Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame
Abstract
:1. Introduction
2. Results
2.1. Quantitative Analysis of Melatonin
2.2. Comparison of Melatonin Contents in Different Oilseeds and Common Plant-Derived Foods
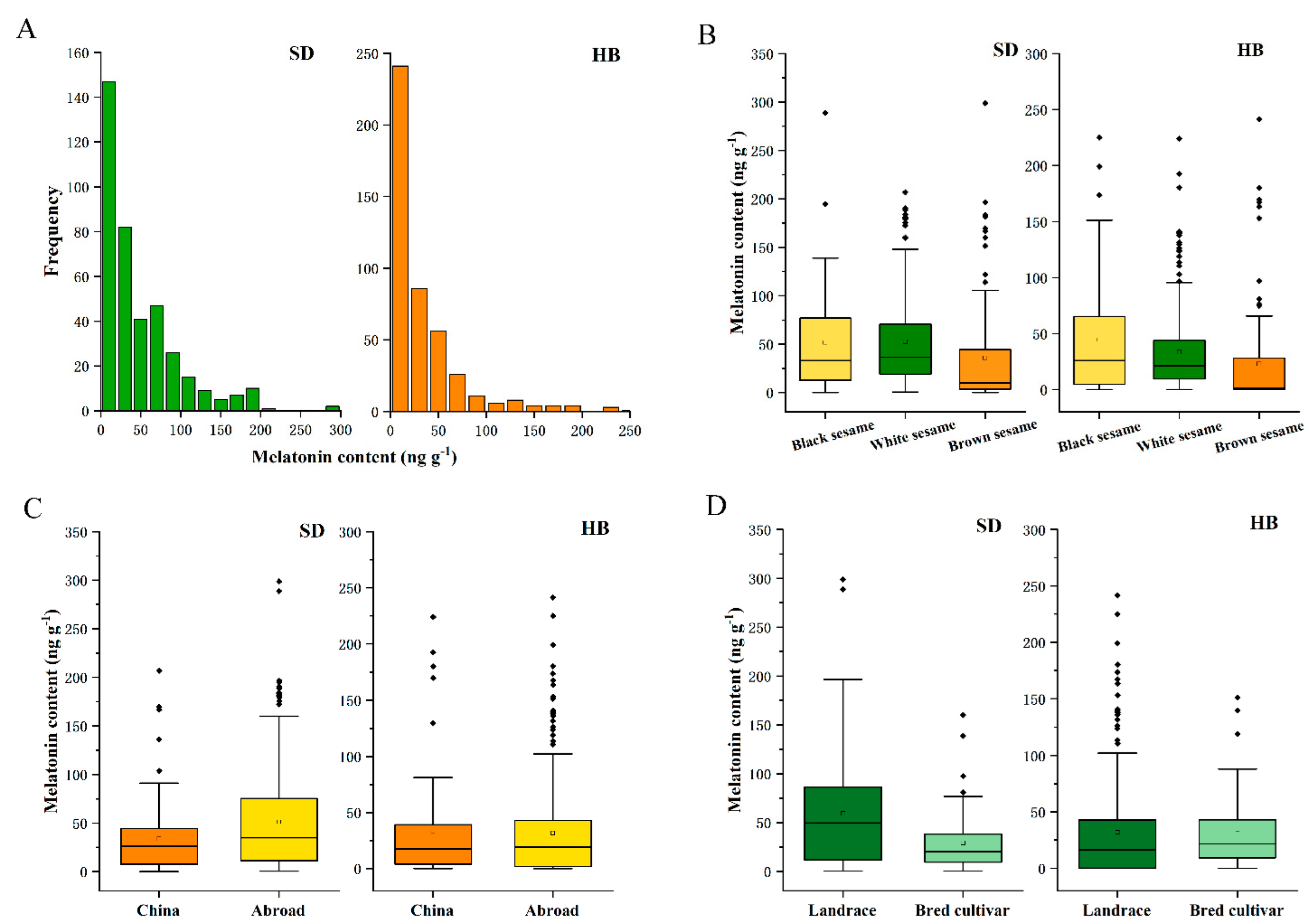
2.3. Distribution of Melatonin Content in Sesame Germplasm Resources
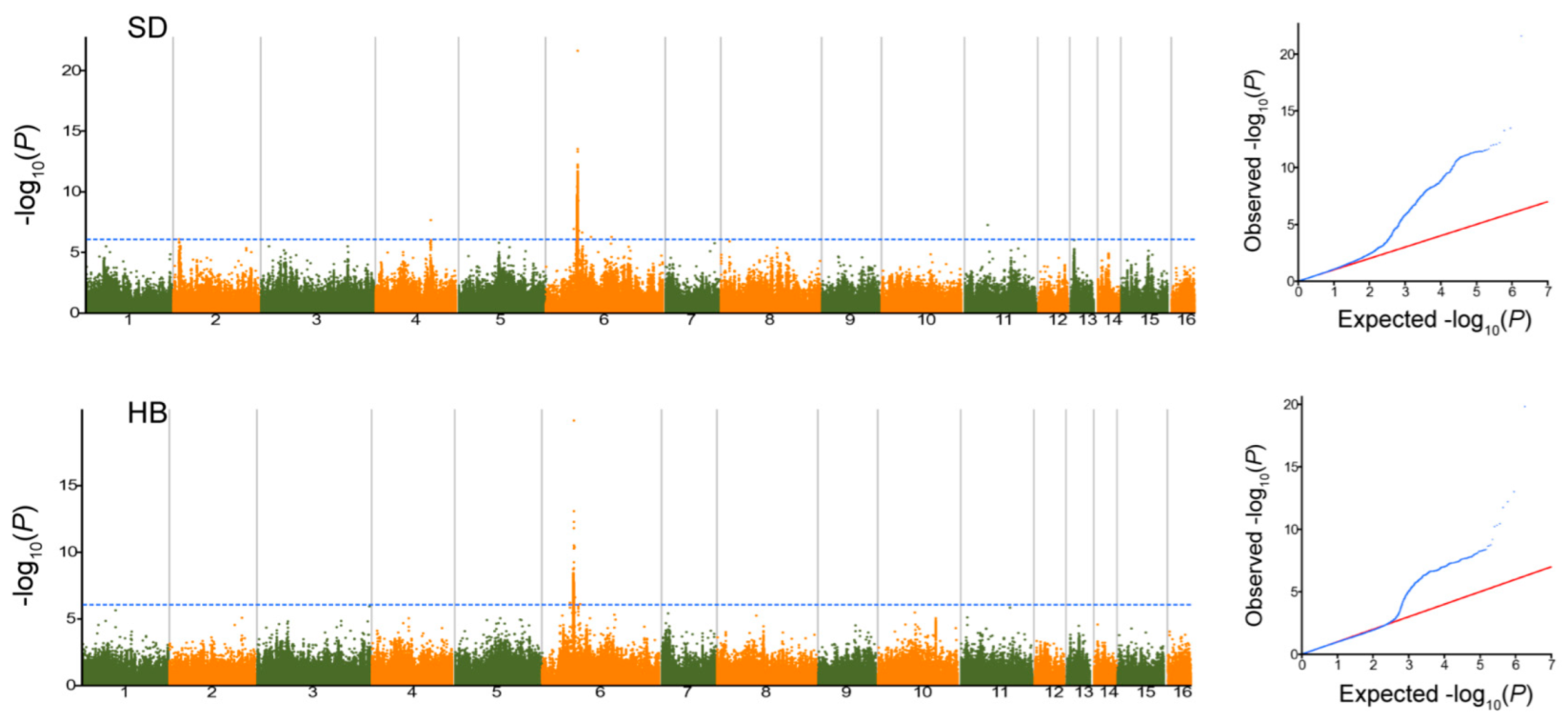
2.4. GWAS Study for Melatonin in Sesame
2.5. Genes Underlying Melatonin Content in Sesame
3. Discussions
4. Material and Methods
4.1. Chemicals and Standards
4.2. Collection of Samples
4.3. Sample Extraction and Preparation
4.4. LC-MS/MS Analysis
4.5. Population Genetics Analysis and GWAS Study
4.6. Candidate Gene Mining
4.7. Plasmid Construction and Hairy Root Transformation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Wang, X.B.; Ni, L.; Di, X.; Ma, B.T.; Niu, S.; Liu, C.W.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Shchetinin, E.; Baturin, V.; Arushanyan, E.; Bolatchiev, A.; Bobryshev, D. Potential and Possible Therapeutic Effects of Melatonin on SARS-CoV-2 Infection. Antioxidants 2022, 11, 140. [Google Scholar] [CrossRef]
- Gooley, J.J.; Rajaratnam, S.M.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, H.J.; Bae, M.K.; Kim, Y.D. Suppression of Osteoclastogenesis by Melatonin: A Melatonin Receptor-Independent Action. Int. J. Mol. Sci. 2017, 18, 1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arribas, R.L.; Romero, A.; Egea, J.; de Los Rios, C. Modulation of serine/threonine phosphatases by melatonin: Therapeutic approaches in neurodegenerative diseases. Br. J. Pharmacol. 2018, 175, 3220–3229. [Google Scholar] [CrossRef] [Green Version]
- McMullan, C.J.; Schernhammer, E.S.; Rimm, E.B.; Hu, F.B.; Forman, J.P. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013, 309, 1388–1396. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Ma, T.; Deng, Z.; Gutierrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gomez, D.; Lozano, M.; Fernandez-Leon, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodriguez, A.B. Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Afzali, D.; Azadmehr, F.; Torkzadeh, M. Vortex-assisted dispersive liquid-liquid microextraction trace amounts of melatonin prior to HPLC determination in fruit juice samples. Sep. Sci. Technol. 2016, 51, 1509–1514. [Google Scholar] [CrossRef]
- Niu, J.H.; Zhang, X.T.; Qin, P.G.; Yang, Y.X.; Tian, S.F.; Yang, H.; Lu, M.H. Simultaneous Determination of Melatonin, l-Tryptophan, and two l-Tryptophan-Derived Esters in Food by HPLC with Graphene Oxide/SiO2 Nanocomposite as the Adsorbent. Food Anal. Methods 2018, 11, 2438–2446. [Google Scholar] [CrossRef]
- Bedigian, D.; Harlan, J.R. Evidence for cultivation of sesame in the ancient world. Econ. Bot. 1986, 40, 137–154. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization Statistical Databases (FAOSTAT) FAOSTAT Provides Free Access to Food and Agriculture Data for over 245 Countries and Territories and Covers All FAO Regional Groupings; FAO: Rome, Italy, 2019. [Google Scholar]
- Choi, G.-H.; Lee, H.Y.; Back, K. Chloroplast overexpression of rice caffeic acid O-methyltransferase increases melatonin production in chloroplasts via the 5-methoxytryptamine pathway in transgenic rice plants. J. Pineal Res. 2017, 63, e12412. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Metabolite-based genome-wide association studies in plants. Curr. Opin. Plant Biol. 2015, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, J.N.; Daly, M.J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 2005, 6, 95–108. [Google Scholar] [CrossRef]
- Wang, W.Y.S.; Barratt, B.J.; Clayton, D.G.; Todd, J.A. Genome-wide association studies: Theoretical and practical concerns. Nat. Rev. Genet. 2005, 6, 109–118. [Google Scholar] [CrossRef]
- Wu, D.; Li, D.; Zhao, X.; Zhan, Y.; Teng, W.; Qiu, L.; Zheng, H.; Li, W.; Han, Y. Identification of a candidate gene associated with isoflavone content in soybean seeds using genome-wide association and linkage mapping. Plant J. 2020, 104, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Gao, Y.; Wei, X.; Wang, L.; Li, D.; Zhou, R.; You, J.; Zhang, X.; Yu, J.; Zhang, Y. Design and establishment of information database of sesame (Sesamum indicum L.) germplasm resources. Chin. J. Oil Crop Sci. 2018, 40, 57–63. [Google Scholar]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W.B. High levels of melatonin in the seeds of edible plants—Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in montmorency and balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high-performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- De la Puerta, C.; Carrascosa-Salmoral, M.P.; Garcia-Luna, P.P.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. Melatonin is a phytochemical in olive oil. Food Chem. 2007, 104, 609–612. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtanikaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Johns, N.P.; Johns, J.; Porasuphatana, S.; Plaimee, P.; Sae-Teaw, M. Dietary Intake of Melatonin from Tropical Fruit Altered Urinary Excretion of 6-Sulfatoxymelatonin in Healthy Volunteers. J. Agric. Food Chem. 2013, 61, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin content in grape: Myth or panacea? J. Sci. Food Agric. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Brunetti, C.; George, R.M.; Tattini, M.; Field, K.; Davey, M.P. Metabolomics in plant environmental physiology. J. Exp. Bot. 2013, 64, 4011–4020. [Google Scholar] [CrossRef] [Green Version]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Wan, L.; Lei, Y.; Yan, L.; Liu, Y.; Pandey, M.K.; Wan, X.; Varshney, R.K.; Fang, J.; Liao, B. Transcriptome and metabolome reveal redirection of flavonoids in a white testa peanut mutant. BMC Plant Biol. 2020, 20, 161. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, 12. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.X.; Liu, G.Y.; Bai, Y.J.; Xia, F.Y.; He, C.Z.; Shi, H.T. Two transcriptional activators of N-acetylserotonin O-methyltransferase 2 and melatonin biosynthesis in cassava. J. Exp. Bot. 2017, 68, 4997–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.X.; Chang, Y.L.; Zeng, H.Q.; Liu, G.Y.; He, C.Z.; Shi, H.T. RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J. Pineal Res. 2018, 64, 10. [Google Scholar] [CrossRef]
- Li, D.H.; Liu, P.; Yu, J.Y.; Wang, L.H.; Dossa, K.; Zhang, Y.X.; Zhou, R.; Wei, X.; Zhang, X.R. Genome-wide analysis of WRKY gene family in the sesame genome and identification of the WRKY genes involved in responses to abiotic stresses. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef]
- Nanjareddy, K.; Zepeda-Jazo, I.; Arthikala, M.K. A protocol for the generation of Arachis hypogaea composite plants: A valuable tool for the functional study of mycorrhizal symbiosis. Appl. Plant Sci. 2022, 10, e11454. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Hkkinen, S.T.; Lemasson, C.; Guillet, M.; Cardon, F. Hairy Root Cultures—A Versatile Tool With Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Xu, S.; Lai, E.; Zhao, L.; Cai, Y.; Ogutu, C.; Cherono, S.; Han, Y.; Zheng, B. Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci. Rep. 2020, 10, 2836. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, X.; Cao, L.; Ji, J.; Liu, T.; Duan, K. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean. Plant Methods 2021, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, T. Biosynthetic Origin of 2,3-Epoxysesamone in a Sesamum indicum Hairy Root Culture. Biosci. Biotechnol. Biochem. 2009, 73, 2535–2537. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Leal, A.; Alvarez-Fernandez, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Quality control and determination of melatonin in food supplements. J. Food Compos. Anal. 2016, 45, 80–86. [Google Scholar] [CrossRef]
- Williamson, B.L.; Johnson, K.L.; Tomlinson, A.J.; Gleich, G.J.; Naylor, S. On-line HPLC tandem mass spectrometry structural characterization of case-associated contaminants of L-tryptophan implicated with the onset of eosinophilia myalgia syndrome. Toxicol. Lett. 1998, 99, 139–150. [Google Scholar] [CrossRef]
- Williamson, B.L.; Tomlinson, A.J.; Mishra, P.K.; Gleich, G.J.; Naylor, S. Structural characterization of contaminants found in commercial preparations of melatonin: Similarities to case-related compounds from L-tryptophan associated with eosinophilia-myalgia syndrome. Chem. Res. Toxicol. 1998, 11, 234–240. [Google Scholar] [CrossRef]
- Wen, W.; Li, D.; Li, X.; Gao, Y.; Li, W.; Li, H.; Liu, J.; Liu, H.; Chen, W.; Luo, J.; et al. Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat. Commun. 2014, 5, 3438. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.M.; Sul, J.H.; Service, S.K.; Zaitlen, N.A.; Kong, S.Y.; Freimer, N.B.; Sabatti, C.; Eskin, E. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010, 42, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Dossa, K.; You, J.; Wang, L.; Zhang, Y.; Li, D.; Zhou, R.; Yu, J.; Wei, X.; Zhu, X.; Jiang, S.; et al. Transcriptomic profiling of sesame during waterlogging and recovery. Sci. Data 2019, 6, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.H.; Dossa, K.; Zhang, Y.X.; Wei, X.; Wang, L.H.; Zhang, Y.J.; Liu, A.L.; Zhou, R.; Zhang, X.R. GWAS Uncovers Differential Genetic Bases for Drought and Salt Tolerances in Sesame at the Germination Stage. Genes 2018, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.H.; Dossou, S.S.K.; Wei, X.; Zhang, Y.X.; Li, D.H.; Yu, J.Y.; Zhang, X.R. Transcriptome Dynamics during Black and White Sesame (Sesamum indicum L.) Seed Development and Identification of Candidate Genes Associated with Black Pigmentation. Genes 2020, 11, 1399. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.A.; Lee, J.W.; Yi, Y.B.; Park, G.Y.; Chung, C.H. Induction of hairy roots and characterization of peroxidase expression as a potential root growth marker in sesame. Prep. Biochem. Biotechnol. 2009, 39, 345–359. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wang, Y.Y.; Zhang, Y.J.; Dossa, K.; Li, D.H.; Zhou, R.; Wang, L.H.; Zhang, X.R. Genome-wide identification and expression analyses of genes involved in raffinose accumulation in sesame. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, M.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Silva, C.; Vacondio, F.; Rivara, M.; Plazzi, P.V.; Franceschinit, D.; et al. Antioxidant and Cytoprotective Activity of Indole Derivatives Related to Melatonin. In Developments in Tryptophan and Serotonin Metabolism; Advances in Experimental Medicine and Biology; Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H., Varesio, L., Eds.; Springer: Boston, MA, USA, 2003; Volume 527. [Google Scholar] [CrossRef]
| Common Name | Scientific Name | Melatonin | Region | Method | Ref. |
|---|---|---|---|---|---|
| Sesame | Sesamum indicum L. | 0.41-25.18 ng g−1 | China | LC-MS/MS | This study |
| Rapeseed | Brassica campestris L. | NA | China | LC-MS/MS | This study |
| Soybean | Glycine max (Linn.) Merr. | NA | China | LC-MS/MS | This study |
| Walnut | Juglans regia L. | 3.60 ng g−1 | China | LC-MS/MS | This study |
| Camellia seed | Theaceae Camellia L. | NA | China | LC-MS/MS | This study |
| Corn | Zea mays L. | 1.52 ng g−1 | China | LC-MS/MS | This study |
| Wheat | Triticum aestivum L. | NA | China | LC-MS/MS | This study |
| Rice | Oryza sativa | 1.00 ng g−1 | China | LC-MS/MS | This study |
| Sunflower seed | Helianthus annuus L. | 29 ng g−1 | America | HPLC-ECD | [23] |
| Flaxseed | Linum usitatissimum L. | 12 ng g−1 | America | HPLC-ECD | [24] |
| Poppy seed | Popaver somniferum L. | 6 ng g−1 | America | HPLC-ECD | [24] |
| Barley | Hordeum vulgare L. | 0.87 ng g−1 | Egypt | GC-MS | [25] |
| Corn | Zea mays L. | 1.88 ng g−1 | Egypt | GC-MS | [25] |
| Extra virgin olive oil | - | 0.071–0.119 ng mL;−1 | Spain | ELISA | [26] |
| Banana | Musa sapientum L. | 0.47 ng g−1 | Germany | RIA and GC-MS | [25] |
| Apple | Malus domestica | 0.05 ng g−1 | Japan | Radioimmunoassay | [27] |
| Strawberry | Fragaria × ananassa Duch | 0.14 ng g−1 | Egypt | GC-MS | [28] |
| Barley | Hordeum vulgare L. | 0.87 ng g−1 | Egypt | GC-MS | [25] |
| Orange | Citrus reticulata Blanco | 0.15 ng g−1 | Thailand | HPLC-FD & ELISA | [29] |
| Pineapple | Ananus comosus M. | 0.28 ng g−1 | Egypt | GC-MS | [25] |
| Montmorency tart cherry | Prunus cerasus L. | 13.46 ng g−1 | Germany | HPLC-ECD | [24] |
| Barbera grape (skin) | Vitis vinifera L. | 0.63 ng g−1 | Italy | HPLC-FD and ELISA | [30] |
| Mango | Mangifera indica L. | 0.70 ng g−1 | Thailand | HPLC-FD & ELISA | [29] |
| Papaya | Carica papyya L. | 0.24 ng g−1 | Thailand | HPLC-FD & ELISA | [29] |
| Cucumber | Cucumis sativus L. | 0.02 ng g−1 | Japan | Radioimmunoassay | [27] |
| Asparagus | Asparagus officinalis L. | 0.01 ng g−1 | Japan | Radioimmunoassay | [27] |
| Welsh onion | Allium fistulosum L. | 0.09 ng g−1 | Japan | Radioimmunoassay | [27] |
| Tomato | Solanum lycopersicum L. | 0.30 ng g−1 | Egypt | GC-MS | [25] |
| Ginger | Zingiber officinale R. | 1.42 ng g−1 | Egypt | GC-MS | [25] |
| Red wine | — | 0.05–0.62 ng mL−1 | Italy | UPLC-HRMS | [31] |
| White wine | — | 0.18 ng mL−1 | Italy | UPLC-HRMS | [31] |
| Samples | Content of Melatonin (ngg−1 FW) | Range of Values (ng g−1 FW) |
|---|---|---|
| VC | ND | - |
| SiWRKY67-OE#1 | 0.329 ± 0.032 | 0.293–0.352 |
| SiWRKY67-OE#2 | 0.406 ± 0.042 | 0.369–0.452 |
| SiWRKY67-OE#3 | 0.350 ± 0.024 | 0.325–0.373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; You, J.; Liu, A.; Qi, X.; Li, D.; Zhao, Y.; Zhang, Y.; Zhang, L.; Zhang, X.; Li, P. Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame. Plants 2022, 11, 2005. https://doi.org/10.3390/plants11152005
Wang X, You J, Liu A, Qi X, Li D, Zhao Y, Zhang Y, Zhang L, Zhang X, Li P. Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame. Plants. 2022; 11(15):2005. https://doi.org/10.3390/plants11152005
Chicago/Turabian StyleWang, Xiao, Jun You, Aili Liu, Xin Qi, Donghua Li, Ya Zhao, Yanxin Zhang, Liangxiao Zhang, Xiurong Zhang, and Peiwu Li. 2022. "Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame" Plants 11, no. 15: 2005. https://doi.org/10.3390/plants11152005









