Soil Fungal Composition Drives Ecosystem Multifunctionality after Long-Term Field Nitrogen and Phosphorus Addition in Alpine Meadows on the Tibetan Plateau
Abstract
:1. Introduction
2. Results
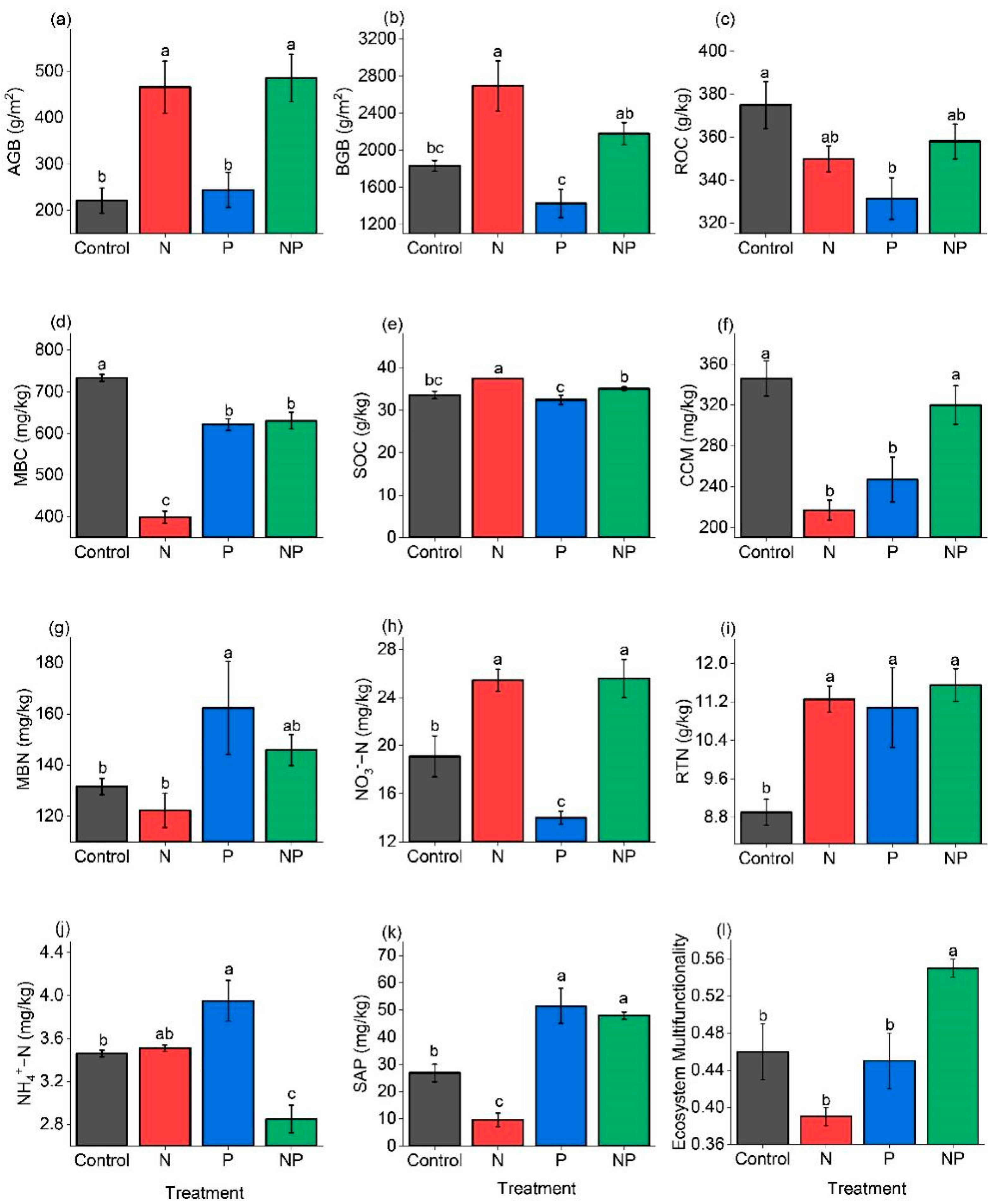
2.1. Effects of N and P Addition on Ecosystem Parameters
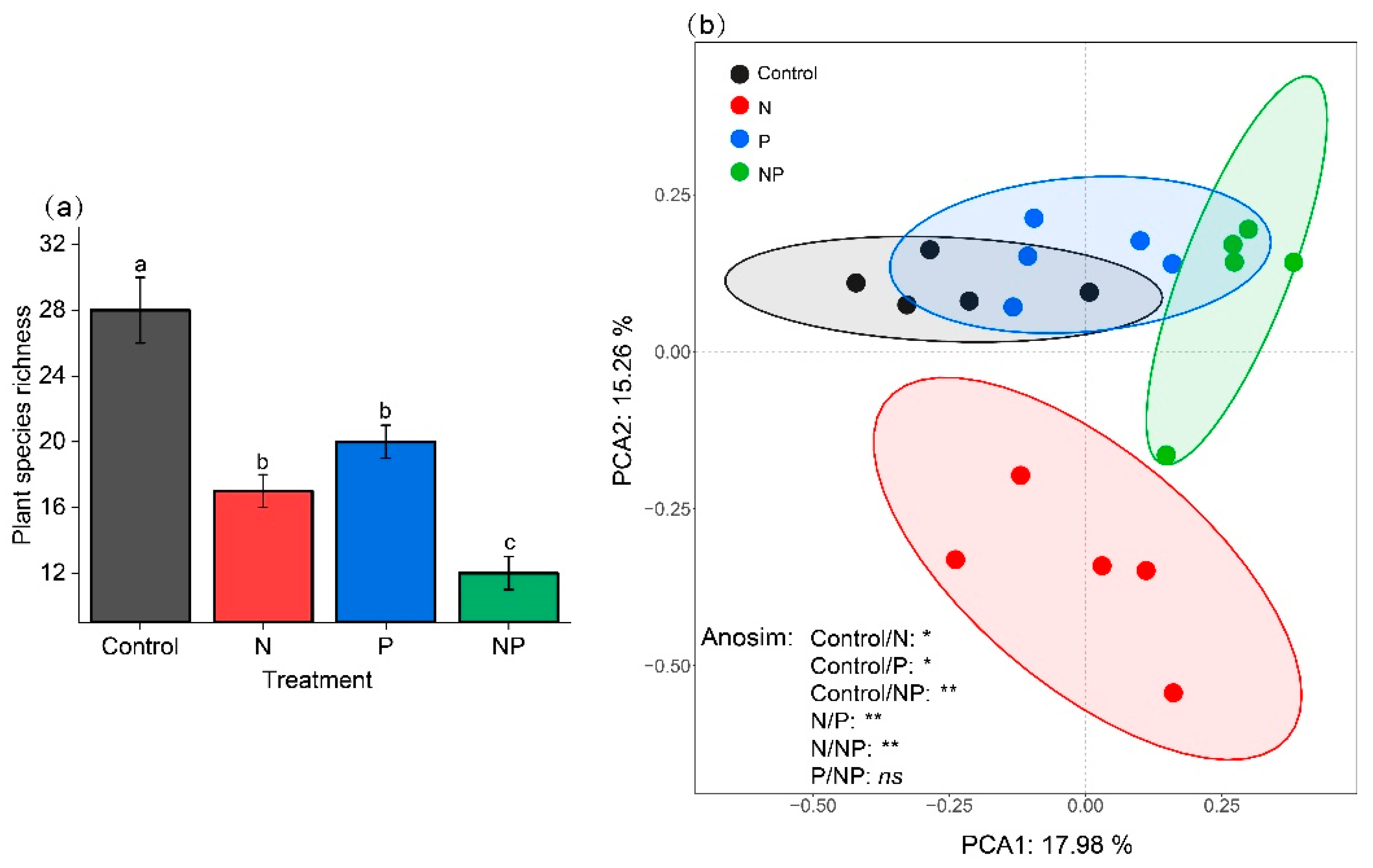
2.2. Effects of N and P Addition on Abiotic Factors and Plant and Microbial Species Diversity
2.3. Relationships between Abiotic Properties, Plant and Microbial Species Diversity, and Ecosystem Parameters
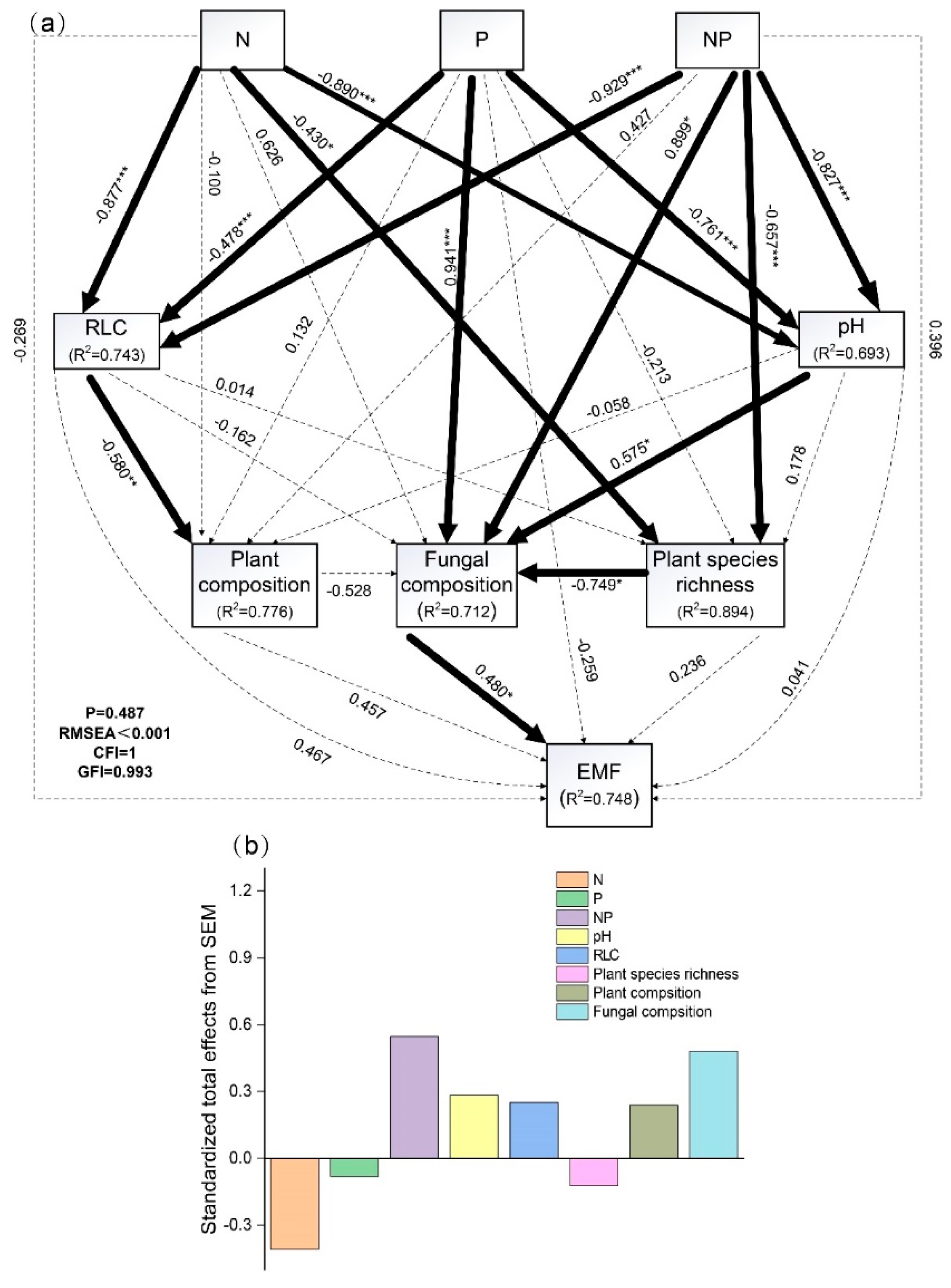
2.4. The Direct and Indirect Mediation Effect of N and P Addition on EMF
3. Discussion
3.1. The Responses of Abiotic Factors to N and P Addition
3.2. The Responses of Plant and Microbial Species Diversity to N and P Addition
3.3. The Responses of Ecosystem Parameters to N and P Addition
3.4. The Effect of the Direct and Indirect Pathways of N and P Addition on EMF
4. Materials and Methods
4.1. Study Site and Experimental Design
4.2. Plant and Soil Sampling
4.3. Soil and Root Samples Properties Analysis and Microbial High-Throughput Sequencing
4.4. Quantifying Ecosystem Multifunctionality and Biodiversity Indices
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Wang, C.; Feng, X.M.; Wu, X.; Fu, B.J. Research progress on the relationship between biodiversity and ecosystem multifunctionality. Sheng Tai Xue Bao 2022, 42, 11–23. [Google Scholar]
- Finney, D.M.; Kaye, J.P. Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J. Appl. Ecol. 2017, 54, 509–517. [Google Scholar] [CrossRef]
- Huang, X.B.; Su, J.R.; Li, S.F.; Liu, W.D.; Lang, X.D. Functional diversity drives ecosystem multifunctionality in a Pinus yunnanensis natural secondary forest. Sci. Rep. 2019, 9, 6979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.B.; Li, S.F.; Su, J.R. Selective logging enhances ecosystem multifunctionality via increase of functional diversity in a Pinus yunnanensis forest in Southwest China. For. Ecosyst. 2020, 7, 55. [Google Scholar] [CrossRef]
- Mensah, S.; Salako, K.V.; Assogbadjo, A.E.; Kaka, R.; Seifert, T. Functional trait diversity is a stronger predictor of multifunctionality than dominance: Evidence from an Afromontane forest in South Africa. Ecol. Indic. 2020, 115, 106415. [Google Scholar] [CrossRef]
- Liu, X.C.; Shi, X.M.; Zhang, S.T. Abiotic factors and plant functional diversity co-regulate the impacts of nitrogen addition on ecosystem multifunctionality in an alpine meadow. Sci. Total Environ. 2021, 780, 146476. [Google Scholar] [CrossRef]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef]
- Maestre, F.T.; Castillo-Monroy, A.P.; Bowker, M.A.; Ochoa-Hueso, R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J. Ecol. 2012, 100, 317–330. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Pasari, J.R.; Levi, T.; Zavaleta, E.S.; Tilman, D. Several scales of biodiversity affect ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2013, 110, 10219–10222. [Google Scholar] [CrossRef] [Green Version]
- Van der Plas, F.; Manning, P.; Soliveres, S.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Ampoorter, E.; Baeten, L.; et al. Biotic homogenization can decrease landscapescale forest multifunctionality. Proc. Natl. Acad. Sci. USA 2016, 113, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Grman, E.; Zirbel, C.R.; Bassett, T.; Brudvig, L.A. Ecosystem multifunctionality increases with beta diversity in restored prairies. Oecologia 2018, 188, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.Y.; Classen, A.T.; Zhao, K.; Chen, L.T.; Shi, Y.; Jiang, Y.X.; He, J.S. The links between ecosystem multifunctionality and above—And belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Ochoa, V.; Gozalo, B.; Berdugo, M.; Val, J.; Singh, B.K. Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytol. 2016, 209, 1540–1552. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Singh, B.K. Fungal richness contributes to multifunctionality in boreal forest soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Schillaci, M.; Raio, A.; Sillo, F.; Zampieri, E.; Mahmood, S.; Anjum, M.; Khalid, A.; Centritto, M. Pseudomonas and Curtobacterium Strains from Olive Rhizosphere Characterized and Evaluated for Plant Growth Promoting Traits. Plants 2022, 11, 2245. [Google Scholar] [CrossRef]
- Jing, X.; Prager, C.M.; Chen, L.T.; Chu, H.Y.; Gotelli, N.J.; He, J.S.; Shi, Y.; Yang, T.; Zhu, B.; Classen, A.T.; et al. The influence of aboveground and belowground species composition on spatial variation in plant- and soil-mediated ecosystem functions in alpine grasslands. Glob. Ecol. Biogeogr. 2022, 31, 486–500. [Google Scholar] [CrossRef]
- Veach, A.M.; Chen, H.; Yang, Z.K.; Labbe, A.D.; Engle, N.L.; Tschaplinski, T.J.; Schadt, C.W.; Cregger, M.A. Plant hosts modify belowground microbial community response to extreme drought. mSystems 2020, 5, e00092-20. [Google Scholar] [CrossRef]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.J.; Allan, E.; Besser, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, Z.Y.; Tong, L.J.; Khalifa, M.; Wang, Q.; Gang, C.C.; Wang, Z.Q.; Li, J.L.; Sun, Z.G. Assessing the effects of climate variation and human activities on grassland degradation and restoration across the globe. Ecol. Indic. 2019, 106, 105504–105515. [Google Scholar] [CrossRef]
- Cui, H.W.; Wagg, C.; Wang, X.T.; Liu, Z.Y.; Liu, K.; Chen, S.Y.; Chen, J.W.; Song, H.X.; Meng, L.H.; Wang, J.J.; et al. The loss of above- and belowground biodiversity in degraded grasslands drives the decline of ecosystem multifunctionality. Appl. Soil Ecol. 2022, 172, 104370. [Google Scholar] [CrossRef]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decades. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Li, J.H.; Yang, Y.J.; Li, B.W.; Li, W.J.; Wang, G.; Knops, J.M.H. Effects of nitrogen and phosphorus fertilization on soil carbon fractions in alpine meadows on the Qinghai-Tibetan Plateau. PLoS ONE 2014, 9, e103266. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Hou, Y.L.; Zhang, S.X.; Li, W.J.; Xu, D.H.; Knops, J.M.H.; Shi, X.M. Fertilization with nitrogen and/or phosphorus lowerssoil organic carbon sequestration in alpine meadows. Land Degrad. Dev. 2018, 29, 1634–1641. [Google Scholar] [CrossRef]
- Li, J.H.; Li, F.; Chen, S.; Li, W.J.; Abbott, L.K.; Knops, J.M.H. Nitrogen additions promote decomposition of soil organic carbon in a Tibetan alpine meadow. Soil Sci. Soc. Am. J. 2018, 82, 614–621. [Google Scholar] [CrossRef]
- Li, J.H.; Cheng, B.H.; Zhang, R.; Li, W.J.; Shi, X.M.; Han, Y.W.; Ye, L.F.; Ostle, N.J.; Bardgett, R.D. Nitrogen and phosphorus additions accelerate decomposition of slow carbon pool and lower total soil organic carbon pool in alpine meadows. Land Degrad. Dev. 2021, 32, 1761–1772. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, R.; Cheng, B.H.; Ye, L.F.; Li, W.J.; Shi, X.M. Effects of nitrogen and phosphorus additions on decomposition and accumulation of soil organic carbon in alpine meadows on the Tibetan Plateau. Land Degrad. Dev. 2021, 32, 1467–1477. [Google Scholar] [CrossRef]
- Chen, Q.L.; Ding, J.; Zhu, D.; Hu, H.W.; Zhu, Y.G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2019, 141, 107686. [Google Scholar] [CrossRef]
- Cammeraat, E.L.H.; Risch, A.C. The impact of ants on mineral soil properties and processes at different spatial scales. J. Appl. Entomol. 2010, 132, 285–294. [Google Scholar] [CrossRef]
- Luo, P.Y.; Han, X.R.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H.Z. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F. Effect of Nitrogen and Phosphorous Additions on Species Diversity and Plant Height in the Sub-alpine Meadow. Master’ Thesis, Lanzhou University, Lanzhou, China, 2014. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10730-1014303705.htm (accessed on 29 November 2014).
- Jutila, H.M.; Grace, J.B. Effects of disturbance on germination and seedling establishment in a coastal prairie grassland: A test of the competitive release hypothesis. J. Ecol. 2001, 90, 291–302. [Google Scholar] [CrossRef]
- Wang, J.Q.; Shi, X.Z.; Zheng, C.Y.; Suter, H.; Huang, Z.Q. Different responses of soil bacterial and fungal community to nitrogen deposition in a subtropical forest. Sci. Total Environ. 2020, 755, 142449. [Google Scholar] [CrossRef] [PubMed]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.M.; Guhr, A.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Yang, L.Y.; Shi, H.Q.; Li, L.H.; Bai, W.M.; Zhao, Y. Soil Acidification Under Long-Term N Addition Decreases the Diversity of Soil Bacteria and Fungi and Changes Their Community Composition in a Semiarid Grassland. Microb. Ecol. 2022. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal community across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.Q.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Grosso, F.; Bååth, E.; de Nicola, F. Bacterial and fungal growth on different plant litter in Mediterranean soils: Effects of C/N ratio and soil pH. Appl. Soil Ecol. 2016, 108, 1–7. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Xu, T.L.; Veresoglou, S.D.; Hu, H.W.; Hao, Z.P.; Hu, Y.J.; Liu, L.; Deng, Y.; Rillig, M.C.; Chen, B.D. Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol. Biochem. 2017, 110, 12–21. [Google Scholar] [CrossRef]
- Baumann, F.; He, J.S.; Schmidt, K.; KÜhn, P.; Scholten, T. Pedogenesis, permafrost, and soil moisture as controlling factors for soil nitrogen and carbon contents across the Tibetan Plateau. Glob. Chang. Biol. 2009, 15, 3001–3017. [Google Scholar] [CrossRef]
- Zhang, S.H.; Chen, D.D.; Sun, D.S.; Wang, X.T.; Smith, J.L.; Du, G.Z. Impacts of altitude and position on the rates of soil nitrogen mineralization and nitrification in alpine meadows on the eastern Qinghai-Tibetan Plateau, China. Biol. Fertil. Soils 2012, 48, 393–400. [Google Scholar] [CrossRef]
- Song, M.H.; Zheng, L.L.; Suding, K.N.; Yin, T.F. Plasticity in nitrogen form uptake and preference in response to long-term nitrogen fertilization. Plant Soil 2015, 394, 215–224. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, R.; Xu, X.L.; Song, M.H.; Ouyang, H. Three Tibetan grassland plant species tend to partition niches with limited plasticity in nitrogen use. Plant Soil 2019, 441, 601–611. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, T.B.; Liu, X.; Nie, M.; Xu, X.L.; Zhou, S.R. Limited inorganic N niche partitioning by nine alpine plant species after long-term nitrogen addition. Sci. Total Environ. 2020, 718, 137270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Sang, C.P.; Yang, J.Y.; Qu, L.R.; Xia, Z.W.; Sun, H.; Jiang, P.; Wang, X.G.; He, H.B.; Wang, C. Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol. Biochem. 2021, 156, 108207. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.G.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Sirulnik, A.G.; Allen, E.B.; Meixner, T.; Allen, M.F. Impacts of anthropogenic N additions on nitrogen mineralization from plant litter in exotic annual grasslands. Soil Biol. Biochem. 2007, 39, 24–32. [Google Scholar] [CrossRef]
- Lu, X.T.; Reed, S.; Yu, Q.; He, N.P.; Wang, Z.W.; Han, X.G. Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob. Chang. Biol. 2013, 19, 2775–2784. [Google Scholar] [CrossRef]
- Hautier, Y.; Isbell, F.; Borer, E.T.; Seabloom, E.W.; Harpole, W.S.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Adler, P.B.; Alberti, J.; et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Li, S.F.; Huang, X.B.; Lang, X.D.; Shen, J.Y.; Xu, F.D.; Su, J.R. Cumulative effects of multiple biodiversity attributes and abiotic factors on ecosystem multifunctionality in the Jinsha River valley of southwestern China. For. Ecol. Manag. 2020, 472, 118281. [Google Scholar]
- Porazinska, D.L.; Farrer, E.C.; Spasojevic, M.J.; de Mesquita, C.P.B.; Sartwell, S.A.; Smith, J.G.; White, C.T.; King, A.J.; Suding, K.N.; Schmidt, S.K. Plant diversity and density predict belowground diversity and function in an early successional alpine ecosystem. Ecology 2018, 99, 1942–1952. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R. Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 2006, 9, 921–933. [Google Scholar] [CrossRef]
- Yasuhara, M.; Doi, H.; Wei, C.L.; Danovaro, R.; Myhre, S.E. Biodiversity-ecosystem functioning relationships in long-term time series and palaeoecological records: Deep sea as a test bed. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150282–20150291. [Google Scholar] [CrossRef] [Green Version]
- Guan, P.T.; Mahamood, M.; Yang, Y.R.; Wu, D.H. Land conversion regulates the effects of long-term climate warming on soil micro-food web community. Agric. Ecosyst. Environ. 2021, 314, 107426–107435. [Google Scholar] [CrossRef]
- Xu, Y.D.; Dong, S.K.; Gao, X.X.; Yang, M.Y.; Li, S.; Shen, H.; Xiao, J.N.; Han, Y.H.; Zhang, J.; Li, Y.; et al. Aboveground community composition and soil moisture play determining roles in restoring ecosystem multifunctionality of alpine steppe on Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 2021, 305, 107163–107169. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Liu, H.; Bai, J.; Li, J. Soil Fungal Composition Drives Ecosystem Multifunctionality after Long-Term Field Nitrogen and Phosphorus Addition in Alpine Meadows on the Tibetan Plateau. Plants 2022, 11, 2893. https://doi.org/10.3390/plants11212893
Cheng B, Liu H, Bai J, Li J. Soil Fungal Composition Drives Ecosystem Multifunctionality after Long-Term Field Nitrogen and Phosphorus Addition in Alpine Meadows on the Tibetan Plateau. Plants. 2022; 11(21):2893. https://doi.org/10.3390/plants11212893
Chicago/Turabian StyleCheng, Bingheng, Hongyan Liu, Juan Bai, and Jinhua Li. 2022. "Soil Fungal Composition Drives Ecosystem Multifunctionality after Long-Term Field Nitrogen and Phosphorus Addition in Alpine Meadows on the Tibetan Plateau" Plants 11, no. 21: 2893. https://doi.org/10.3390/plants11212893



