresses. In Sustaining Future Food Security in Changing Environments; Nova Science Publishers, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Akanmu, A.M.; Hassen, A. The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Anim. Prod. Sci. 2017, 58, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Burrell-Saward, H.; Harris, A.J.; de LaFlor, R.; Sallam, H.; Alavijeh, M.S.; Ward, T.H.; Croft, S.L. Dose-dependent effect and pharmacokinetics of fexinidazole and its metabolites in a mouse model of human African trypanosomiasis. Int. J. Antimicrob. Agents 2017, 50, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Gokulan, K.; Kolluru, P.; Cerniglia, C.E.; Khare, S. Dose-dependent effects of Aloin on the intestinal bacterial community structure, short chain fatty acids metabolism and intestinal epithelial cell permeability. Front. Microbiol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Macheboeuf, D.; Morgavi, D.; Papon, Y.; Mousset, J.-L.; Arturo-Schaan, M. Dose–response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol. 2008, 145, 335–350. [Google Scholar] [CrossRef]
- Ghamkhar, K.; Rochfort, S.; Banik, B.K.; Revell, C. Candidate metabolites for methane mitigation in the forage legume biserrula. Agron. Sustain. Dev. 2018, 38, 30. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Paul, S.; Pandey, P.; Rathore, R. Potential of Moringa oleifera leaves in modulating in vitro methanogenesis and fermentation of wheat straw in buffalo. Indian J. Anim. Sci. 2014, 84, 533–538. Available online: https://www.academia.edu/download/35343705/Moringa_Paper._2014._IJAS.pdf (accessed on 23 July 2020).
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2019, 129, 181–190. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Castillo-Lopez, R.I.; Leon-Felix, J.; Angulo-Escalante, M.; Gutierrez-Dorado, R.; Muy-Rangel, M.D.; Heredia, J.B. Nutritional and phenolic characterization of Moringa oleifera leaves grown in Sinaloa, Mexico. Pak. J. Bot. 2017, 49, 161–168. [Google Scholar]
- Omede, A. Total polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts. Anim. Res. Int. 2016, 13, 2454–2462. Available online: https://www.ajol.info/index.php/ari/article/view/145371 (accessed on 27 June 2019).
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Harry-Asobara, J.L.; Samson, E. Comparative study of the phytochemical properties of Jatropha curcas and Azadirachta indica plant extracts. J. Poisonous Med. Plants Res. 2014, 2, 20–24. Available online: https://www.semanticscholar.org/paper/Comparative-Study-of-the-Phytochemical-Properties-Harry-Asobara-Linda/184871ff1fccfc78c820bda13f43c78b9eb9c83b (accessed on 16 August 2020).
- Zeru, A.E.; Hassen, A.; Apostolides, Z.; Tjelele, J. Screening of Candidate Bioactive Secondary Plant Metabolite Ion-Features from Moringa oleifera Accessions Associated with High and Low Enteric Methane Inhibition from Ruminants. Metabolites 2022, 12, 501. [Google Scholar] [CrossRef]
- Tarawali, S.A.; Tarawali, G.; Larbi, A.; Hanson, J. Methods for the Evaluation of Forage Legumes, Grasses and Fodder Trees for Use as Livestock Feed; International Livestock Research Institute (ILRI): Nairobi, Kenya, 1995; pp. 1–43. [Google Scholar]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Madaan, R.; Bansal, G.; Kumar, S.; Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci. 2011, 73, 666. [Google Scholar] [CrossRef] [Green Version]
- Bag, G.; Devi, P.G.; Bhaigyabati, T. Assessment of total flavonoid content and antioxidant activity of methanolic rhizome extract of three Hedychium species of Manipur valley. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 154–159. Available online: http://www.globalresearchonline.net/ (accessed on 24 December 2018).
- Mould, F.L.; Morgan, R.; Kliem, K.E.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123, 155–172. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P. Forage fiber analysis. Apparatus, reagents, procedures and some applications. Agric Handb. 1970, 379, 20. [Google Scholar]
- Akanmu, A.M. Effect of Medicinal Plant Extracts from West Africa on Rumen Fermentation Parameters, Enteric Methane Emission and Growth Performance in Merino Sheep. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Akanmu, A.M.; Hassen, A.; Adejoro, F.A. Gas production, digestibility and efficacy of stored or fresh plant extracts to reduce methane production on different substrates. Animals 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, E.; Van der Merwe, F. Application of an in vitro technique to South African forages with special reference to the effect to certain factors on the results. S. Afr. J. Agric. Sci. 1967, 10, 983–995. [Google Scholar]
- Tilley, J.; Terry, R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Ankom, T. Pressure to Gas Production Conversion, User Manual Service Procedure 005; Calculation of Gas Volume in mL at 39 °C with Pressure Measured in psi; ANKOM Technology Macedon: New York, NY, USA, 2014; Available online: https://www.manualsdir.com/manuals/655546/ankom-rf.html?page=46 (accessed on 12 September 2018).
- SAS Institute Inc. SAS/ACCESS® 9.4 Interface to ADABAS: Reference; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 19 September 2019).



| Accession Number/Name/Code | PH (cm) | SD (cm) | CD (cm) | NPB | FLY (kg/ha) | DLY (kg/ha) | PVS | PGS | PCS | SS (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bulk (A1) | 153.9 A | 2.3 | 72.9 AB | 0.53 B | 2063.8 AB | 553.2 AB | 5.33 AB | 5.67 AB | 2.67 | 52.5 AB |
| 07229 (A2) | 163.6 A | 2.8 | 78.8 AB | 1.40 AB | 2981.3 A | 827.6 A | 7.00 A | 6.00 AB | 2.67 | 78.1 A |
| 07633 (A3) | 188.1 A | 3.01 | 105.3 A | 2.67 A | 2144.6 AB | 608.4 AB | 7.67 A | 6.33 A | 3.67 | 74.0 AB |
| 07632 (A4) | 160.3 A | 2.64 | 95.7 AB | 0.80 B | 1573.3 AB | 470.5 AB | 7.00 A | 6.33 A | 4.00 | 75.2 AB |
| 07627 (A5) | 166.2 A | 2.51 | 82.6 AB | 0.20 B | 2149.2 AB | 589.8 AB | 6.33 A | 5.00 AB | 3.33 | 74.1 AB |
| 03295 (A6) | 127.6 AB | 1.89 | 68.2 AB | 0.68 B | 590.4 B | 168.2 B | 5.67 AB | 5.33 AB | 2.67 | 61.9 AB |
| 05536 (A7) | 125.9 AB | 1.95 | 69.1 AB | 0.47 B | 1458.3 AB | 423.0 AB | 4.67 AB | 5.33 AB | 3.33 | 51.9 AB |
| 07717 (A8) | 150.8 A | 2.47 | 74.5 AB | 0.73 B | 2107.5 AB | 604.1 AB | 7.00 A | 6.33 A | 3.33 | 75.4 AB |
| 07316 (A9) | 138.3 AB | 2.14 | 74.3 AB | 1.07 B | 1697.9 AB | 468.2 AB | 5.67 AB | 6.33 A | 4.00 | 58.2 BC |
| 07216 (A10) | 105.1 AB | 1.59 | 58.9 B | 0.27 B | 805.0 B | 220.2 B | 4.33 AB | 5.67 AB | 3.00 | 44.9 B |
| Pretoria (A11) | 146.7 A | 2.12 | 69.1 AB | 0.87 B | 1975.4 AB | 552.4 AB | 6.00 AB | 5.67 AB | 4.00 | 73.7 AB |
| M. stenopetala (MS) | 46.7 B | 1.67 | 60.0 B | 1.27 AB | 1452.9 AB | 421.2 AB | 2.00 B | 4.33 B | 1.67 | 59.2 AB |
| p value | 0.002 | 0.073 | 0.009 | 0.001 | 0.006 | 0.009 | 0.006 | 0.027 | 0.328 | 0.0053 |
| Agronomic Traits | PC 1 × 100 | PC 2 × 100 | PC 3 × 100 | PC 4 × 100 |
|---|---|---|---|---|
| Canopy diameter | 33.88 | −7.02 | 16.75 | 12.44 |
| Chlorosis score | 23.90 | −30.53 | −41.19 | 10.59 |
| Dry leaf yield | 24.45 | 49.67 | −1.67 | −53.63 |
| Fresh leaf weight | 23.70 | 50.57 | −3.87 | −6.37 |
| Number of primary branches | 20.78 | 2.88 | 66.56 | −5.15 |
| Plant greenness score | 27.69 | −16.65 | −29.44 | −12.39 |
| Plant height | 33.72 | −1.41 | −27.96 | 43.72 |
| Plant vigor score | 34.81 | −4.44 | −23.9 | −8.61 |
| Stem diameter | 35.42 | 17.61 | 4.44 | 62.66 |
| Seedling survival rate | 28.35 | 21.87 | 7.18 | 3.73 |
| Total flavonoids | 26.47 | −40.42 | 24.97 | 1.93 |
| Total phenolics | 28.56 | −35.58 | 25.70 | −26.59 |
| Eigenvalue | 7.09 | 2.14 | 1.21 | 0.58 |
| % variance | 59.09 | 17.8 | 10.07 | 4.81 |
| Accession Number/Code | Mean Value ± SEM | ||||
|---|---|---|---|---|---|
| TGP (mL/g DM) | CH4 (mL/g DM) | CH4/IVOMD (%) | IVOMD (%) | CH4 Inhibition (%) | |
| Bulk (A1) | 170.5 ± 1.2 BCD | 5.23 ± 0.13 B | 8.81 ± 0.18 BCD | 59.3 ± 0.4 A | 17.9 ± 2.0 B |
| 07229 (A2) | 172.7 ± 1.6 B | 5.21 ± 0.17 B | 8.70 ± 0.36 DE | 60.0 ± 0.9 A | 18.2 ± 2.0 B |
| 07633 (A3) | 160.8 ± 2.2 E | 4.56 ± 0.15 C | 7.61 ± 0.37 F | 60.03 ± 1.0 A | 28.4 ± 2.1 A |
| 07632 (A4) | 164.4 ± 0.5 CDE | 4.71 ± 0.14 C | 8.69 ± 0.21 DE | 54.2 ± 0.7 BC | 25.9 ± 2.0 A |
| 07627 (A5) | 163.7 ± 2.8 DE | 4.72 ± 0.17 C | 8.79 ± 0.18 CD | 53.7 ± 0.9 C | 26.0 ± 2.3 A |
| 03295 (A6) | 169.9 ± 3.4 BCD | 5.06 ± 0.23 B | 9.41 ± 0.35 BCD | 53.8 ± 0.5 C | 20.6 ± 3.4 B |
| 05536 (A7) | 170.4 ± 2.7 BCD | 5.04 ± 0.18 B | 9.38 ± 0.36 BC | 53.7 ± 0.9 C | 20.9 ± 2.7 B |
| 07717 (A8) | 164.2 ± 1.6 CDE | 4.68 ± 0.16 C | 8.06 ± 0.21 EF | 58.4 ± 0.5 A | 26.6 ± 2.3 A |
| 07316 (A9) | 170.6 ± 1.9 BC | 5.01 ± 0.13 B | 9.22 ± 0.20 BCD | 54.3 ± 1.1 BC | 21.4 ± 2.0 B |
| 07216 (A10) | 170.2 ± 1.6 BCD | 5.04 ± 0.18 B | 9.22 ± 0.35 BCD | 54.7 ± 1.3 BC | 20.9 ± 2.8 B |
| Pretoria (A11) | 164.1 ± 0.4 CDE | 4.52 ± 0.18 C | 7.85 ± 0.28 F | 57.6 ± 1.0 A | 29.1 ± 2.6 A |
| M. stenopetala (MS) | 175.0 ± 1.1 B | 5.17 ± 0.15 B | 9.45 ± 0.24 B | 54.7 ± 0.9 BC | 18.8 ± 2.1 B |
| Control | 203.8 ± 5.3 A | 6.33 ± 0.18 A | 11.65 ± 0.40 A | 54.7 ± 0.4 BC | - |
| Accessions Number/Name | Total Phenolic (mg GAE/100 g Extract) | Total Flavonoid (mg QE/100 g Extract) |
|---|---|---|
| Bulk (A1) | 3510 ± 83 CD | 1834 ± 70 D |
| 07229 (A2) | 3489 ± 258 CD | 1894 ± 168 CD |
| 07633 (A3) | 4698 ± 75 A | 2915 ± 68 A |
| 07632 (A4) | 3927 ± 71 BC | 2391 ± 42 B |
| 07627 (A5) | 3671 ± 122 BCD | 2311 ± 120 B |
| 03295 (A6) | 3661 ± 90 BCD | 2159 ± 84 BCD |
| 05536 (A7) | 3586 ± 157 BCD | 2102 ± 43 BCD |
| 07717 (A8) | 3998 ± 93 B | 2250 ± 126 B |
| 07316 (A9) | 3578 ± 7 BCD | 2083 ± 78 BCD |
| 07216 (A10) | 3770 ± 101 BC | 2190 ± 149 BC |
| Pretoria (A11) | 3800 ± 89 BC | 2275 ± 105 B |
| M. stenopetala (MS) | 3270 ± 181 D | 1834 ± 144 D |
| Pearson correlation coefficients (2-tailed) | ||
| Total gas | −0.70551 * | −0.73034 * |
| CH4 | −0.62109 * | −0.70695 * |
| % CH4 inhibition | 0.62057 * | 0.70699 * |
| IVOMD | 0.32318 | 0.1105 |
| CH4/IVOMD | −0.67362 * | −0.61938 * |
| Agronomic Traits | Metabolites | In Vitro Fermentation Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| TP | TF | TGP | CH4 | IVOMD | CH4/IVOMD | CH4/TGP | % CH4 Inhibition | |
| CD | 0.452 ** | 0.438 ** | −0.475 ** | −0.307 | 0.203 | −0.366 * | −0.143 | 0.308 |
| DLY | 0.044 | −0.065 | −0.206 | −0.143 | 0.424 ** | −0.369 * | −0.09 | 0.143 |
| FLY | 0.027 | −0.08 | −0.178 | −0.105 | 0.452 ** | −0.356 * | −0.054 | 0.105 |
| NPB | 0.421 * | 0.375 * | −0.243 | −0.249 | 0.404 * | −0.422 ** | −0.226 | 0.25 |
| PCS | 0.203 | 0.246 | −0.369 * | −0.244 | −0.007 | −0.194 | −0.166 | 0.246 |
| PGS | 0.360 * | 0.258 | −0.426 ** | −0.428 ** | 0.219 | −0.470 ** | −0.361 * | 0.428 ** |
| PH | 0.430 ** | 0.363 * | −0.476 ** | −0.299 | 0.361 * | −0.459 ** | −0.161 | 0.3 |
| PVS | 0.453 ** | 0.425 ** | −0.464 ** | −0.311 | 0.318 | −0.441 ** | −0.188 | 0.312 |
| SD | 0.332 * | 0.261 | −0.372 * | −0.242 | 0.356 * | −0.408 * | −0.143 | 0.243 |
| SS | 0.294 | 0.291 | −0.367 * | −0.359 * | 0.273 | −0.455 ** | −0.318 | 0.359 * |
| Parameters | PC 1 × 100 | PC 2 × 100 | PC 3 × 100 | PC 4 × 100 |
|---|---|---|---|---|
| Canopy diameter (CD) | 26.21 | 6.86 | 20.92 | 13.74 |
| CH4 | −25.63 | 27.39 | 22.32 | 7.11 |
| % CH4 inhibition | 25.56 | −27.61 | −22.02 | −7.75 |
| CH4/IVOMD | −28.13 | −2.99 | 21.77 | −7.59 |
| CH4/TGP | −20.67 | 29.93 | 38.51 | 7.92 |
| Chlorosis score (ChS) | 21.69 | −21.17 | 24.67 | −31.23 |
| Dry leaf yield (DLY) | 17.51 | 39.91 | −23.20 | −18.36 |
| Fresh leaf yield (FLY) | 16.78 | 40.97 | −22.00 | −19.51 |
| IVOMD | 15.82 | 35.77 | −9.44 | 24.32 |
| Number of primary branches (NPB) | 15.88 | 15.81 | −8.19 | 64.02 |
| Plant greenness score (PGS) | 21.16 | 5.91 | 45.18 | −5.15 |
| Plant height (PH) | 26.16 | 11.54 | 29.78 | −20.72 |
| Plant vigor score (PVS) | 27.48 | 8.47 | 26.10 | −17.71 |
| Stem diameter (SD) | 26.44 | 24.96 | 9.95 | −1.81 |
| Seedling survival (SS) | 23.75 | 12.15 | −29.17 | −16.33 |
| Total flavonoids (TF) | 25.00 | −24.89 | 8.57 | 28.03 |
| Total gas production (TGP) | −27.75 | 20.75 | 3.01 | 6.55 |
| Total phenolics (TP) | 26.02 | −15.05 | 13.89 | 36.48 |
| Eigenvalue | 10.26 | 3.27 | 1.42 | 1.27 |
| % variance | 57.00 | 18.19 | 7.89 | 7.08 |
| Species Name | Accession Code/Number | Collection Area | Country of Origin |
|---|---|---|---|
| Moringa oleifera | A1 (Bulk) | Meru | Kenya |
| Moringa oleifera | A2 (7229) | Machakos | Kenya |
| Moringa oleifera | A3 (7633) | Segou | Mali |
| Moringa oleifera | A4 (7632) | Bamako | Mali |
| Moringa oleifera | A5 (7627) | NA | Kenya |
| Moringa oleifera | A6 (3295) | Mbbololo | Kenya |
| Moringa oleifera | A7 (5536) | Busia | Kenya |
| Moringa oleifera | A8 (7717) | Ramogi | Kenya |
| Moringa oleifera | A9 (7316) | Kibwezl | Kenya |
| Moringa oleifera | A10 (7216) | Ramisi | Kenya |
| Moringa oleifera | A11 (NA) | Pretoria | South Africa |
| Moringa stenopetala | MS (NA) | NA | Kenya |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeru, A.E.; Hassen, A.; Apostolides, Z.; Tjelele, J. Relationships between Agronomic Traits of Moringa Accessions and In Vitro Gas Production Characteristics of a Test Feed Incubated with or without Moringa Plant Leaf Extracts. Plants 2022, 11, 2901. https://doi.org/10.3390/plants11212901
Zeru AE, Hassen A, Apostolides Z, Tjelele J. Relationships between Agronomic Traits of Moringa Accessions and In Vitro Gas Production Characteristics of a Test Feed Incubated with or without Moringa Plant Leaf Extracts. Plants. 2022; 11(21):2901. https://doi.org/10.3390/plants11212901
Chicago/Turabian StyleZeru, Addisu Endalew, Abubeker Hassen, Zeno Apostolides, and Julius Tjelele. 2022. "Relationships between Agronomic Traits of Moringa Accessions and In Vitro Gas Production Characteristics of a Test Feed Incubated with or without Moringa Plant Leaf Extracts" Plants 11, no. 21: 2901. https://doi.org/10.3390/plants11212901







