Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives
Abstract
:1. Epidemiology and Pathology of Colletotrichum spp.
1.1. Interaction between Colletotrichum spp. and Their Hosts
1.2. Antracnhose Disease: A Challenge for the Agri-Food Sector
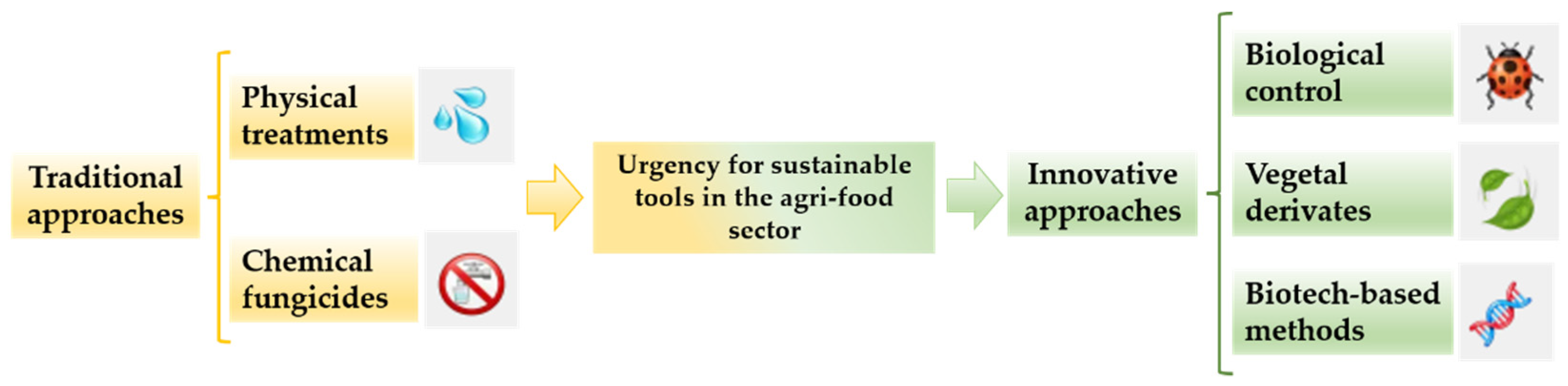
2. Traditional Approaches
3. Innovative and Sustainable Approaches
3.1. Biological Strategies
3.1.1. Biological Control
3.1.2. Plant Derivates
3.2. Biotechnology-Based Strategies
3.2.1. Bidirectional Cross-Kingdom RNA Interference
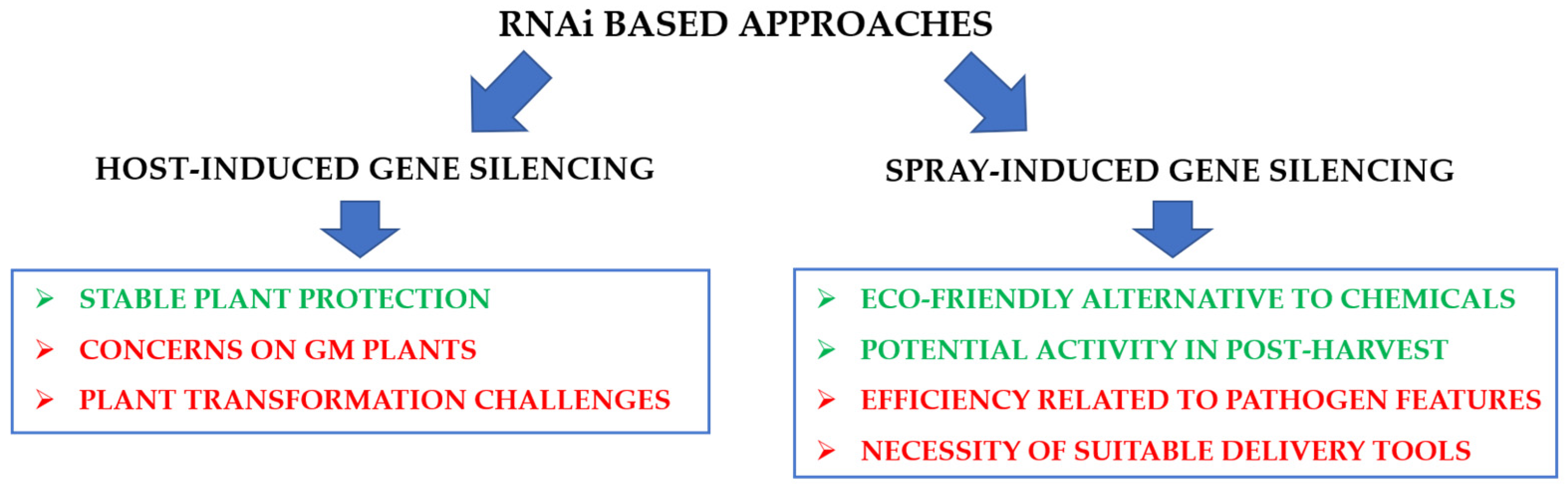
3.2.2. Host-Induced Gene Silencing Approaches
3.2.3. Spray-Induced Gene Silencing Approaches
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Bordoh, P.K.; Ali, A.; Dickinson, M.; Siddiqui, Y.; Romanazzi, G. A review on the management of postharvest anthracnose in dragon fruits caused by Colletotrichum spp. Crop Prot. 2020, 130, 105067. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose in various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, A.P.; Satapathy, R.R.; Rout, M. Management of Colletotrichum gloeosporioides causing papaya anthracnose with best resulting plant extract and bio-agents. Pharma Innov. J. 2021, 10, 629–630. [Google Scholar]
- Banya, M.; Garg, S.; Lal Meena, N. A review: Chilli anthracnose, its spread and management. J. Pharmacogn. Phytochem. 2020, 9, 1432–1438. [Google Scholar]
- Wharton, P.S.; Diéguez-Uribeondo, J. The biology of Colletotrichum acutatum. An. Jard. Bot. Madr. 2004, 61, 3–22. [Google Scholar] [CrossRef]
- Jeffries, P.; Dodd, J.C.; Jeger, M.J.; Plumbey, R.A. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol. 1990, 39, 343–366. [Google Scholar] [CrossRef]
- Prusky, D.; Kobiler, I.; Ardi, R.; Beno-Moalem, D.; Yakoby, N.; Keen, N.T. Resistance mechanisms of subtropical fruits to Colletotrichum gloeosporioides. In Colletotrichum: Host Specificity, Pathology, and Host-Pathogen Interaction; Prusky, D., Freeman, S., Dickman, M.B., Eds.; The American Phytopathological Society Press: St. Paul, MN, USA, 2000; pp. 232–244. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Filho, J.G.; da Cruz Silva, G.; Cipriano, L.; Gomes, M.; Buranelo Egea, M. Control of postharvest fungal diseases in fruits using external application of RNAi. J. Food Sci. 2021, 86, 3341–3348. [Google Scholar] [CrossRef]
- Van der Bruggen, P.; Maraite, H. Histopathology of cassava anthracnose disease caused by Colletotrichum gloeosporioides f. sp. manihotis. Parasitica 1987, 43, 3–21. [Google Scholar]
- Zulfiqar, M.; Brlansky, R.H.; Timmer, L.W. Infection of flower and vegetative tissues of citrus by Colletotrichum acutatum and C. gloeosporioides. Mycologia 1996, 88, 121–128. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O.; O’Connell, R.J.; Nash, C.; Lucas, J.A. Stomatal penetration of cowpea (Vigna unguiculata) leaves by a Colletotrichum species causing latent anthracnose. Plant Pathol. 1999, 48, 777–784. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, R.R. Postharvest disinfection of fruits and vegetables and their management. In Postharvest Disinfection of Fruits and Vegetables, 1st ed.; Siddiqui, M.W., Ed.; Elsevier: Oxford, UK; pp. 1–52.
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correl, J.C. Lifestyle of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.A.; O’Connell, R.J.; Pring, R.J.; Nash, C. Infection strategies of Colletotrichum species. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 88–120. [Google Scholar]
- Skipp, R.A.; Beever, R.E.; Sharrock, K.R.; Rikkerink, E.H.A.; Templeton, M.D. Histopathological, genetic, Biochemical and molecular basis in Colletotrichum. In Pathogens and Host Parasite Specificity in Plant Disease; Singh, U.S., Kohmoto, K., Singh, R.P., Eds.; Elsevier Science Ltd.: Oxford, UK, 1995; pp. 119–143. [Google Scholar]
- O’Connell, R.J.; Bailey, J.A.; Richmond, D.V. Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum. Physiol. Mol. Plant Pathol. 1985, 27, 75–98. [Google Scholar] [CrossRef]
- Latunde-Dada, A.O.; O’Connell, R.J.; Nash, C.; Pring, R.J.; Lucas, J.A.; Bailey, J.A. Infection process and identity of the hemibiotrophic anthracnose fungus (Colletotrichum destructivum) from cowpea (Vigna unguiculata). Mycol. Res. 1996, 100, 1133–1141. [Google Scholar] [CrossRef]
- Wharton, P.S.; Julian, A.M. A cytological study of compatible and incompatible interactions between Sorghum bicolor and Colletotrichum sublineolum. N. Phytol. 1996, 134, 25–34. [Google Scholar] [CrossRef]
- Wharton, P.S.; Julian, A.M.; O’Connell, R.J. Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology 2001, 91, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Arauz, L.F. Mango anthracnose: Economic impact and current option for integrated management. Plant Dis. 2000, 84, 600–611. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Prusky, D.; Barad, S.; Ment, D.; Bi, F. The pH modulation by fungal secreted molecules: A mechanism affecting pathogenicity by postharvest pathogens. Isr. J. Plant Sci. 2016, 63, 22–30. [Google Scholar] [CrossRef]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. N. Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef]
- Md Nor, S.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Roberts, R.G.; Snow, J.P. Histopathology of cotton boll rot caused by Colletotrichum capsici. Phytopathology 1984, 74, 390–397. [Google Scholar] [CrossRef]
- Pring, R.J.; Nash, C.; Zakaria, M.; Bailey, J.A. Infection process and host range of Colletotrichum capsici. Physiol. Mol. Plant Pathol. 1995, 46, 137–152. [Google Scholar] [CrossRef]
- Smith, B.J. Epidemiology and pathology of strawberry anthracnose: A North American perspective. Hortscience 2008, 43, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Higuera, J.J.; Garrido-Gala, J.; Lekhbou, A.; Arjona-Girona, I.; Amil-Ruiz, F.; Mercado, J.A.; Pliego-Alfaro, F.; Muñoz-Blanco, J.; López-Herrera, C.J.; Caballero, J.L. The strawberry FaWRKY1 transcription factor negatively regulates resistance to Colletotrichum acutatum in fruit upon infection. Front. Plant Sci. 2019, 10, 480. [Google Scholar] [CrossRef]
- Sreenivasaprasad, S.; Talhinhas, P. Genotypic and phenotypic diversity in Colletotrichum acutatum, a cosmopolitan pathogen causing anthracnose on a wide range of hosts. Mol. Plant Pathol. 2005, 6, 361–378. [Google Scholar] [CrossRef]
- Marian, M.; Ohno, T.; Suzuki, H.; Kitamura, H.; Kuroda, K.; Shimizu, M. A novel strain of endophytic Streptomyces for the biocontrol of strawberry anthracnose caused by Glomerella cingulata. Microbiol. Res. 2020, 234, 126428. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Appl. Environ. Microbiol. 1996, 62, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Raghuwanshi, R.; Gupta, V.K.; Singh, H.B. Chilli anthracnose: The epidemiology and management. Front. Microbiol. 2016, 7, 1527. [Google Scholar] [CrossRef] [Green Version]
- Fischer, I.; Moraes, M.; Palharini, M.; Cruz, J.; Firmino, A. Ocorrência de antracnose em abacate, agressividade e sensibilidade de Colletotrichum gloeosporioides a fungicidas. Agropecu. Cient. Semiárido 2019, 13, 130–137. [Google Scholar]
- Lakshmi, B.K.M.; Reddy, P.N.; Prasad, R.D. Cross-infection potential of Colletotrichum gloeosporioides Penz. isolates causing anthracnose in subtropical fruit crops. Trop. Agric. Res. 2011, 22, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.; Ali, A.; Ramachandran, S.; Smith, D.R.; Alderson, P.G. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot. 2010, 29, 1136–1141. [Google Scholar] [CrossRef]
- Paull, R.E.; Nishijima, W.; Reyes, M.; Cavaletto, C. Postharvest handling and losses during marketing of papaya (Carica papaya). Postharvest Biol. Technol. 1997, 11, 165–179. [Google Scholar] [CrossRef]
- Darshan, K.; Vanitha, S.; Venugopala, K.M.; Parthasarathy, S. Strategic eco-friendly management of post-harvest fruit rot in papaya caused by Colletotrichum gloeosporioides. J. Biol. Control 2019, 33, 225–235. [Google Scholar] [CrossRef]
- Freeman, S.; Shabi, E. Cross-infection of subtropical and temperature fruits by Colletotrichum species from various hosts. Physiol. Mol. Plant Path. 1996, 49, 395–404. [Google Scholar] [CrossRef]
- Nelson, S.C. Mango anthracnose (Colletotrichum gloeosporioides). Plant Dis. 2008, PD-48. [Google Scholar]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef]
- Masyahit, M.; Kamaruzaman, S.; Yahya, A.; Ghazali, M. First report of the occurrence of the anthracnose disease caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. on dragon fruit (Hylocereus spp.) in Peninsular Malaysia. Am. J. Appl. Sci. 2009, 6, 902–912. [Google Scholar]
- Deng, L.; Zeng, K.; Zhou, Y.; Huang, Y. Effects of postharvest oligochitosan treatment on anthracnose disease in citrus (Citrus sinensis L. Osbeck) fruit. Eur. Food Res. Technol. 2015, 240, 795–804. [Google Scholar] [CrossRef]
- Martínez-Blay, V.; Pérez-Gago, M.B.; de la Fuente, B.; Carbó, R.; Palou, L. Edible coatings formulated with antifungal GRAS salts to control citrus anthracnose caused by Colletotrichum gloeosporioides and preserve postharvest fruit quality. Coatings 2020, 10, 730. [Google Scholar] [CrossRef]
- Lum, M.S.; Norazira, M.A. Effects of hot water, submergence time and storage duration on quality of dragon fruit (Hylocereus polyrhizus). J. Agric. Sci. 2011, 3, 146. [Google Scholar] [CrossRef] [Green Version]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M.; Hamzah, M.H.; Shukor, N.I.A. The causal agent of anthracnose in papaya fruit and control by three different Malaysian stingless bee honeys, and the chemical profile. Sci. Hortic. 2019, 257, 108590. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Scientia Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Sanders, G.M.; Korsten, L.; Wehner, F.C. Survey of fungicide sensitivity in Colletotrichum gloeosporioides from different avocado and mango production areas in South Africa. Eur. J. Plant Pathol. 2000, 106, 745–752. [Google Scholar] [CrossRef]
- Chung, W.H.; Ishii, H.; Nishimura, K.; Fukaya, M.; Yano, K.; Kajitani, Y. Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Dis. 2006, 90, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Inada, M.; Ishii, H.; Chung, W.H.; Yamada, T.; Yamaguchi, J.; Furuta, A. Occurrence of strobilurin-resistant strains of Colletotrichum gloeosporioides (Glomerella cingulata), the causal fungus of strawberry anthracnose. Jpn. J. Phytopathol. 2008, 74, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, M.; Ogawara, T.; Hashimoto, Y.; Miyamoto, T.; Kaneda, M.; Tomita, Y. Identification of Colletotrichum species causing strawberry anthracnose and distribution of fungal strains resistant to some fungicides in Ibaraki prefecture. Bull. Hortic. Inst. Ibaraki Agric. Cent. 2010, 17, 35–42. [Google Scholar]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messean, A. Conventional pesticides in agriculture: Benefits versus risks. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Wu, D.; Zhang, Q.; Chen, H.; Li, H.; Han, Q.; Lai, X.; Wang, H.; Wu, Y.; Yuan, J.; et al. Efficacy and mechanism of cinnamon essential oil on inhibition of Colletotrichum acutatum isolated from “Hongyang” kiwifruit. Front. Microbiol. 2018, 9, 1288. [Google Scholar] [CrossRef] [Green Version]
- Chechi, A.; Stahlecker, J.; Dowling, M.E.; Schnabel, G. Diversity in species composition and fungicide resistance profiles in Colletotrichum isolates from apples. Pestic. Biochem. Phys. 2019, 158, 18–24. [Google Scholar] [CrossRef]
- Kimaru, K.S.; Muchemi, K.P.; Mwangi, J.W. Effects of anthracnose disease on avocado production in Kenya. Cogent Food Agric. 2020, 6, 1799531. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Xu, X.; Zou, X.; Duan, K.; Gao, Q. Characterization and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in Eastern China. Plant Dis. 2020, 104, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Yokosawa, S.; Eguchi, N.; Kondo, K.I.; Sato, T. Phylogenetic relationship and fungicide sensitivity of members of the Colletotrichum gloeosporioides species complex from apple. J. Gen. Plant Pathol. 2017, 83, 291–298. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. Trends Analyt. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quiros-Alcala, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Ncama, K.; Mditshwa, A.; Tesfay, S.Z.; Mbili, N.C.; Magwaza, L.S. Topical procedures adopted in testing and application of plant-based extracts as bio-fungicides in controlling postharvest decay of fresh produce. Crop Prot. 2019, 115, 142–151. [Google Scholar] [CrossRef]
- Diskin, S.; Sharir, T.; Feygenberg, O.; Maurer, D.; Alkan, N. Fludioxonil—A potential alternative for postharvest disease control in mango fruit. Crop Prot. 2019, 124, 104855. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Bommuraj, V.; Chen, Y.; Sperling, R.; Barel, S.; Feygenberg, O.; Maurer, D.; Alkan, N. Postharvest fungicide for avocado fruits: Antifungal efficacy and peel to pulp distribution kinetics. Foods 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokare, P.; Fatima, S.; Jagdale, P.E. A review on the management practices of Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. causes anthracnose disease of mango. Int. J. Botany Stud. 2021, 6, 742–746. [Google Scholar]
- Fiankor, D.-D.D.; Curzi, D.; Olper, A. Trade, price and quality upgrading effects of agri-food standards Eur. Rev. Agric. Econ. 2021, 48, 835–877. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Droby, S.; Wisniewski, M.; Teixido, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mahunu, G.K.; Castoria, R.; Yang, Q.Y.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Shi, X.-C.; Wang, S.-Y.; Duan, X.C.; Wang, Y.-Z.; Liu, F.-Q.; Laborda, P. Biocontrol strategies for the management of Colletotrichum species in postharvest fruits. Crop Prot. 2021, 141, 105454. [Google Scholar] [CrossRef]
- Arroyave-Toro, J.J.; Mosquera, S.; Villegas-Escobar, V. Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol. Control 2017, 114, 195–200. [Google Scholar] [CrossRef]
- Lee, G.-W.; Ko, J.-A.; Oh, B.-T.; Choi, J.-R.; Lee, K.-J.; Chae, J.-C.; Kamala-Kannan, S. Biological control of postharvest diseases of apples, peaches and nectarines by Bacillus subtilis S16 isolated from halophytes rhizosphere. Biocontrol Sci. Technol. 2012, 22, 351–361. [Google Scholar] [CrossRef]
- Kim, Y.S.; Balaraju, K.; Jeon, Y. Biological control of apple anthracnose by Paenibacillus polymyxa APEC128, an antagonistic rhizobacterium. Plant Pathol. J. 2016, 32, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Boukaew, S.; Petlamul, W.; Bunkrongcheap, R.; Chookaew, T.; Kabbua, T.; Thippated, A.; Prasertsan, P. Fumigant activity of volatile compounds of Streptomyces philanthi RM-1-138 and pure chemicals (acetophenone and phenylethyl alcohol) against anthracnose pathogen in postharvest chili fruit. Crop Protect. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Sandani, H.B.P.; Ranathunge, N.P.; Lakshman, P.L.N.; Weerakoon, W.M.W. Biocontrol potential of five Burkholderia and Pseudomonas strains against Colletotrichum truncatum infecting chilli pepper. Biocontrol Sci. Technol. 2019, 29, 727–745. [Google Scholar] [CrossRef]
- Freeman, S.; Minz, D.; Kolesnik, I.; Barbul, O.; Zveibil, A.; Maymon, M.; Nitzani, Y.; Kirshner, B.; Rav-David, D.; Bilu, A.; et al. Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. Eur. J. Plant Pathol. 2004, 110, 361–370. [Google Scholar] [CrossRef]
- Oliveri, C.; Distefano, G.; La Malfa, S.; La Rosa, R.; Deng, Z.N.; Gentile, A. Lemon fruits from endochitinase transgenic plants exhibit resistance against postharvest fungal pathogens. Acta Hortic. 2015, 1065, 1639–1645. [Google Scholar] [CrossRef]
- Sangeetha, G.; Usharani, S.; Muthukumar, A. Biocontrol with Trichoderma species for the management of postharvest crown rot of banana. Phytopathol. Mediterr. 2009, 48, 214–225. [Google Scholar]
- Conway, W.S.; Leverentz, B.; Janisiewicz, W.J.; Saftner, R.A.; Camp, M.J. Improving biocontrol using antagonist mixtures with heat and/or sodium bicarbonate to control postharvest decay of apple fruit. Postharvest Biol. Technol. 2005, 36, 235–244. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Leverentz, B.; Conway, W.S.; Saftner, R.A.; Reed, A.N.; Camp, M.J. Control of bitter rot and blue mold of apples by integrating heat and antagonist treatments on 1-MCP treated fruit stored under controlled atmosphere conditions. Postharvest Biol. Technol. 2003, 29, 129–143. [Google Scholar] [CrossRef]
- Mewa-Ngongang, M.; du Plessis, H.W.; Ntwampe, S.K.O.; Chidi, B.S.; Hutchinson, U.F.; Mekuto, L.; Jolly, N.P. The use of Candida pyralidae and Pichia kluyveri to control spoilage microorganisms of raw fruits used for beverage production. Foods 2019, 8, 454. [Google Scholar] [CrossRef] [Green Version]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 90, 71–118. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Vera, M.; Jimenez, B.; Balderas, K.; Ortiz, M.; Allende, R.; Carrillo, A.; Galindo, E. Pilot-scale production and liquid formulation of Rhodotorula minuta, a potential biocontrol agent of mango anthracnose. J. Appl. Microbiol. 2005, 99, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.C.; Wu, H.Y.; Wang, Y.W.; Ariyawansa, H.A.; Hu, H.P.; Hung, T.H.; Tzean, S.S.; Chung, C.L. Diversity and pathogenecity of Colletotrichum species causing strawberry anthracnose in Taiwan and description of a new species, Colletotrichum miaoliense sp. nov. Sci. Rep. 2020, 10, 14664. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Yusuf, M.; Chowdhury, J.U.; Khan, S.; Anwar, M.N. Antifungal activity of forty higher plants against phytopathogenic fungi. Bangl. J. Microbiol. 2008, 24, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Nduagu, C.; Ekefan, E.J.; Nwankiti, A.O. Effect of some crude plant extracts on growth of Colletotrichum capsici (Synd) & Bisby, causal agent of pepper anthracnose. J. Appl. Biosci. 2008, 6, 184–190. [Google Scholar]
- Johnny, L.; Yusuf, U.; Nulit, R. Antifungal activity of selected plant leaves crude extracts against a pepper anthracnose fungus, Colletotrichum capsici (Sydow) butler and bisby (Ascomycota: Phyllachorales). Afr. J. Biotechnol. 2011, 10, 4157–4165. [Google Scholar]
- Saravanakumar, P.; Karthikeyan, V.; Patharajan, S.; Kannabiran, B. Antifungal activity of Plumbago species against anthracnose fungus Colletotrichum gloeosporioides (Penz.) of chilli. Arch. Phytopathol. Plant Prot. 2011, 44, 287–297. [Google Scholar] [CrossRef]
- Bordoh, P.K.; Ali, A.; Dickinson, M.; Siddiqui, Y. Antimicrobial effect of rhizome and medicinal herb extract in controlling postharvest anthracnose of dragon fruit and their possible phytotoxicity. Sci. Hortic. 2020, 265, 109249. [Google Scholar] [CrossRef]
- López-Velázquez, J.G.; Delgado-Vargas, F.; Ayón-Reyna, L.E.; López-Angulo, G.; Bautista-Baños, S.; Uriarte-Gastelum, Y.G.; López-López, M.E.; Vega-García, M.O. Postharvest application of partitioned plant extracts from Sinaloa, Mexico for controlling papaya pathogenic fungus Colletotrichum gloeosporioides. J. Plant Pathol. 2021, 103, 831–842. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Freiesleben, S.; Jager, A.K. Correlation between plant secondary metabolites and their antifungal mechanisms—A review. J. Med. Aromat. Plants 2014, 3, 2167-0412. [Google Scholar]
- Gutierrez-del-Rio, I.; Fernandez, J.; Lombo, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gonҫalves, D.C.; Rodrigues Ribeiro, W.; Gonҫalves, D.C.; Menini, L.; Costa, H. Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Res. Int. 2021, 150, 110758. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Junior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Ali, A.; Wee Pheng, T.; Mustafa, M.A. Application of lemongrass oil in vapour phase for the effective control of anthracnose of “Sekaki” papaya. J. Appl. Microbiol. 2015, 118, 1456–1464. [Google Scholar] [CrossRef]
- Ali, A.; Hei, G.K.; Keat, Y.W. Efficacy of ginger oil and extract combined with gum arabic on anthracnose and quality of papaya fruit during cold storage. J. Food Sci. Technol. 2016, 53, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hort. 2017, 34, 18–26. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Vargas, A.I.; Schaffer, B.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Postharvest management of anthracnose in avocado (Persea americana Mill.) fruit with plant extracted oils. Food Packag. 2017, 12, 16–22. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Antifungal activity of five different essential oils in vapour phase for the control of Colletotrichum gloeosporioides and Lasiodiplodia theobromae in vitro and on mango. Inter. J. Food Sci. Technol. 2016, 51, 411–418. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J. Essent. Oil Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Madjouko, M.A.; Tchameni, S.N.; Tchinda, E.S.; Dongmo Jazet, P.M.; Kamsu, P.N.; Medzue Souop Kamga, V.A.; Sameza, M.S.; Tchoumbougnang, F.; Menut, C. Inhibitory effects of essential oils from Ocimum basilicum and Ocimum gratissimum on Colletotrichum musae: The causal agent of bananas anthracnose. J. Phytopath. 2019, 167, 257–264. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Jiménez, M.; Jiménez, A.; Atarés, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987. [Google Scholar] [CrossRef]
- Scremin, F.R.; Veiga, R.S.; Silva-Buzanello, R.A.; Becker-Algeri, T.A.; Corso, M.P.; Torquato, A.S.; Bittencourt, P.R.S.; Flores, E.L.M.; Canan, C. Synthesis and characterization of protein microcapsules for eugenol storage. J. Therm. Anal. Calorim. 2016, 131, 653–660. [Google Scholar] [CrossRef]
- Shin, J.; Na, K.; Shin, S.; Seo, S.-M.; Youn, H.J.; Park, I.-K.; Hyun, J. Biological activity of thyme white essential oil stabilized by cellulose nanocrystals. Biomolecules 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT 2019, 113, 108290. [Google Scholar] [CrossRef]
- Weisany, W.; Samadi, S.; Amini, J.; Hossaini, S.; Yousefi, S.; Maggi, F. Enhancement of the antifungal activity of thyme and dill essential oils against Colletotrichum nymphaeae by nano-encapsulation with copper NPs. Ind. Crops Prod. 2019, 132, 213–225. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Ambika, S.; Sujatha, K. Antifungal activity of aqueous and ethanol extracts of seaweeds against sugarcane red rot pathogen (Colletotrichum falcatum). Sci. Res. Essays 2015, 10, 232–235. [Google Scholar]
- Melo, V.M.M.; Medeiros, D.A.; Rios, F.J.B.; Castelar, L.I.M.; de, F.F.U. Carvalho, A. Antifungal Properties of Proteins (Agglutinins) from the Red Alga Hypnea musciformis (Wulfen) Lamouroux. Bot. Mar. 1997, 40, 281–284. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Stadnik, M.J. Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiol. Mol. Plant Pathol. 2012, 78, 8–13. [Google Scholar] [CrossRef]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Smania, A.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerré-Tugaye, M.-T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, E.J.; Hong, J.K.; Jeun, Y.C. Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. Plant Pathol. J. 2018, 34, 113–120. [Google Scholar] [CrossRef]
- Aguado, A.; Pastrana, A.M.; de los Santos, B.; Romero, F.; Sánchez, M.C.; Capotea, N. Efficiency of natural products in the control of Colletotrichum acutatum monitored by real-time PCR. Acta Hortic. 2014, 1049, 329–334. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Borrelli, V.M.G.; Brambilla, V.; Rogowsky, P.; Marocco, A.; Lanubile, A. The enhancement of plant disease resistance using CRISPR/Cas9 technology. Front. Plant Sci. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Mishra, R.; Mohanty, J.N.; Mahanty, B.; Joshi, R.K. A single transcript CRISPR/Cas9 mediated mutagenesis of CaERF28 confers anthracnose resistance in chilli pepper (Capsicum annuum L.). Planta 2021, 254, 5. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Joshi, R.K.; Zhao, K. Genome editing in rice: Recent advances, challenges, and future implications. Front. Plant Sci. 2018, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Mohanty, J.N.; Chand, S.K.; Joshi, R.K. Can-miRn37a mediated suppression of ethylene response factors enhances the resistance of chilli against anthracnose pathogen Colletotrichum truncatum L. Plant Sci. 2018, 267, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Ketting, R.F. The many faces of RNAi. Dev. Cell 2011, 20, 148–161. [Google Scholar] [CrossRef] [Green Version]
- Castel, S.E.; Martienssen, R.A. RNA interference in the nucleus: Roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Kogel, K.-H.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi—Nature’s blueprint for modern crop protection strategies. Curr. Opin. Microbiol. 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs—Big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Gebremichael, D.E.; Haile, Z.M.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef]
- Lax, C.; Tahiri, G.; Patiño-Medina, J.A.; Cánovas-Márquez, J.T.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Navarro, E.; Calo, S. The evolutionary significance of RNAi in the fungal kingdom. Int. J. Mol. Sci. 2020, 21, 9348. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Kumar, M.; Mysore, K.S. RNAi in plants: Recent developments and applications in agriculture. In Gene Silencing: Theory Techniques and Applications; Catalano, A.J., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 183–199. [Google Scholar]
- Nunes, C.C.; Dean, R.A. Host-induced gene silencing: A tool for understanding fungal host interaction and for developing novel disease control strategies. Mol. Plant Pathol. 2012, 13, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, L.; Baraldi, E.; Mezzetti, B.; Limera, C.; Sabbadini, S. Biotechnological approaches: Gene overexpression, gene silencing and genome editing to control fungal and oomycete diseases in grapevine. Int. J. Mol. Sci. 2020, 21, 5701. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, C.; Kuo, Y.-W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef] [PubMed]
- Vetukuri, R.R.; Dubey, M.; Kalyandurg, P.B.; Carlsson, A.S.; Whisson, S.C.; Ortiz, R. Spray-induced gene silencing: An innovative strategy for plant trait improvement and disease control. Crop Breed. Appl. Biotechnol. 2021, 21, e387921S11. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant–microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Bielska, E.; Birch, P.R.J.; Buck, A.H.; Abreu-Goodger, C.; Innes, R.W.; Jin, H.; Pfaffl, M.W.; Robatzek, S.; Regev-Rudzki, N.; Tisserant, C.; et al. Highlights of the mini-symposium on extracellular vesicles in inter-organismal communication, held in Munich, Germany, August 2018. J. Extracell. Vesicles 2019, 8, 1590116. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; He, B.; Weiberg, A.; Buck, A.H.; Jin, H. Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 2019, 15, e1008090. [Google Scholar] [CrossRef] [Green Version]
- Šečić, E.; Kogel, K.H. Requirements for fungal uptake of dsRNA and gene silencing in RNAi-based crop protection strategies. Curr. Opin. Biotechnol. 2021, 70, 136–142. [Google Scholar] [CrossRef]
- Laurie, J.D.; Linning, R.; Bakkeren, G. Hallmarks of RNA silencing are found in the smut fungus Ustilago hordei but not in its close relative Ustilago maydis. Curr. Genet. 2008, 53, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Li, L.; Gu, W.; Xue, Z.; Crosthwaite, S.K.; Pertsemlidis, A.; Lewis, Z.; Freitag, M.; Selker, E.U.; Mello, C.C.; et al. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol. Cell. 2010, 38, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz Vázquez, R.M. A non-canonical RNA silencing pathway promotes mRNA degradation in basal fungi. PLoS Genet. 2015, 11, e1005168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulin, E.H.; de Lima, T.A.; dos Santos, P.J.C.; Machado, M.A. RNAi-induced silencing of the succinate dehydrogenase subunits gene in Colletotrichum abscissum, the causal agent of postbloom fruit drop (PFD) in citrus. Microbiol. Res. 2022, 260, 126938. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, P.M.; Graham, M.W.; Wang, M.-B. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA 1998, 95, 13959–13964. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jin, H. Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Wassenegger, M. Host-induced gene silencing—Mechanisms and applications. New Phytol. 2021, 231, 54–59. [Google Scholar] [CrossRef]
- Van Schie, C.C.N.; Takken, F.L.W. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A new frontier in crop protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.C.; Veluthambi, K.; Subramaniam, K. Host-generated double stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol. Biochem. Parasitol. 2006, 148, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kogel, K.-H. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Song, X.-S.; Li, H.-P.; Cao, L.-H.; Sun, K.; Qiu, X.-L.; Xu, Y.-B.; Yang, P.; Huang, T.; Zhang, J.-B.; et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015, 13, 1335–1345. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Y.; Zhao, J.-H.; Gao, F.; Zhou, B.-J.; Fang, Y.-Y.; Guo, H.-S. Host-Induced Gene Silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae. Mol. Plant 2016, 9, 939–942. [Google Scholar] [CrossRef] [Green Version]
- Panwar, V.; Jordan, M.; McCallum, B.; Bakkeren, G. Host-induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnol. J. 2018, 16, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Biotechnol. J. 2014, 12, 541–553. [Google Scholar] [CrossRef]
- He, F.; Zhang, R.; Zhao, J.; Qi, T.; Kang, Z.; Guo, J. Host-induced silencing of Fusarium graminearum genes enhances the resistance of Brachypodium distachyon to Fusarium head blight. Front. Plant. Sci. 2019, 10, 1362. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.-Y.; Zhang, C.; Ganiger, M. Reduction of Phakopsora pachyrhizi infection on soybean through host- and spray-induced gene silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Mahto, B.K.; Singh, A.; Pareek, M.; Rajam, M.V.; Dhar-Ray, S.; Reddy, P.M. Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato. Plant Mol. Biol. 2020, 104, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Sun, J.; Kang, Z.; Ding, S.; Xu, J.-R.; Peng, Y.-L. A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae. Mol. Plant Microbe Interact. 2010, 23, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, A.; Höfle, L.; Werner, B.T.; Imani, J.; Schmidt, A.; Jelonek, L.; Kogel, K.H. SIGS vs. HIGS: A study on the efficacy of two dsRNA delivery strategies to silence Fusarium FgCYP51 genes in infected host and non-host plants. Mol. Plant Pathol. 2019, 20, 1636–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; de Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Rosa, S.; Pesaresi, P.; Mizzotti, C.; Bulone, V.; Mezzetti, B.; Baraldi, E.; Masiero, S. Game-changing alternatives to conventional fungicides: Small RNAs and short peptides. Trends Biotechnol. 2022, 40, 320–337. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef]
- Chaloner, T.; van Kan, J.A.L.; Grant-Downton, R.T. RNA ‘Information Warfare’ in pathogenic and mutualistic interactions. Trends Plant Sci. 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Lichtenberg, S.S.; Tsyusko, O.V.; Palli, S.R.; Unrine, J.M. Uptake and bioactivity of chitosan/double-stranded RNA polyplex nanoparticles in Caenorhabditis elegans. Environ. Sci. Technol. 2019, 53, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Miguel-Rojas, C.; Perez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Chen, J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo suppressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef]
- Kupferschmidt, K. Lethal dose of RNA. Science 2013, 341, 732–733. [Google Scholar] [CrossRef]
- Taning, C.N.T.; Mezzetti, B.; Kleter, G.; Smagghe, G.; Baraldi, E. Does RNAi-based technology fit within EU sustainability goals? Trends Biotechnol. 2021, 31, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.-X.; Song, X.-S.; Xiao, X.-M.; Duan, X.-X.; Wang, J.-X.; Duan, Y.-B.; Hou, Y.-P.; Zhou, M.-G. A β2-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pestic. Biochem. Phys. 2019, 153, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Suarez, I.B.; Sanchez-Salas, J.L.; Ragazzo-Sanzhez, J.A.; Calder’on-Santoyo, M. Evaluation of the toxicity and pathogenecity of biocontrol agents in murine models, chicken embryos and dermal irritation in rabbits. Toxicol. Res. 2017, 6, 188–198. [Google Scholar]
- Bahadou, S.A.; Ouijja, A.; Karfach, A.; Tahiri, A.; Lahlali, R. New potential bacterial antagonists for the biocontrol of fire blight disease (Erwinia amylovora) in Morocco. Microb. Pathog. 2018, 117, 7–15. [Google Scholar] [CrossRef]
- Bachman, P.M.; Bolognesi, R.; Moar, W.J.; Mueller, G.M.; Paradise, M.S.; Ramaseshadri, P.; Tan, J.; Uffman, J.P.; Warren, J.; Wiggins, B.E.; et al. Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). Transgenic Res. 2013, 22, 1207–1222. [Google Scholar] [CrossRef] [Green Version]
- Ramon, M.; Devos, Y.; Lanzoni, A.; Liu, Y.; Gomes, A.; Gennaro, A.; Waigmann, E. RNAi-based GM plants: Food for thought for risk assessors. Plant Biotechnol. J. 2014, 12, 1271–1273. [Google Scholar] [CrossRef]
- Casacuberta, J.M.; Devos, Y.; Du Jardin, P.; Ramon, M.; Vaucheret, H.; Noguè, F. Biotechnological uses of RNAi in plants: Risk assessment considerations. Trends Biotechnol. 2015, 33, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Levine, S.L.; Bachman, P.M.; Jensen, P.D.; Mueller, G.M.; Uffman, J.P.; Meng, C.; Song, Z.; Richards, K.B.; Beevers, M.H. No impact of DvSnf7 RNA on honey bee (Apis mellifera L.) adults and larvae in dietary feeding tests. Environ. Toxicol. Chem. 2016, 35, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Mezzetti, B.; Smagghe, G.; Arpaia, S.; Christiaens, O.; Pfeilstetter, A.D.; Jones, H.; Kostov, K.; Sabbadini, S.; Opsahl-Sorteberg, H.-G.; Ventura, V.; et al. RNAi: What is its position in agriculture? J. Pest Sci. 2020, 93, 1125–1130. [Google Scholar] [CrossRef]

| Biological Agent (Species and Strain/Isolate) | Colletotrichum Species | Host Species | Reference |
|---|---|---|---|
| Bacillus subtilis EA-CB0015 | C. acutatum species complex | Cyphomandra betacea | [74] |
| Bacillus subtilis S16 | C. acutatum species complex | Malus pumila | [75] |
| Prunus persica cv. Chunjungdo | |||
| P. persica cv. Sunfre | |||
| Paenibacillus polymyxa APEC128 | C. acutatum species complex | M. pumila | [76] |
| C. gloeosporioides species complex | |||
| Streptomyces philanthi RM-1-138 | C. gloeosporioides species complex | Capsicum annuum | [77] |
| Burkholderia rinojensis F2 | C. truncatum | C. annuum | [78] |
| B. rinojensis F80 | |||
| Burkholderia gladioli F79 | |||
| Burkholderia arboris F35 | |||
| Pseudomonas aeruginosa F65 | |||
| Trichoderma harzianum T-39 | C. acutatum sensu lato | Fragaria × ananassa | [79] |
| Trichoderma hamatum T-105 | |||
| Trichoderma atroviride T-161 | |||
| Trichoderma longibrachiatum T-166 | |||
| T. harzanium1 | C. gloeosporioides species complex | Citrus limon L. | [80] |
| T. harzanium TH-1 | C. musae | Musa acuminata | [81] |
| T. viridae TV-3 | |||
| T. viridae TV-4 | |||
| Trichoderma pseudokomngii2 | |||
| Metchnikowia pulcherrima FMB-24H-2 | C. acutatum sensu lato | Malus domestica | [82] |
| M. pulcherrima T5-A2 | C. acutatum sensu lato | M. domestica | [83] |
| Pichia kluyveri Y1125 | C. acutatum species complex | M. domestica | [84] |
| Essential Oil | Colletotrichum Species | Host Species | Reference |
|---|---|---|---|
| Lemongrass oil | C. gloeosporioides species complex | Carica papaya L. | [99] |
| Ginger oil | C. gloeosporioides species complex | C. papaya L. | [100] |
| Savory oil | C. gloeosporioides species complex | C. papaya L. | [101] |
| C. gloeosporioides species complex | Persea americana | [102] | |
| Thyme oil | C. gloeosporioides species complex | C. papaya | [101] |
| C. gloeosporioides species complex | P. americana | [101] | |
| C. gloeosporioides species complex | Mangifera indica L. | [103] | |
| C. acutatum species complex | Fragaria x ananassa | [104] | |
| Cinnamon bark oil | C. acutatum species complex | Fragaria x ananassa | [104] |
| Ocinum basilicum oil | C. musae | Musa spp. | [105] |
| Ocinum gratissimus oil | C. musae | Musa spp. | [105] |
| Seaweed Derivate-Species | Colletotrichum Species | Host Species | Reference |
|---|---|---|---|
| “Ulvan”-Ulva spp. | C. lindemuthianum | Phaseolus vulgaris L. | [117] |
| “Ulvan”-Ulva fasciata | C. lindemuthianum | P. vulgaris L. | [118] |
| Ethanolic fraction-Ulva spp. | C. trifolii | Medicago truncatula | [119] |
| Algal suspension-Chlorella fusca | C. orbiculare | Cucumis sativus | [120] |
| Seaweed-based biofungicide-Ascophyllum nodosum | C. acutatum species complex | Fragaria × ananassa | [121] |
| Approach | Outcome | Reference |
|---|---|---|
| HIGS | C. gloeosporioides species complex inhibition in GM plants carrying a CgCOM1 RNA expressing cassette | [166] |
| SIGS | C. truncatum silencing by means of interfering dsRNAs | [187] |
| Existence of the RNAi machinery in C. abscissum | [149] | |
| Absence of dsRNA uptake efficiency in C. gloeosporioides species complex | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants 2022, 11, 1856. https://doi.org/10.3390/plants11141856
Ciofini A, Negrini F, Baroncelli R, Baraldi E. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants. 2022; 11(14):1856. https://doi.org/10.3390/plants11141856
Chicago/Turabian StyleCiofini, Alice, Francesca Negrini, Riccardo Baroncelli, and Elena Baraldi. 2022. "Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives" Plants 11, no. 14: 1856. https://doi.org/10.3390/plants11141856






