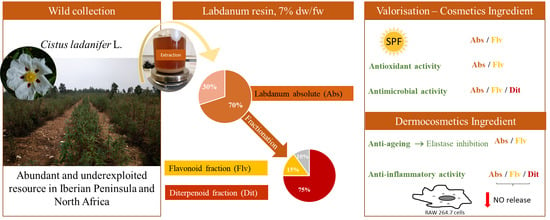
Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities
Abstract
:1. Introduction
2. Results and discussion
2.1. Extraction and Yields of Labdanum Absolute and Its Fractions
2.2. Chemical Profile of Labdanum Absolute and of Its Fractions
2.3. Assessment of Labdanum Absolute and Labdanum Fractions Sun Protection Factor
2.4. Antioxidant Activity of Labdanum Resin and Its Fractions
2.5. Anti-Elastase Activity
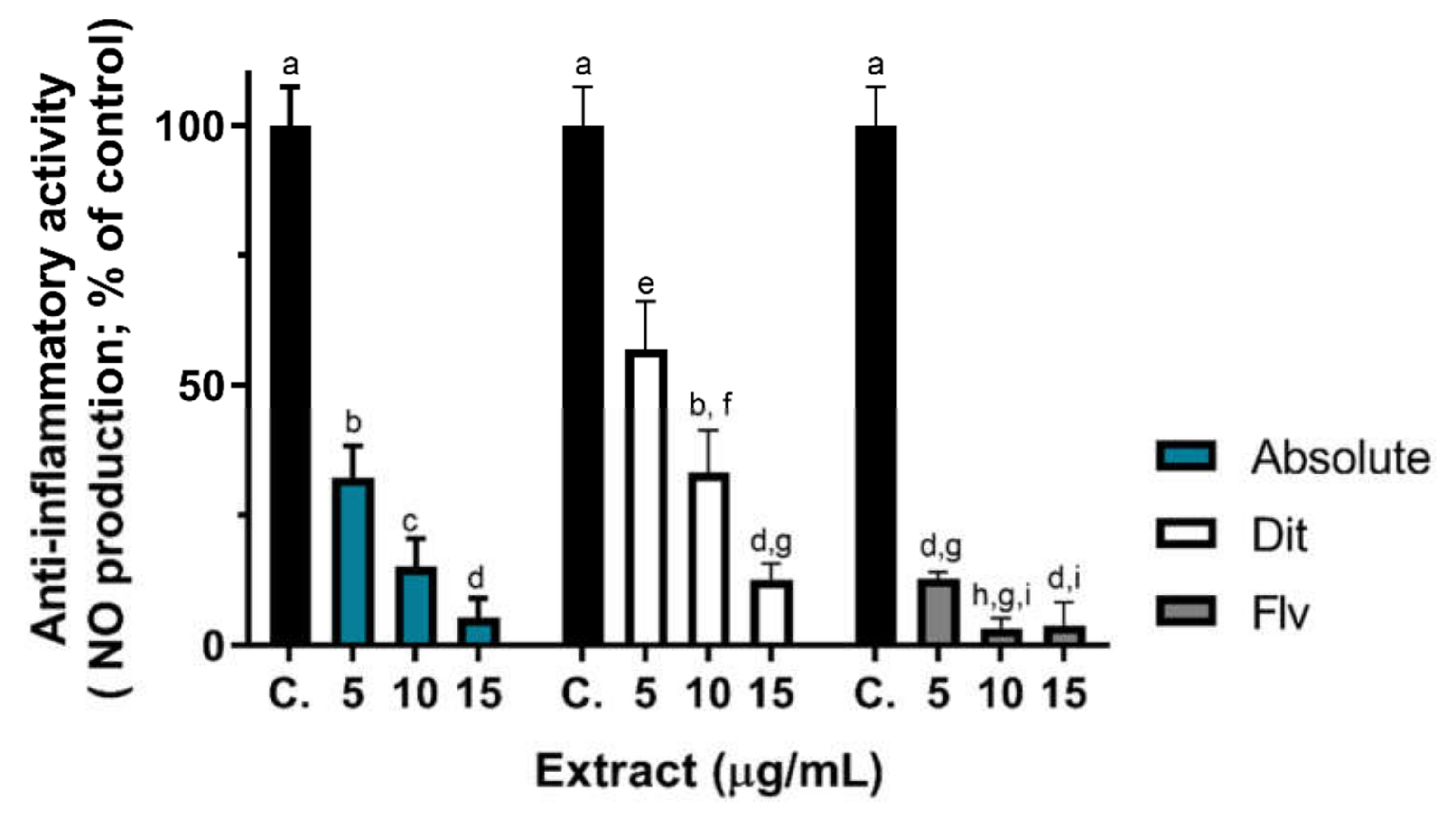
2.6. Anti-Inflammatory Activity
2.7. Antimicrobial Activity
3. Materials and Methods
3.1. Labdanum Absolute
3.2. Diterpenoid and Flavonoid Fractions
3.3. Chemical Characterization of Absolute and Fractions
3.4. UV Radiation Absorption
3.5. Antioxidant Activity
3.6. Elastase Inhibition Activity
3.7. Anti-Inflammatory Activity
3.8. Antimicrobial Activity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godinho-Ferreira, P.; Azevedo, A.; Rego, F. Carta Da Tipologia Florestal De Portugal Continental. Silva Lusit. 2005, 13, 1–34. [Google Scholar]
- Demoly, J.P.; Montserrat, P. Cistus. In Flora Ibérica; Castroviejo, S., Ed.; CSIC (Centro Superior Investigacion Cientifica): Madrid, Spain, 1993; pp. 319–337. [Google Scholar]
- Masa, C.V.; Díaz, T.S.; Gallego, J.C.A.; Lobón, N.C. Quantitative Variation of Flavonoids and Diterpenes in Leaves and Stems of Cistus ladanifer L. At Different Ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langenheim, J.H. Plant Resins. Am. Sci. 1990, 78, 16–24. [Google Scholar]
- Morgado, J.M.; Tapias, R.; Alesso, P. Producción de goma bruta de jara (Cistus ladanifer L.) en el suroeste de la península Ibérica. In Proceedings of the Congresos Forestales 2005, Zaragoza, Spain, 26–30 September 2005. [Google Scholar]
- Burguer, L. Investigação E Comparação De Metodologias De Extração De Lábdano Obtido a Partir De Cistus ladanifer L. Master’s Thesis, Instituto Politécnico de Bragança, Universidadad de Salamanca, Bragança, Portugal, 2016. [Google Scholar]
- Greche, H.; Mrabet, N.; Ismaili-Alaoui, M.; Hajajji, N.; Bousta, D.; Dahchour, A.; Benjilali, B. Chemical composition, antibacterial and antifungal activities of Moroccan Cistus ladanifer L. leaves extracts. In Recherches sur les Plantes Aromatiques et Médicinales; Ennabili, H.G.A., Ed.; Imprimerie Al Maarif Al Jadida: Rabat, Morocco, 2009; pp. 201–213. [Google Scholar]
- Gallego, J.C.A. Influencia De Los Factores Climáticos En La Síntesis Y Actividad De Compuestos Fitotóxicos Secretados Por Cistus Ladanifer.L. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 2006. [Google Scholar]
- De Pascual, J.T.; Urones, J.G.; Basabe, M.P.; Marcos, I.S.; Montaña, A. Nuevo Studio Sobre Componentes De Cistus Ladaniferus L. Stud. Chem. IX 1984, 9, 31–47. [Google Scholar]
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational Variation in the Flavonoid Composition of Cistus ladanifer L. Exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Vogt, T.; Proksch, P.; Gülz, P.-G. Epicuticular Flavonoid Aglycones in the Genus Cistus, Cistaceae. J. Plant Physiol. 1987, 131, 25–36. [Google Scholar] [CrossRef]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal Variation of Cistus ladanifer L. Diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean Plant Species Are Valuable Resources: The Example of Cistus Ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef]
- L’ORÉAL. 2020 Annual Report—Cosmetics Market. Available online: https://www.loreal-finance.com/en/annual-report-2020/cosmetics-market-2-1-0/ (accessed on 20 February 2022).
- CosmeticsEurope. Cosmetics and Personal Care Industry Overview. Available online: https://cosmeticseurope.eu/cosmetics-industry/ (accessed on 21 February 2022).
- González, J.; Ramón Vallejo, J.; Amich, F. Cistus ladanifer L. In Inventario Español de los Conocimientos Tradicionales Relativos a la Biodiversidad; de Santayana, M.P., Morales, R., Tardío, J., Aceituno, L., Molina, M., Eds.; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018; Volume 2, pp. 47–55. [Google Scholar]
- CBI. Centre for the Promotion of Imports of the Netherlands Ministry of Foreign Affairs. What Is the Demand for Natural Ingredients for Cosmetics on the European Market? Available online: https://www.cbi.eu/market-information/natural-ingredients-cosmetics/what-demand (accessed on 30 January 2022).
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Minkis, K.; Harvey Swary, J.; Alam, M. Skin physiology pertinent to cosmetic dermatology: Photoaging. In Cosmetic Dermatology; Draelos, Z., Ed.; John Wiley & Sons Ltd.: Oxford, UK, 2016; pp. 13–22. [Google Scholar]
- Linden, K.G. Commentary: Sunscreen Sun Protection Factor (Spf): Is Higher Better? J. Am. Acad. Dermatol. 2018, 78, 911–912. [Google Scholar] [CrossRef]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet Radiation and the Skin: Photobiology and Sunscreen Photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.; Azulay, R.D. Determinação Do Fator De Proteção Solar Por Espectrofotometria /Determination of Sun Protection Factor by Spec Trophotometric Methods. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Dutra, E.A.; Gonçalves da Costa e Oliveira, D.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of Sun Protection Factor (Spf) of Sunscreens by Ultraviolet Spectrophotometry. Rev. Bras. Ciências Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus× Incanus L. And Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Ultrav. Light Hum. Health Dis. Environ. 2017, 996, 71–87. [Google Scholar]
- Zhong, Q.-Y.; Lin, B.; Chen, Y.-T.; Huang, Y.-P.; Feng, W.-P.; Wu, Y.; Long, G.-H.; Zou, Y.-N.; Liu, Y.; Lin, B.-Q.; et al. Gender Differences in Uv-Induced Skin Inflammation, Skin Carcinogenesis and Systemic Damage. Environ. Toxicol. Pharmacol. 2021, 81, 103512. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus Zygis Subsp. Zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Gomi, T.; Shishido, M.; Watanabe, H.; Suenobu, N. Neutrophil Elastase Contributes to Extracellular Matrix Damage Induced by Chronic Low-Dose Uv Irradiation in a Hairless Mouse Photoaging Model. J. Dermatol. Sci. 2010, 60, 151–158. [Google Scholar] [CrossRef]
- Weyrich, L.S.; Dixit, S.; Farrer, A.G.; Cooper, A.J. The Skin Microbiome: Associations between Altered Microbial Communities and Disease. Australas. J. Dermatol. 2015, 56, 268–274. [Google Scholar] [CrossRef]
- Coates, R.; Moran, J.; Horsburgh, M.J. Staphylococci: Colonizers and Pathogens of Human Skin. Future Microbiol. 2014, 9, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N.; Lin, A.N. Pseudomonas Skin Infection. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef]
- Kashem, S.; Kaplan, D.H. Skin Immunity to Candida Albicans. Trends Immunol. 2016, 37, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhdev Swami, H. An overview of extraction techniques for medicinal and aromatic plants. In Extraction Technologies for Medicinal and Aromatic Plants; Handa, S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; ICS-UNIDO: Trieste, Italy, 2008; pp. 21–54. [Google Scholar]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- De Pascual, J.T.; Bellido, I.; Basabe, P.; Marcos, I.; Ruano, I.; Urones, J. Labdane Diterpenoids from Cistus ladaniferus. Phytochemistry 1982, 21, 899–901. [Google Scholar] [CrossRef]
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of Secreted Flavonoids of Cistus ladanifer L. by High-Performance Liquid Chromatography–Particle Beam Mass Spectrometry. J. Chromatogr. A 1998, 799, 111–115. [Google Scholar] [CrossRef]
- Proksch, P.; Gülz, P.G. Methylated Flavonoids from Cistus ladanifer and Cistus palhinhae and Their Taxonomic Implications. Phytochemistry 1984, 23, 470–471. [Google Scholar] [CrossRef]
- Masa, C.V.; Gallego, J.C.A.; Lobón, N.C.; Díaz, T.S. Intra-Population Variation of Secondary Metabolites in Cistus ladanifer L. Molecules 2016, 21, 945. [Google Scholar] [CrossRef] [Green Version]
- De Pascual, J.T.; Portela, C.M.; Sánchez, I.B. Estudio De La Gomorresina Del Cistus ladaniferus (L.). Ii. 3,7-Dimethyl-Kampferol O Jaranol. Anales Quimica LXIV 1968, 64, 623–632. [Google Scholar]
- De Pascual, J.T.; Urones, J.G.; Basabe, M.P. Flavonoides Del Cistus ladaniferus (L). An. Quim. 1974, 70, 155–157. [Google Scholar]
- Chaves, N.; Escudero, J.; Gutiérrez-Merino, C. Quantitative Variation of Flavonoids among Individuals of a Cistus ladanifer Population. Biochem. Syst. Ecol. 1997, 25, 429–435. [Google Scholar] [CrossRef]
- De Pascual, J.T.; Urones, J.G.; Marcos, I.S.; Nuñez, L.; Basabe, P. Diterpenoids and Flavonoids from Cistus palhinhae. Phytochemistry 1983, 22, 2805–2808. [Google Scholar] [CrossRef]
- Tabacik, C.; Bard, M. Etude Chimio-Taxonomique Dans Le Genre Cistus. Phytochemistry 1971, 10, 3093–3106. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Pérez-Alvarez, J.A.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of Flavonoid Content and Chemical Composition of the Essential Oils of Moroccan Herbs: Myrtle (Myrtus communis L.), Rockrose (Cistus ladanifer L.) and Montpellier Cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Cocker, J.D.; Halsall, T.G. 820. The Chemistry of Gum Labdanum—Part II—The Structure of Labdanolic Acid. J. Chem. Soc. 1956, 4262–4271. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Weirauch, M.; Thefeld, K.; Surburg, H. Constituents of Commercial Labdanum Oil. Flavour Fragr. J. 1998, 13, 295–318. [Google Scholar] [CrossRef]
- Lobón, N.C.; de la Cruz, I.F.; Gallego, J.C.A. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a Hundred Plant Extracts as Tyrosinase and Elastase Inhibitors, Two Enzymatic Targets of Cosmetic Interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Knowles, R.G.; Moncada, S. Nitric Oxide Synthases in Mammals. Biochem J 1994, 298 Pt 2, 249–258. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L.-J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef] [PubMed]
- Man, M.-Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory Role of Nitric Oxide in Cutaneous Inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The Role of Macrophages in Skin Homeostasis. Pflug. Arch. Eur. J. Physiol. 2017, 469, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Youbi, A.E.H.E.; El Mansouri, L.; Boukhira, S.; Daoudi, A.; Bousta, D. In Vivo Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Cistus ladanifer L. From Morocco. Am. J. Ther. 2016, 23, e1554–e1559. [Google Scholar] [CrossRef] [PubMed]
- Rauwald, H.W.; Liebold, T.; Grötzinger, K.; Lehmann, J.; Kuchta, K. Labdanum and Labdanes of Cistus Creticus and C. Ladanifer: Anti-Borrelia Activity and Its Phytochemical Profiling. Phytomedicine 2019, 60, 152977. [Google Scholar] [CrossRef]
- Ramiro, F.B.; Casquete, R.; Martínez, A.; de Guía Córdoba, M.; Ruíz-Moyano, S.; José Benito, M. Antioxidant, Antihypertensive and Antimicrobial Properties of Phenolic Compounds Obtained from Native Plants by Different Extraction Methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldan-Segura, C.M.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef]
- Lekbach, Y.; Xu, D.; El Abed, S.; Dong, Y.; Liu, D.; Khan, M.S.; Koraichi, S.I.; Yang, K. Mitigation of Microbiologically Influenced Corrosion of 304l Stainless Steel in the Presence of Pseudomonas aeruginosa by Cistus ladanifer Leaves Extract. Int. Biodeterior. Biodegrad. 2018, 133, 159–169. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal Activity and Detailed Chemical Characterization of Cistus ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Deniz, K.M.; Tekin, B.N.; Bayraktar, O.; Duman, E.T.; Başpınar, Y. Antioxidant and Antimicrobial Properties of Cistus ladanifer. Int. J. Second. Metab. 2017, 4, 434–444. [Google Scholar]
- Ferreira, S.; Santos, J.; Duarte, A.; Duarte, A.P.; Queiroz, J.A.; Domingues, F. Screening of Antimicrobial Activity of Cistus ladanifer and Arbutus unedo Extracts. Nat. Prod. Res. 2012, 26, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Amarendra, P.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals; StatPearls [Internet]: Tampa, FL, USA, 2021. [Google Scholar] [PubMed]
- Vogt, T.; Gülz, P.-G. Isocratic Column Liquid Chromatographic Separation of a Complex Mixture of Epicuticular Flavonoid Aglycones and Intracellular Flavonol Glycosides from Cistus laufifolius L. J. Chromatogr. A 1991, 537, 453–459. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. A Comparison of in Vivo and in Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus Pulegioides L. As a Rich Source of Antioxidant, Anti-Proliferative and Neuroprotective Phenolic Compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Borges, A.; Carvalho, A.P.; Monteiro, M.J.; Pintado, M.M.E. Nutritional Characterization of Acorn Flour (a Traditional Component of the Mediterranean Gastronomical Folklore). J. Food Meas. Charact. 2016, 10, 584–588. [Google Scholar] [CrossRef]

| Resin | Absolute | Dit | Flv | Remainder |
|---|---|---|---|---|
| 7.44 ± 0.41 | 73.3 ± 0.9 | 74.2 ± 1.8 | 15.6 ± 4.6 | 10.1 ± 2.9 |
| Peak | Retention Time (min) | ESI Ions | Literature Compounds Match | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [M-H]− (m/z) | [M + H]+(m/z) | Absolute | Dit | Flv | Compound | MW (g/mol) | Reference | ||
| 1 | 16.8 | 269 | 271 | + | + | Apigenin | 270 | [10,36,38,39,40] | |
| 2 | 18.4 | 299 | 301 | ++ | ++ | Kaempferol-3-methylether (isokaempferide) | 300 | [10,36,38,39,40] | |
| 3 | 24.7 | 283 | 285 | + | + | Apigenin-4′-methylether (acacetin) | 284 | [10,36,38,39,40] | |
| 4 | 24.9 | 283 | 285 | + | + | Apigenin-7-methylether (genkwanin) | 284 | [9,10,36,38,39,40] | |
| 5 | 26.6 | 313 | 315 | ++ | ++ | Kaempferol-dimethylether (3,7, jaranol, or 3,4′) | 314 | [10,36,38,39,40,41,42] | |
| 6 | 28.6 | 335 | 337 | + | - | (336) | |||
| 7 | 30.9 | 379 | 363 (292/303/321) | + | - | (380) | |||
| 8 | 32.0 | 381 | - | + | - | (382) | |||
| 9 | 32.2 | - | - | + | - | ||||
| 10 | 33.1 | 319 | 321 | + | Oxo-labdenoic acid | 320 | [7,8,9,12,37,43] | ||
| 11 | 33.5 | 309 (379) | - | + | Labdandiol (p.e. 8,15) | 310 | [9,44,45] | ||
| 12 | 33.9 | - | 299 | + | + | Apigenin-7,4′-dimethylether | 298 | [9,36,38] | |
| 13 | 35.4 | 319 | 321 | + | ++ | Oxo-labdenoic acid | 320 | [3,7,8,9,12,37] | |
| 14 | 35.7 | - | 329 | + | + | Kaempferol-3,7,4′-trimethylether (methyljaranol) | 328 | [9,36,38,46] | |
| 15 | 37.4 | 323 | - | ++ | Labdanolic acid | 324 | [6,7,8,9,37,44,47] | ||
| 16 | 37.9 | - | 307 | + | Labdenoic acid (8(17) ladenic, 7 cativic acids) | 306 | [6,7,44,48] | ||
| 17 | 38.4 | - | 257/351 (375) | + | 8α-methoxy-labdan-15-oic acid | 256 | [9,37,44] | ||
| 18 | 39.6 | - | 257 | + | - | (256) | |||
| 19 | 40.1 | 371 | 338 (398/241) | + | - | (372) | |||
| 20 | 42.3 | - | 456 (393) | ++ | - | (455) | |||
| 21 | 43.6 | 381/433 | 335 (376/435/269) | + | - | (382/434) | |||
| 22 | 44.4 | - | 384 | + | - | (383) | |||
| 23 | 45.9 | - | 335 (376) | ++ | + | - | (334) | ||
| 24 | 47.1 | - | 271 | + | - | (270) | |||
| 25 | 48.1 | - | 398 (165) | + | - | (397) | |||
| Total Absorbance (a.u.) | |||
|---|---|---|---|
| Extract | SPF | UVB | UVA |
| Absolute | 4.98 ± 0.19 b | 9.05 ± 0.36 b | 16.7 ± 0.6 b |
| Dit | 0.736 ± 0.015 c | 1.44 ± 0.02 c | 1.67 ± 0.03 c |
| Flv | 13.0 ± 1.4 a | 22.0 ± 2.3 a | 47.1 ± 5.3 a |
| Extract | Folin–Ciocalteu | DPPH | FRAP | ABTS |
|---|---|---|---|---|
| (mgGAE/mgExt) | (mgTE/mgExt) | |||
| Absolute | 0.128 ± 0.015 a,b,c | 0.031 ± 0.008 a,b,c | 0.038 ± 0.008 a,b,c | 0.142 ± 0.017 a,b,c |
| Flv | 0.203 ± 0.044 a,b | 0.054 ± 0.005 a,b | 0.049 ± 0.004 a,b | 0.379 ± 0.039 a,b |
| Dit | 0.011 ± 0.001 b,c | 0.005 ± 0.001 b,c | 0.020 ± 0.003 b,c | 0.010 ± 0.003 b,c |
| Elastase Inhibition (% of Control) | ||
|---|---|---|
| Extract | 1 mg/mL | 0.5 mg/mL |
| Absolute | 22.074 ± 0.292 * | n.a. |
| Dit | n.a. | - |
| Flv | 13.711 ± 0.313 * | n.a. |
| E. coli | P. aeruginosa | S. aureus | C. albicans | ||
|---|---|---|---|---|---|
| Extracts | MIC | MMC | |||
| Absolute | n.a. | n.a. | 1.2 | n.a. | n.a. |
| Flv | n.a. | n.a. | ≤0.3 | 2.5 | n.a. |
| Dit | n.a. | n.a. | ≤0.3 | 2.5 | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. https://doi.org/10.3390/plants11111477
Frazão DF, Martins-Gomes C, Steck JL, Keller J, Delgado F, Gonçalves JC, Bunzel M, Pintado CMBS, Díaz TS, Silva AM. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants. 2022; 11(11):1477. https://doi.org/10.3390/plants11111477
Chicago/Turabian StyleFrazão, David F., Carlos Martins-Gomes, Jan L. Steck, Judith Keller, Fernanda Delgado, José C. Gonçalves, Mirko Bunzel, Cristina M. B. S. Pintado, Teresa Sosa Díaz, and Amélia M. Silva. 2022. "Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities" Plants 11, no. 11: 1477. https://doi.org/10.3390/plants11111477