Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurements of Seed-Germination and Growth Parameters
2.3. Measurements of Reactive Oxygen Species and Antioxidant Enzyme Activities
2.4. Measurements of Genes Expression Levels
2.5. Statistical Analysis
3. Results
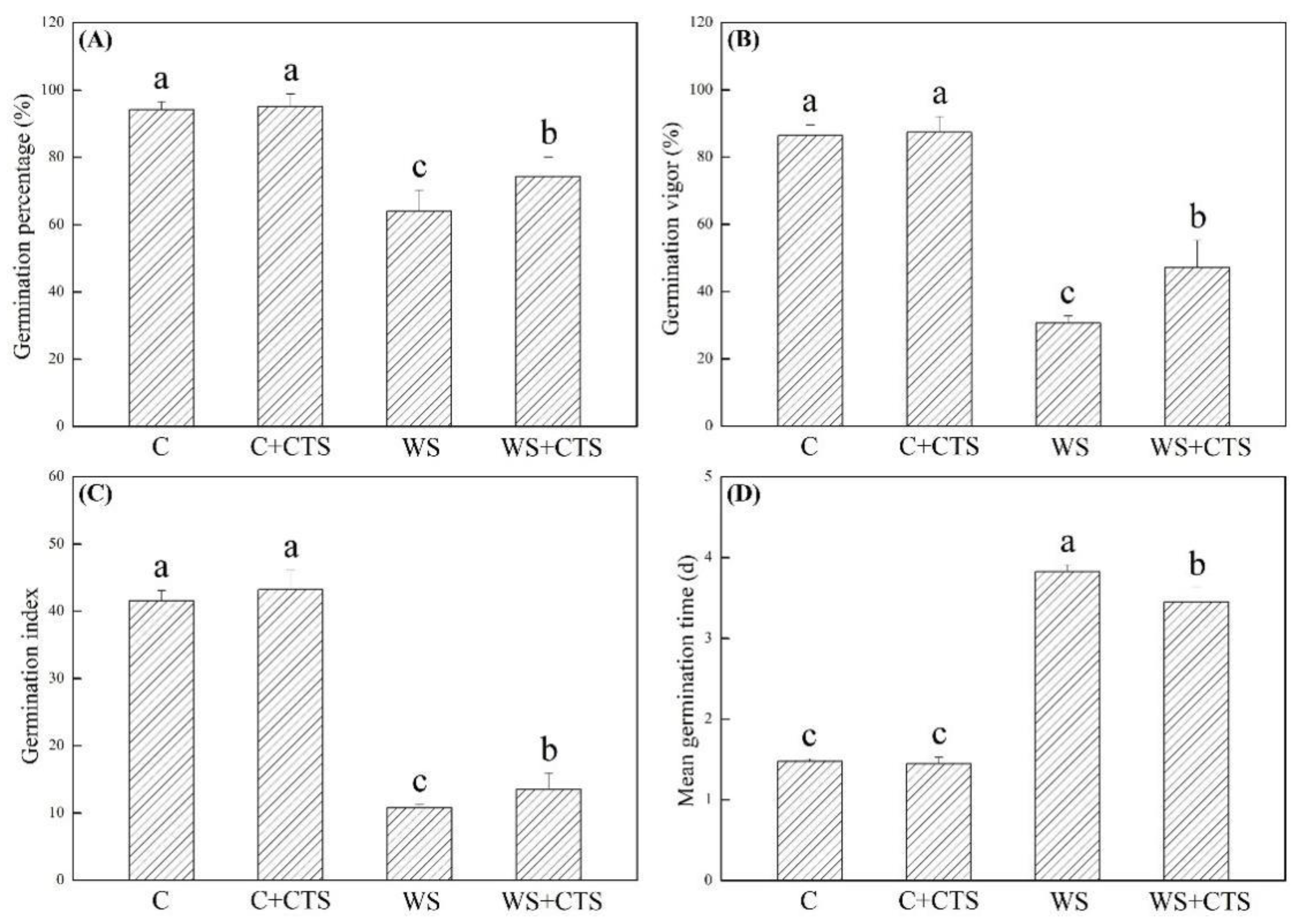
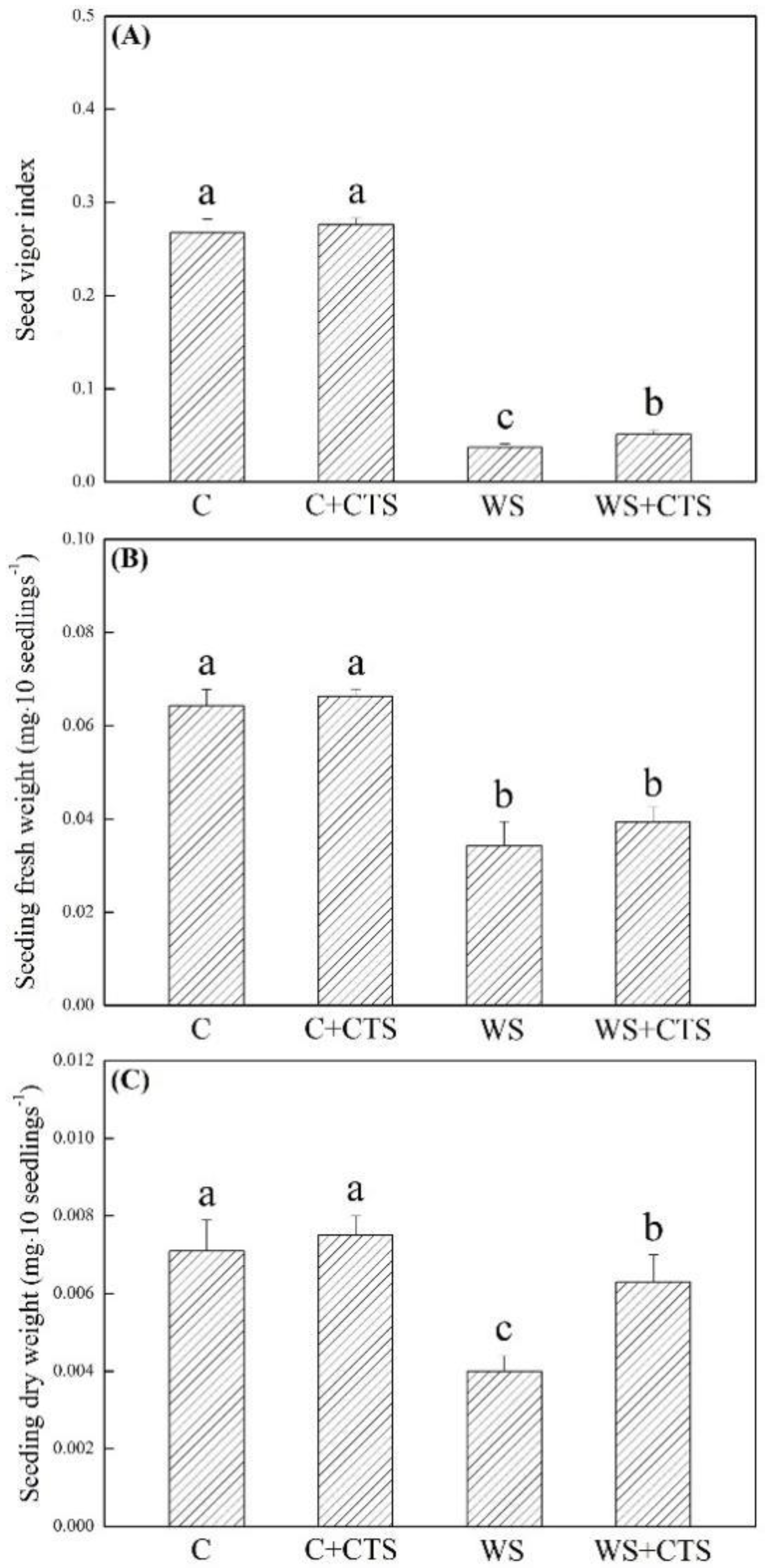
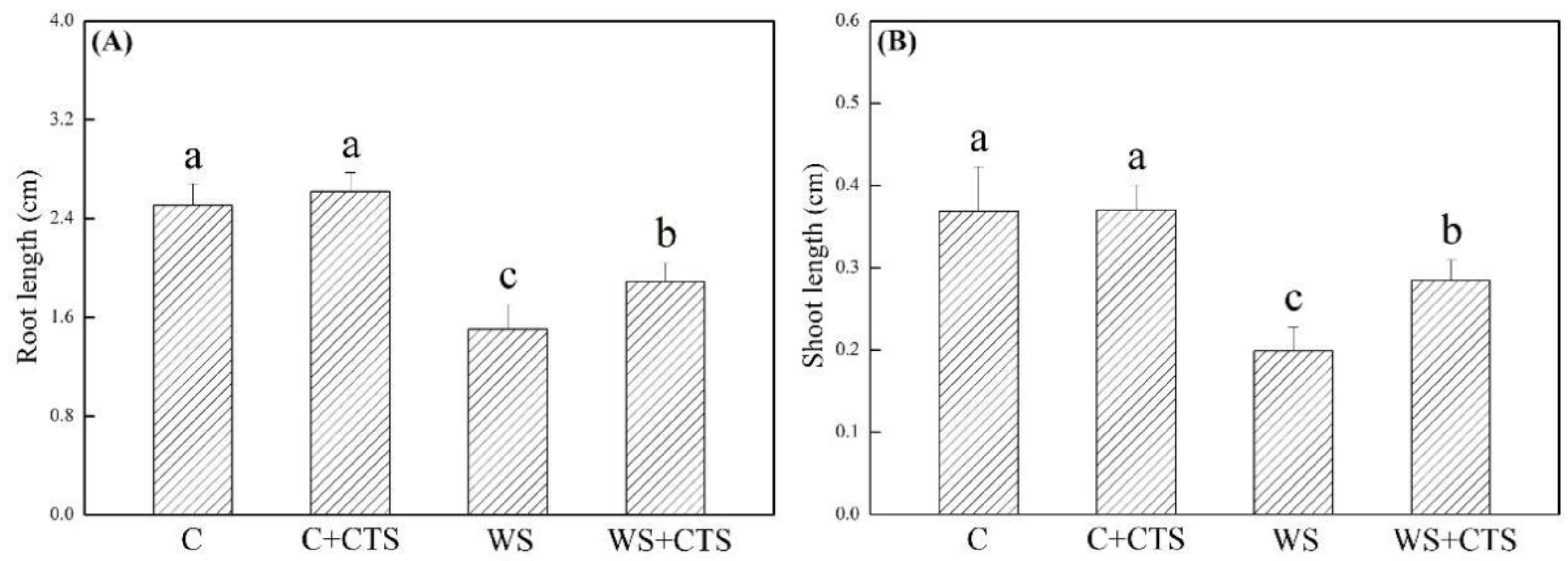
3.1. Seeds Priming with CTS Affected Germination Characteristics under Water Stress
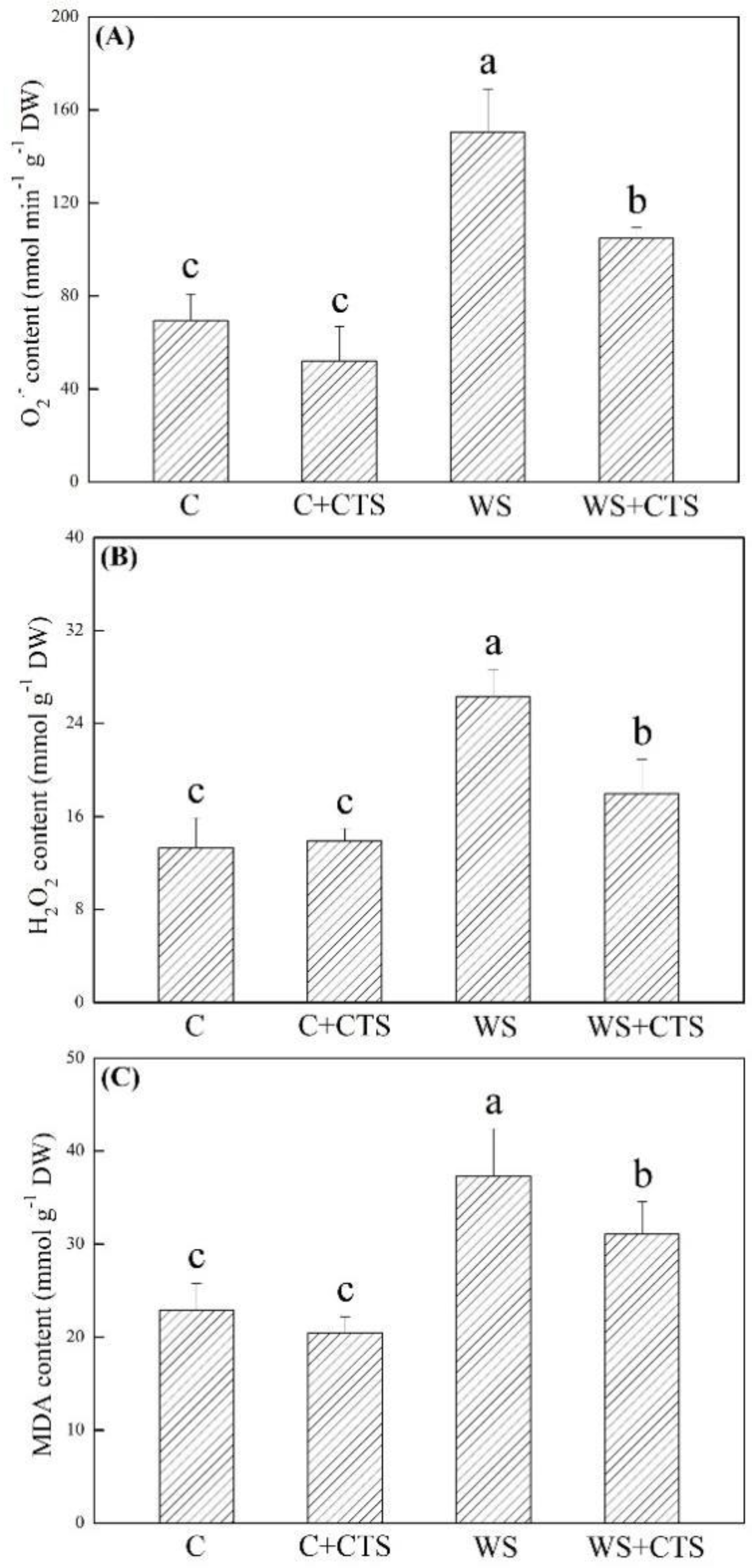
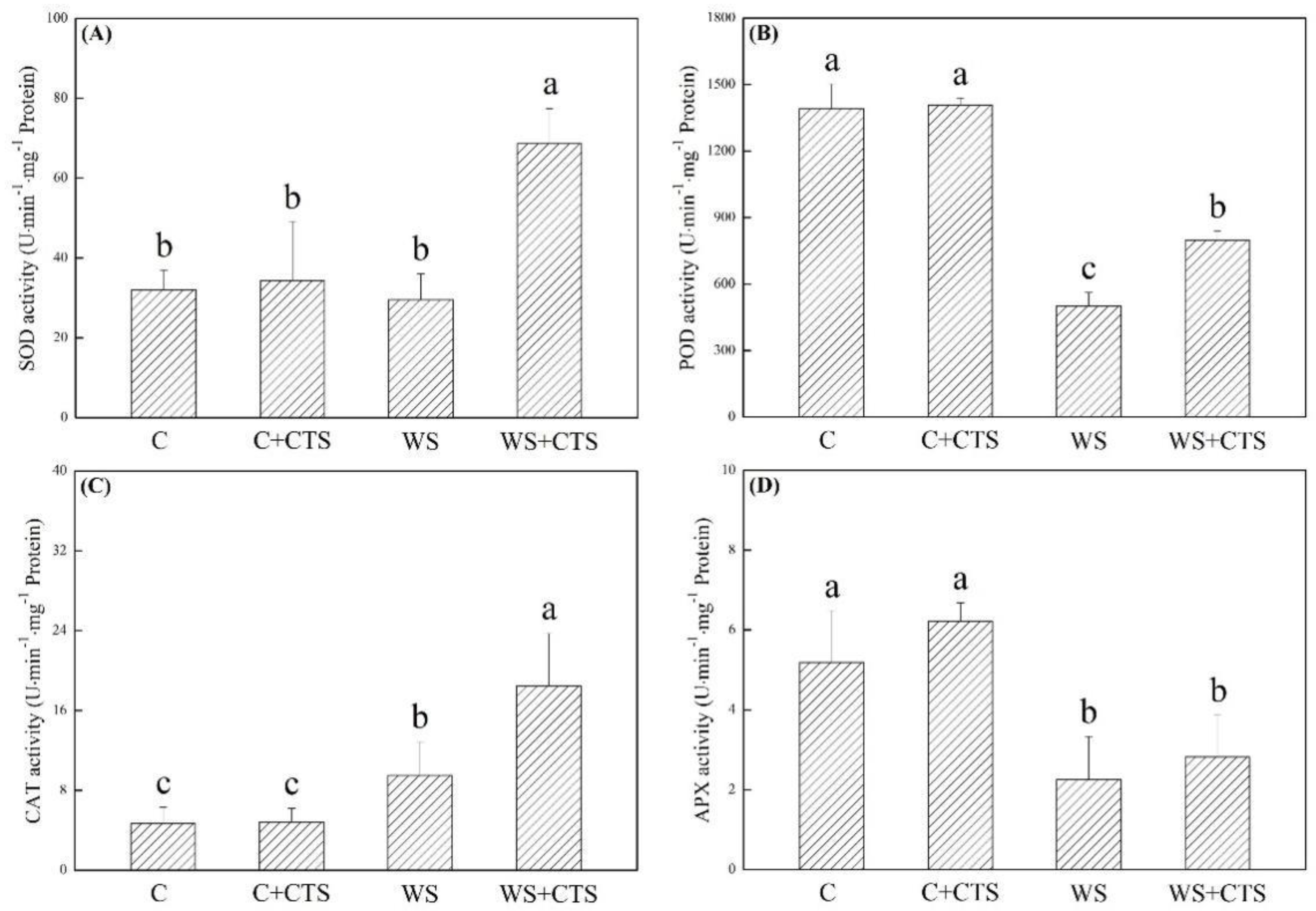
3.2. Seed Priming with CTS Affected Oxidative Damage and Antioxidant Defense under Water Stress
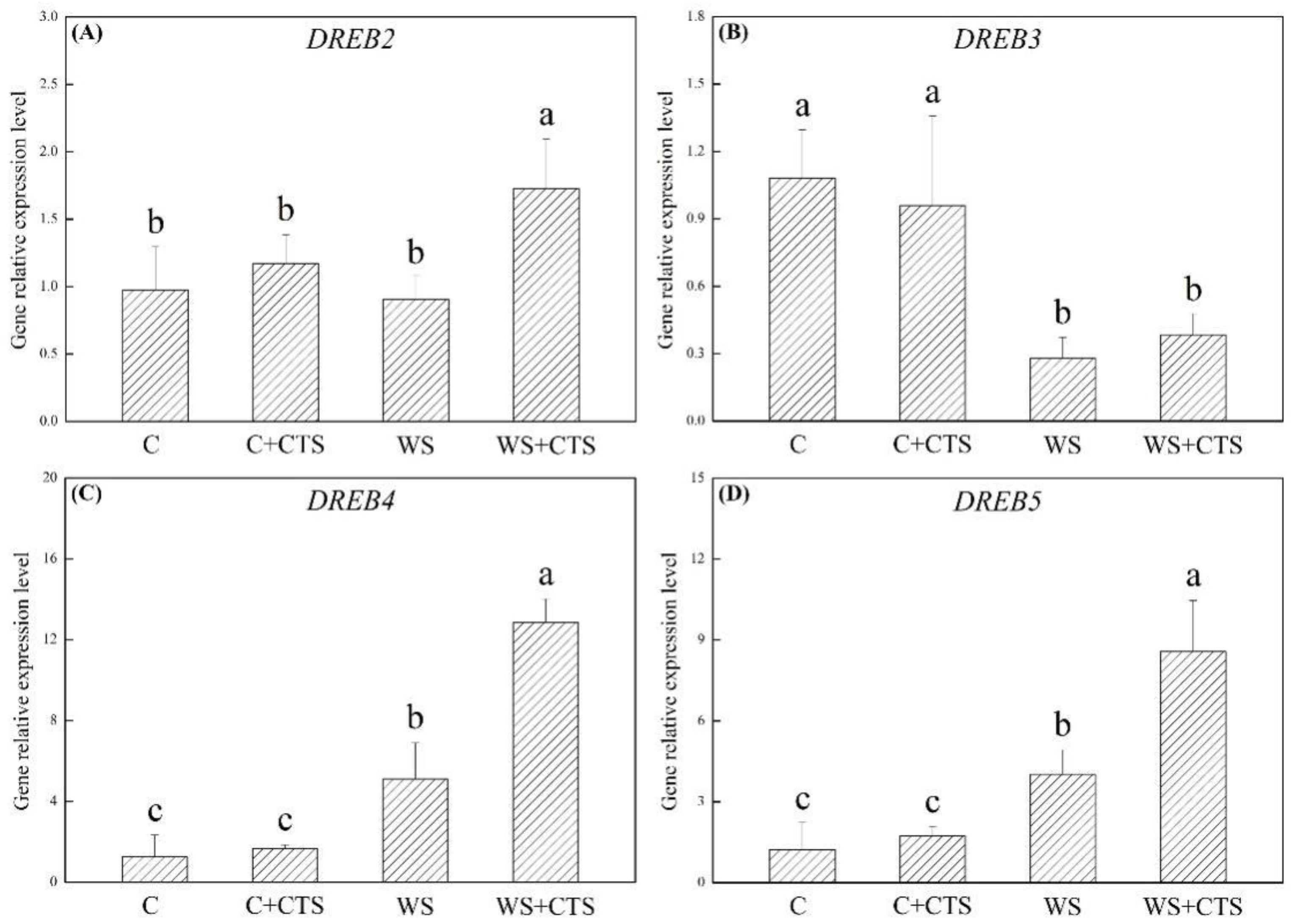
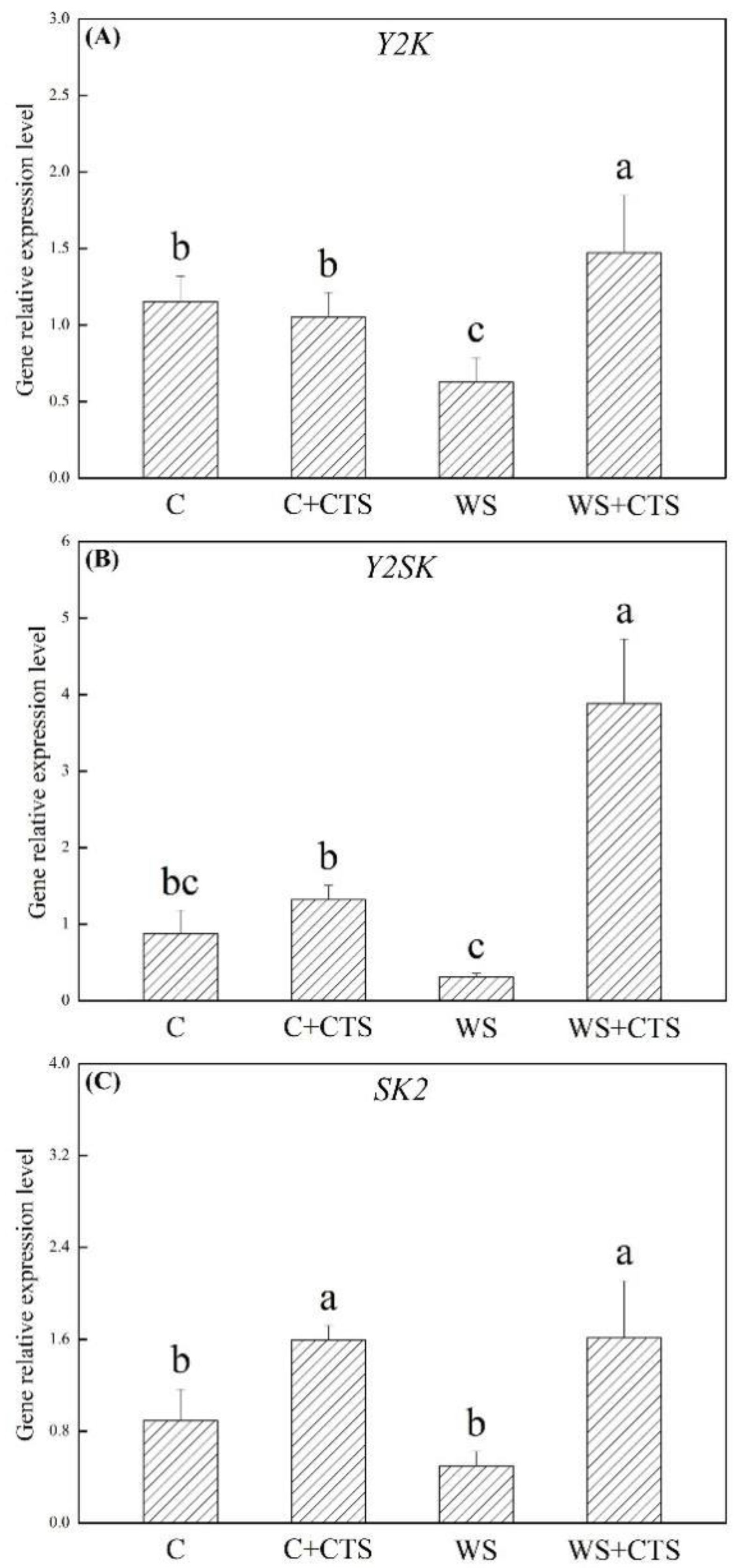
3.3. Seeds Priming with CTS Affected Genes Expression Levels Involved in Dehydration-Responsive Pathway under Water Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aroca, R. Plant responses to drought stress. In From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–5. [Google Scholar]
- Ahmad, S.; Ahmad, R.; Ashraf, M.Y.; Ashraf, M.; Waraich, E.A. Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak. J. Bot. 2009, 41, 647–654. [Google Scholar]
- Sucre, B.; Suarez, N. Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation and leaf growth in Ipomoea pes-caprae. Environ. Exp. Bot. 2011, 70, 2. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Gujjar, R.S.; Akhtar, M.; Singh, M. Transcription factors in abiotic stress tolerance. Indian J. Plant Physiol. 2014, 19, 306–316. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Yadav, J.; Chakdar, H.; Saxena, A. Deciphering the mechanisms of microbe mediated drought stress alleviation in wheat. Res. Sq. Prepr. 2021, 1–30. [Google Scholar]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressfull conditions. J. Bot. 2012, 2012, 217037. [Google Scholar]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Hasanuzzaman, M.; Li, Y.; Akhtar, K.; Zhang, C.; Zhao, T. Seed germination behavior, growth, physiology and antioxidant metabolism of four contrasting cultivars under combined drought and salinity in soybean. Antioxidants 2022, 11, 498. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Yao, H.; Wu, J.; Sun, H.; Gong, H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Biochem. 2014, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kocięcka, J.; Liberacki, D. The potential of using chitosan on cereal crops in the face of climate change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; El-Shehawi, A.; Ibrahim, O.; Abdul-Hafeez, E.; Moussa, M.; Hassan, F. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.; Bakhoum, G.; Tawfic, M. Chitosan and chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinus termis L. plant under drought conditions. Egypt. J. Chem. 2022, 65, 1–2. [Google Scholar] [CrossRef]
- Nurliana, S.; Fachriza, S.; Hemelda, N.; Yuniati, R. Chitosan Application for Maintaining the Growth of Lettuce (Lactuca sativa) under Drought Condition; IOP Conference Series: Earth and Environmental Science, 2022; IOP Publishing: Bristol, UK, 2022; p. 012013. [Google Scholar]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S. Chitosan-induced physiological and biochemical regulations confer drought tolerance in pot marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Farooq, M.; Almamari, S.A.D.; Rehman, A.; Al-Busaidi, W.M.; Wahid, A.; Al-Ghamdi, S.S. Morphological, physiological and biochemical aspects of zinc seed priming-induced drought tolerance in faba bean. Sci. Hortic. 2021, 281, 109894. [Google Scholar] [CrossRef]
- Zhou, M.; Hassan, M.J.; Peng, Y.; Liu, L.; Liu, W.; Zhang, Y.; Li, Z. γ-Aminobutyric acid (GABA) priming improves seeds germination and stress tolerance associated with enhanced antioxidant metabolism, DREB expression, and dehydrin accumulation in white clover under drought stress. Front. Plant Sci. 2021, 12, 2675. [Google Scholar] [CrossRef]
- Cheng, B.; Hassan, M.J.; Feng, G.; Zhao, J.; Liu, W.; Peng, Y.; Li, Z. Metabolites reprogramming and Na+/K+ transportation associated with putrescine-regulated white clover seed germination and seedling tolerance to salt toxicity. Front. Plant Sci. 2022, 13, 856007. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.J.; Geng, W.; Zeng, W.; Raza, M.A.; Khan, I.; Iqbal, M.Z.; Peng, Y.; Zhu, Y.; Li, Z. Diethyl aminoethyl hexanoate priming ameliorates seed germination via involvement in hormonal changes, osmotic adjustment, and dehydrins accumulation in white clover under drought stress. Front. Plant Sci. 2021, 12, 709187. [Google Scholar] [CrossRef]
- Lizárraga-Paulín, E.G.; Miranda-Castro, S.P.; Moreno-Martínez, E.; Lara-Sagahón, A.V.; Torres-Pacheco, I. Maize seed coatings and seedling sprayings with chitosan and hydrogen peroxide: Their influence on some phenological and biochemical behaviors. J. Zhejiang Univ. Sci. 2013, 14, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Method Enzym. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Colmer, T.D.; Barton, L. A review of warm-season turfgrass evapotranspiration, responses to deficit irrigation, and drought resistance. Crop Sci. 2017, 57 (Suppl. S1), S98–S110. [Google Scholar] [CrossRef]
- McCurdy, J.D.; McElroy, J.S.; Guertal, E.A. White clover (Trifolium repens) establishment within dormant bermudagrass turf: Cultural considerations, establishment timing, seeding rate, and cool-season companion grass species. HortScience 2013, 48, 1556–1561. [Google Scholar] [CrossRef] [Green Version]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liang, L.; Cheng, B.; Dong, Y.; Wei, J.; Tian, X.; Peng, Y.; Li, Z. Pretreatment with NaCl promotes the seed germination of white clover by affecting endogenous phytohormones, metabolic regulation, and dehydrin-encoded genes expression under water stress. Int. J. Mol. Sci. 2018, 19, 3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songlin, R.; Qingzhong, X. Effects of chitosan coating on seed germination and salt-tolerance of seedling in hybrid rice (Oryza sativa L.). Zuo Wu Xue Bao 2002, 28, 803–808. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Luo, X. Physiological effects of chitosan coating on wheat growth and activities of protective enzyme with drought tolerance. Open J. Soil Sci. 2012, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Moolphuerk, N.; Lawson, T.; Pattanagul, W. Chitosan mitigates the adverse effects and improves photosynthetic activity in rice (Oryza sativa L.) seedlings under drought condition. J. Crop Improv. 2021, 36, 638–655. [Google Scholar] [CrossRef]
- Mustafa, G.; Shehzad, M.A.; Tahir, M.; Nawaz, F.; Akhtar, G.; Bashir, M.A.; Ghaffar, A. Pretreatment with chitosan arbitrates physiological processes and antioxidant defense system to increase drought tolerance in alfalfa (Medicago sativa L.). J. Soil Sci. Plant Nutr. 2022, 22, 2169–2186. [Google Scholar] [CrossRef]
- Manna, M.; Thakur, T.; Chirom, O.; Mandlik, R.; Deshmukh, R.; Salvi, P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol. Plant. 2021, 172, 847–868. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [Green Version]
- do Rego, T.F.C.; Santos, M.P.; Cabral, G.B.; de Moura Cipriano, T.; de Sousa, N.L.; de Souza Neto, O.A.; Aragão, F.J.L. Expression of a DREB 5-A subgroup transcription factor gene from Ricinus communis (RcDREB1) enhanced growth, drought tolerance and pollen viability in tobacco. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 146, 493–504. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Wu, Z.; Chen, M.; Chai, T.; Wang, H. Overexpression of the Polygonum cuspidatum PcDREB2A gene encoding a DRE-Binding transcription factor enhances the drought tolerance of transgenic Arabidopsis thaliana. J. Plant Biol. 2021, 1–11. [Google Scholar] [CrossRef]
- Tao, L.; Yu, G.; Chen, H.; Wang, B.; Jiang, L.; Han, X.; Lin, G.; Cheng, X.-G. SlDREB2 gene specifically recognizing to the universal DRE elements is a transcriptional activator improving drought tolerance in tomato. Sci. Hortic. 2022, 295, 110887. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Zhang, H.; Zhang, Y.; Xue, M.; Ren, M.; Tang, K.; Guo, H.; Wang, M. Constitutive expression of two A-5 subgroup DREB transcription factors from desert shrub Ammopiptanthus mongolicus confers different stress tolerances in transgenic Arabidopsis. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 147, 351–363. [Google Scholar] [CrossRef]
- Han, Y.; Xiang, L.; Song, Z.; Lu, S. Overexpression of SgDREB2C from Stylosanthes guianensis leads to increased drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3520. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2021, 41, 519–533. [Google Scholar] [CrossRef]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the past four decades: From chaperones to transcription co-regulators in regulating abiotic stress response. Curr. Res. Biotechnol. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Sun, S.; Han, W.; Irfan, M.; Zhang, X.; Zhang, L.; Chen, L. AnDHN, a dehydrin protein from Ammopiptanthus nanus, mitigates the negative effects of drought stress in plants. Front. Plant Sci. 2021, 12, 3067. [Google Scholar] [CrossRef]
- Meng, Y.C.; Zhang, H.F.; Pan, X.X.; Chen, N.; Hu, H.F.; Haq, S.U.; Khan, A.; Chen, R.G. CaDHN3, a pepper (Capsicum annuum L.) dehydrin gene enhances the tolerance against salt and drought stresses by reducing ROS accumulation. Int. J. Mol. Sci. 2021, 22, 3205. [Google Scholar] [CrossRef] [PubMed]
- Decena, M.A.; Gálvez-Rojas, S.; Agostini, F.; Sancho, R.; Contreras-Moreira, B.; Des Marais, D.L.; Hernandez, P.; Catalán, P. Comparative genomics, evolution, and drought-induced expression of dehydrin genes in model Brachypodium grasses. Plants 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.S.; Robbins, M.D.; Thorsted, K.; Robins, J.G.; Warnke, S.E.; Martin, R.; Harris-Shultz, K. Transcript responses to drought in Kentucky bluegrass (Poa pratensis L.) germplasm varying in their tolerance to drought stress. Environ. Exp. Bot. 2021, 190, 104571. [Google Scholar] [CrossRef]
| Target Gene | Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Tm (°C) |
|---|---|---|---|---|
| SK2 | GU443960.1 | TGGAACAGGAGTAACAACAGGTGGA | TGCCAGTTGAGAAAGTTGAGGTTGT | 58 |
| Y2K | JF748410.1 | AGCCACGCAACAAGGTTCTAA | TTGAGGATACGGGATGGGTG | 60 |
| Y2SK | GU443965.1 | GTGCGATGGAGATGCTGTTTG | CCTAATCCAACTTCAGGTTCAGC | 60 |
| DREB2 | EU846194.1 | CAAGAACAAGATGATGATGGTGAAC | AAGAAGAAGAATTGGAGGAGTCATG | 58 |
| DREB3 | EU846196.1 | GCTCAATAGGACTCAACCAACTCAC | TGACGTTGTCTAACTCCACGGTAA | 58 |
| DREB4 | EU846198.1 | CTTGGTTGTGGAGATAATGGAGC | AAGTTGCAATCTGAATTCTGAGGAC | 58 |
| DREB5 | EU846200.1 | GCGATAGGTTCAAAGAAAGGGTG | AGAGCAGCATCTTGAGCAGTAGG | 58 |
| β-Actin | JF968419 | TTACAATGAATTGCGTGTTG | AGAGGACAGCCTGAATGG | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Zhao, Y.; Cheng, B.; Tan, M.; Zhang, Y.; Li, Z. Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress. Plants 2022, 11, 2015. https://doi.org/10.3390/plants11152015
Ling Y, Zhao Y, Cheng B, Tan M, Zhang Y, Li Z. Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress. Plants. 2022; 11(15):2015. https://doi.org/10.3390/plants11152015
Chicago/Turabian StyleLing, Yao, Yue Zhao, Bizhen Cheng, Meng Tan, Yan Zhang, and Zhou Li. 2022. "Seed Priming with Chitosan Improves Germination Characteristics Associated with Alterations in Antioxidant Defense and Dehydration-Responsive Pathway in White Clover under Water Stress" Plants 11, no. 15: 2015. https://doi.org/10.3390/plants11152015






