Plant Macrofossils Reveal Aquatic Macrophyte Successions of a Typical Shallow Lake (Huanggai Lake, China) in the Past Century
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
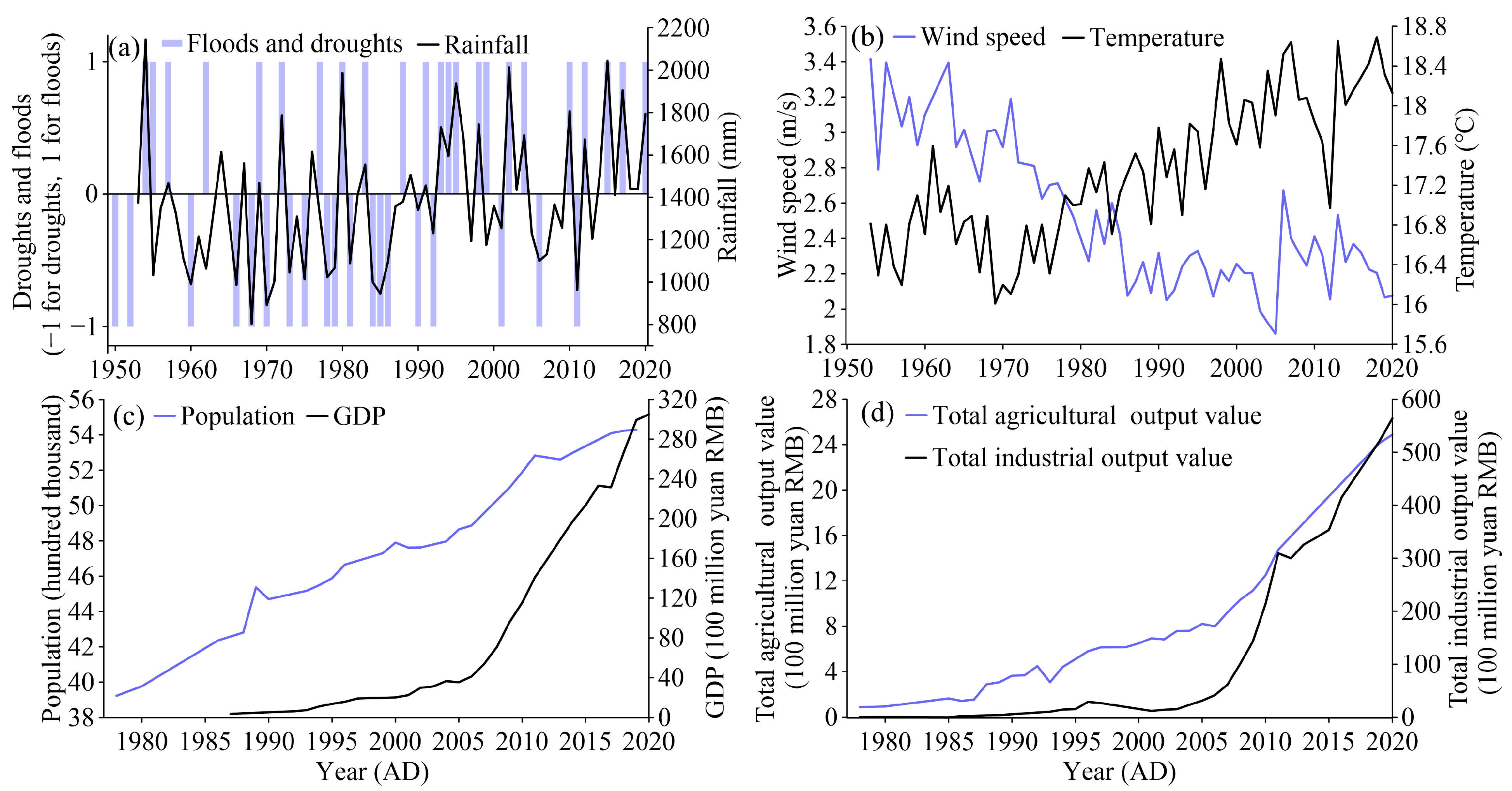
2.2. Documentary Data on Local Social and Environmental Background
2.3. Sediment Sampling and Analyses
2.4. Statistical Methods
3. Results
3.1. Chronology
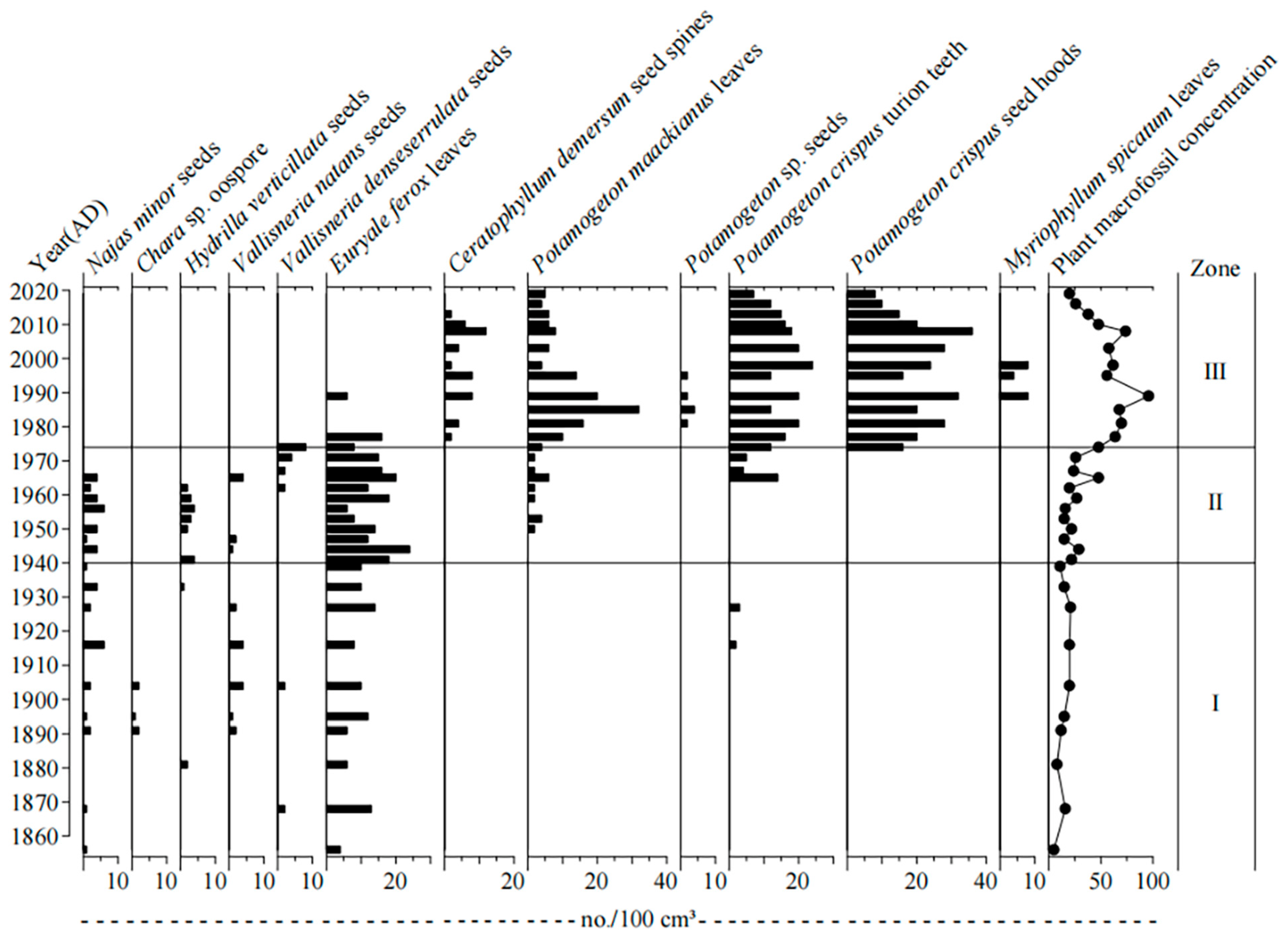
3.2. Macrophyte Flora
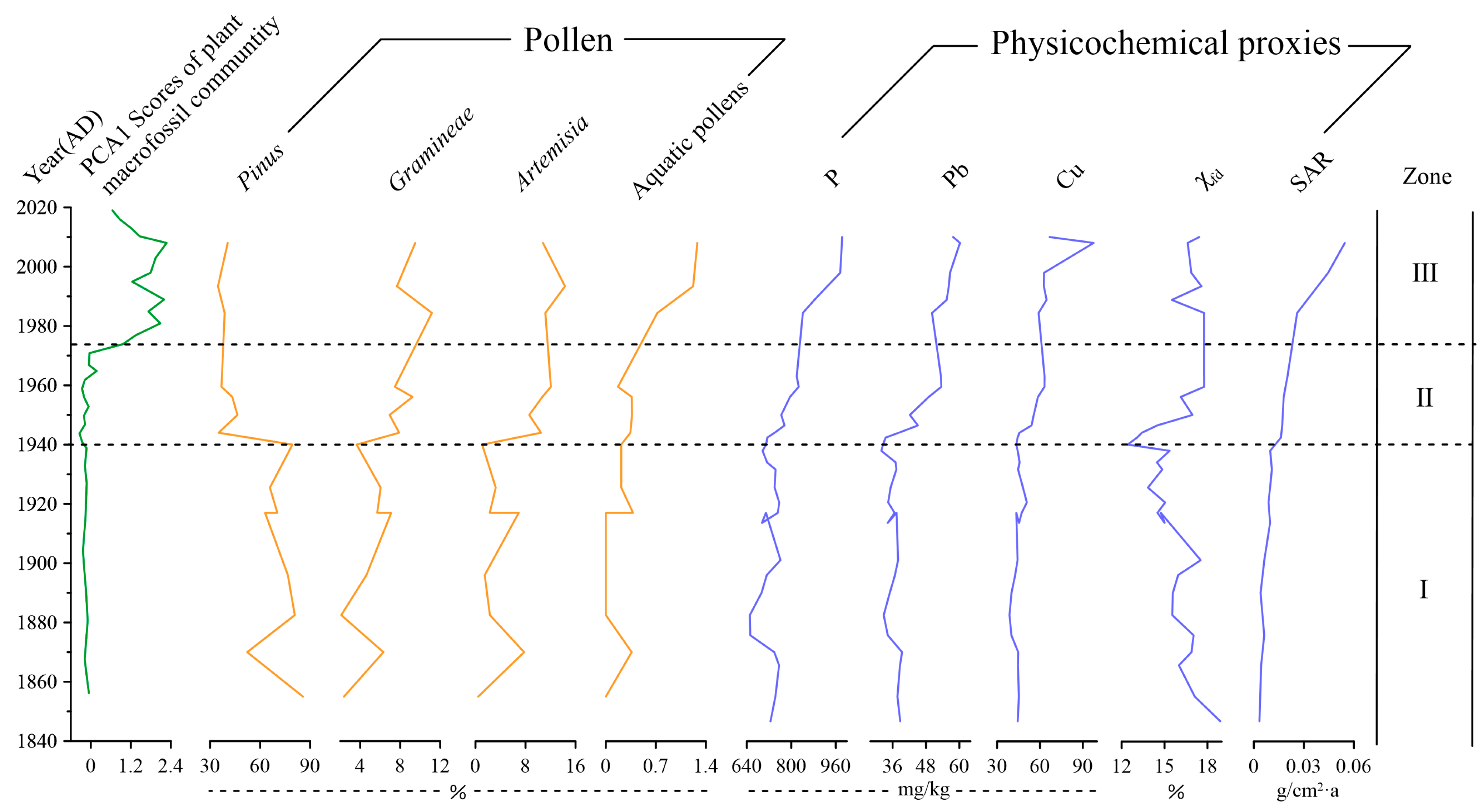
3.3. Ordination of Macrofossil Records
3.4. Multiple Sedimentary Proxy Analysis
4. Discussion
4.1. Ecological Habits of Aquatic Macrophytes in Huanggai Lake
4.2. Succession of Aquatic Macrophyte in Huanggai Lake: Patterns and Drivers
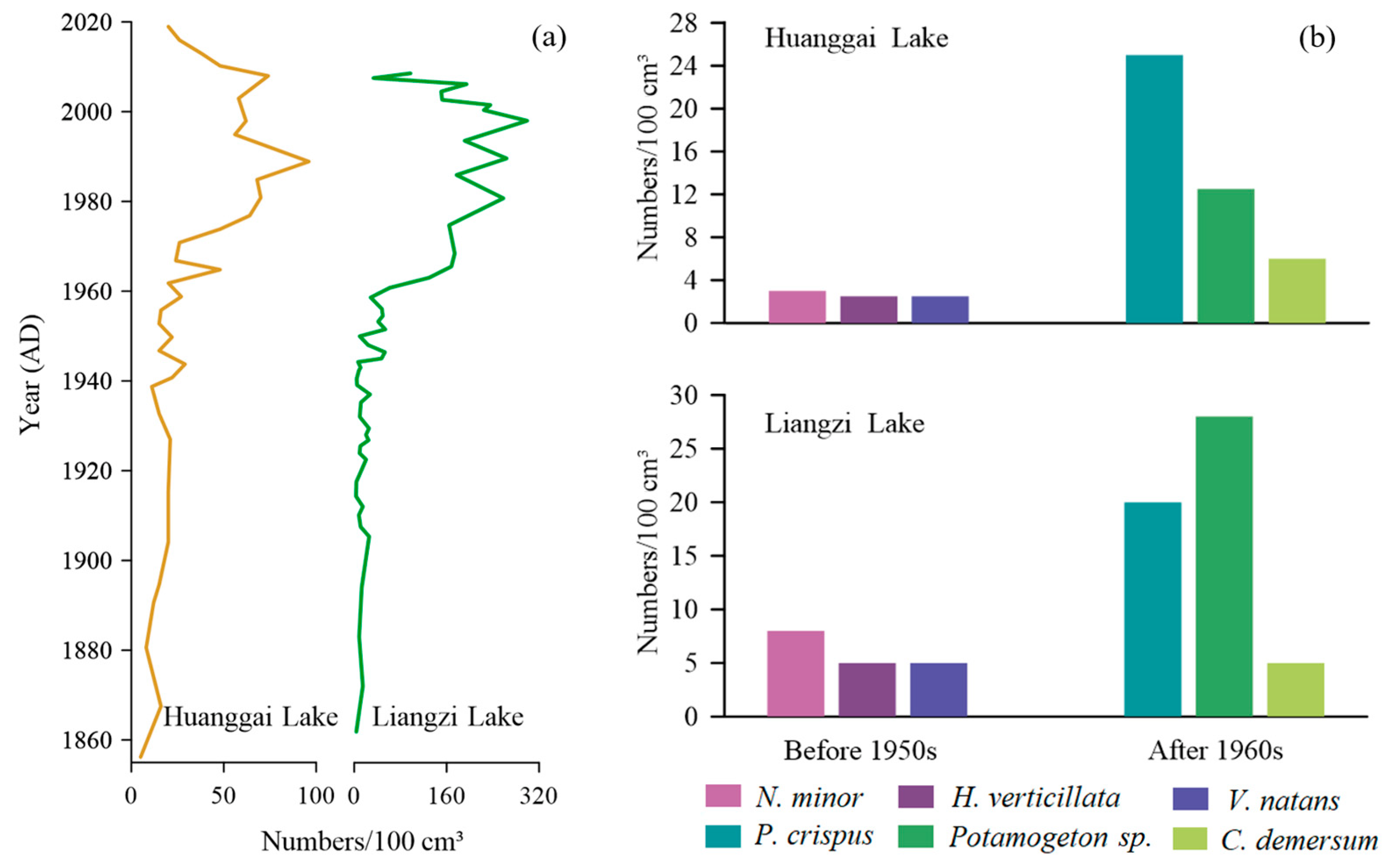
4.3. Comparison of Plant Macrofossil Records with Liangzi Lake in the Same Region
4.4. Implications for Lake Environmental Governance and Restoration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, M.; Jeppesen, E. Regime shifts in shallow lakes. Ecosystems 2007, 10, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; He, Q.; Yang, B.; He, W.; Xu, F.; Janssen, A.B.; Kuiper, J.J.; van Gerven, L.P.; Qin, N.; Jiang, Y. Hydrological regulation drives regime shifts: Evidence from paleolimnology and ecosystem modeling of a large shallow Chinese lake. Glob. Chang. Biol. 2017, 23, 737–754. [Google Scholar] [CrossRef]
- Wang, S.; Meng, W.; Jin, X.; Zheng, B.; Zhang, L.; Xi, H. Ecological security problems of the major key lakes in China. Environ. Earth Sci. 2015, 74, 3825–3837. [Google Scholar] [CrossRef]
- Dong, X.; Yang, X.; Chen, X.; Liu, Q.; Yao, M.; Wang, R.; Xu, M. Using sedimentary diatoms to identify reference conditions and historical variability in shallow lake ecosystems in the Yangtze floodplain. Mar. Freshw. Res. 2015, 67, 803–815. [Google Scholar] [CrossRef]
- Smol, J.P. Pollution of Lakes and Rivers: A Paleoenvironmental Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Birks, H.H. Plant macrofossil introduction. Encycl. Quat. Sci. 2007, 3, 2266–2288. [Google Scholar]
- Birks, H.H.; Peglar, S.M.; Boomer, I.; Flower, R.J.; Ramdani, M.; Appleby, P.; Bjune, A.E.; Patrick, S.T.; Kraïem, M.M.; Fathi, A.A.; et al. Palaeolimnological responses of nine North African lakes in the CASSARINA Project to recent environmental changes and human impact detected by plant macrofossil, pollen, and faunal analyses. Aquat. Ecol. 2001, 35, 405–430. [Google Scholar] [CrossRef] [Green Version]
- Birks, J.; Battarbee, R.; Mackay, A.; Oldfield, F. Reconstructing Holocene climates from pollen and plant macrofossils. In Global Change in the Holocene; Routledge: London, UK, 2014; pp. 358–373. [Google Scholar]
- Madgwick, G.; Emson, D.; Sayer, C.D.; Willby, N.J.; Rose, N.L.; Jackson, M.J.; Kelly, A. Centennial-scale changes to the aquatic vegetation structure of a shallow eutrophic lake and implications for restoration. Freshw. Biol. 2011, 56, 2620–2636. [Google Scholar] [CrossRef]
- Qin, B.; Gao, G.; Zhu, G.; Zhang, Y.; Song, Y.; Tang, X.; Xu, H.; Deng, J. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2012, 58, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of lake Taihu, China. In Eutrophication of Shallow Lakes with Special Reference to Lake Taihu, China; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–14. [Google Scholar]
- Xie, J.; Wu, D.; Chen, X.; Kong, H.; Pu, X.; Yang, X.; Minamoto, T.; Yamanaka, H.; Honio, M.; Kawabata, Z. Relationship between aquatic vegetation and water quality in littoral zones of Lake Dianchi and Lake Erhai. Environ. Sci. Technol. 2013, 36, 55–59. (In Chinese) [Google Scholar]
- Greulich, S.; Chevalier, R.; Villar, M. Soil seed banks in the floodplain of a large river: A test of hypotheses on seed bank composition in relation to flooding and established vegetation. J. Veg. Sci. 2019, 30, 732–745. [Google Scholar] [CrossRef]
- Bishop, I.J.; Bennion, H.; Patmore, I.R.; Sayer, C.D. How effective are plant macrofossils as a proxy for macrophyte presence? The case of Najas flexilis in Scotland. J. Paleolimnol. 2018, 60, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Sayer, C.D.; Burgess, A.; Kari, K.; Davidson, T.A.; Peglar, S.; Yang, H.; Rose, N. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: Implications for the stability of macrophyte-dominance. Freshw. Biol. 2010, 55, 565–583. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Kattel, G.R.; Dong, X.; Yang, X. A century-scale, human-induced ecohydrological evolution of wetlands of two large river basins in Australia (Murray) and China (Yangtze). Hydrol. Earth Syst. Sci. 2016, 20, 2151–2168. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Chen, X.; Dong, X.; Yang, X. Spatiotemporal patterns of carbon sequestration in a large shallow lake, Chaohu Lake: Evidence from multiple-core records. Limnologica 2020, 81, 125748. [Google Scholar] [CrossRef]
- Dong, X.; Anderson, N.J.; Yang, X.; Chen, X.; Shen, J. Carbon burial by shallow lakes on the Y angtze floodplain and its relevance to regional carbon sequestration. Glob. Chang. Biol. 2012, 18, 2205–2217. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, K.; Huang, S.; Lin, Q. Patterns and trajectories of macrophyte change in East China’s shallow lakes over the past one century. Sci. China Earth Sci. 2021, 64, 1735–1745. [Google Scholar] [CrossRef]
- Wang, S.; Dou, H. Records of Lakes in China; Science Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Yang, G.; Bao, X.; Zeng, H.; Chen, Y. Report of Lake Survey in China; Science Press: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Editor Board of Lingxiang City. Records of Linxiang City; Hunan Publishing House: Changsha, China, 2013. (In Chinese) [Google Scholar]
- Appleby, P. Chronostratigraphic techniques in recent sediments. In Tracking Environmental Change Using Lake Sediments; Springer: Berlin/Heidelberg, Germany, 2001; pp. 171–203. [Google Scholar]
- Berggren, G. Atlas of seeds and small fruits of northwest European plant species with morphological descriptions. Part 2: Cyperaceae. Oikos 1969, 20, 1315–1318. [Google Scholar] [CrossRef]
- Haas, J.N. First identification key for charophyte oospores from central Europe. Eur. J. Phycol. 1994, 29, 227–235. [Google Scholar] [CrossRef]
- Faegri, K.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1989. [Google Scholar]
- Grimm, E. TILIA and TILIA GRAPH. PC spreadsheet and graphics software for pollen data. J. Mol. Struct. 1990, 4, 5–7. [Google Scholar]
- Zhang, M.; Liu, Z.; Li, X.; Zhu, G.; Xu, J.; Li, Z. Studies of the selection and application of suitable hydrophyte species on lake restoration in the middle and lower reaches of Yangtze River. Log. Sci. 2014, 33, 344–352. [Google Scholar]
- Fu, W.; Li, Z.; Liu, Y.; Cao, Y.; Li, W. Naming history, classification and characteristics of species from the genus Vallisneria in China. Plant Sci. J. 2019, 37, 448–453. (In Chinese) [Google Scholar]
- Hao, A.; Kobayashi, S.; Huang, H.; Mi, Q.; Iseri, Y. Effects of substrate and water depth of a eutrophic pond on the physiological status of a submerged plant, Vallisneria natans. PeerJ 2020, 8, e10273. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, G.; Xing, W. Functional traits of submerged macrophytes in eutrophic shallow lakes affect their ecological functions. Sci. Total Environ. 2021, 760, 143332. [Google Scholar] [CrossRef]
- Cao, X.; Wan, L.; Xiao, J.; Chen, X.; Zhou, Y.; Wang, Z.; Song, C. Environmental effects by introducing Potamogeton crispus to recover a eutrophic Lake. Sci. Total Environ. 2018, 621, 360–367. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, J.; Bao, W.; Chen, J.; Li, D.; Li, Y. Comparison of nitrogen and phosphorus uptake and water purification ability of five submerged macrophytes. Environ. Sci. 2017, 38, 156–161. (In Chinese) [Google Scholar]
- Li, W.; Zhong, Y. Theories and Methods of the Study of Aquatic Vegetation; Central China Normal University Press: Wuhan, China, 1992. [Google Scholar]
- Zhang, M.; Cao, T.; Guo, L.; Ni, L.; Xie, P. Restoration of constructed hydrophytes community in East Lake of Wuhan and experimental study on water quality improvement. Environ. Sci. Technol. 2010, 33, 154–159. (In Chinese) [Google Scholar]
- Yang, X.; Shen, J.; Jones, R.T.; Wang, S.; Tong, G.; Zhang, Z. Pollen evidence of early human activities in Erhai basin, Yunnan Province. Chin. Sci. Bull. 2005, 50, 569–577. [Google Scholar] [CrossRef]
- Song, M.; Liu, Q.; LI, N. Preliminary study on wetland resources and comprehensive management countermeasures in Huanggai Lake. Yangtze River 2013, 44, 114–117. (In Chinese) [Google Scholar]
- Yao, W.; Zhang, L. Study on flood control planning of Huanggai Lake Basin. Yangtze River 2017, 48, 6–10. (In Chinese) [Google Scholar]
- Chen, Y.; Chen, S.; Ma, C.; Yu, S.; Yang, L.; Zhang, Z.; Yao, M. Palynological evidence of natural and anthropogenic impacts on aquatic environmental changes over the last 150 years in Dongping Lake, North China. Quat. Int. 2014, 349, 2–9. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Dong, X.; Liu, E.; Wang, S. Historical trophic evolutions and their ecological responses from shallow lakes in the middle and lower reaches of the Yangtze River: Case studies on Longgan Lake and Taibai Lake. Sci. China Ser. D 2006, 49, 51–61. [Google Scholar] [CrossRef]
- Anderson, N. Reconstructing historical phosphorus concentrations in rural lakes using diatom models. In Phosphorus Loss from Soil to Water; CAB International: Wallingford, UK, 1997; pp. 95–118. [Google Scholar]
- Deng, J.; Paerl, H.W.; Qin, B.; Zhang, Y.; Zhu, G.; Jeppesen, E.; Cai, Y.; Xu, H. Climatically-modulated decline in wind speed may strongly affect eutrophication in shallow lakes. Sci. Total Environ. 2018, 645, 1361–1370. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Zhou, Y.; Jiang, Z.; Hu, J.; Zhang, X.; Zhou, J.; Wang, G. Changes of aquatic vegetation in Lake Taihu since 1960s. J. Lake Sci. 2017, 29, 351–362. (In Chinese) [Google Scholar]
- Bennion, H.; Sayer, C.D.; Clarke, S.J.; Davidson, T.A.; Rose, N.L.; Goldsmith, B.; Rawcliffe, R.; Burgess, A.; Clarke, G.; Turner, S. Sedimentary macrofossil records reveal ecological change in English lakes: Implications for conservation. J. Paleolimnol. 2018, 60, 329–348. [Google Scholar] [CrossRef] [Green Version]
- Sand-Jensen, K.; Riis, T.; Vestergaard, O.; Larsen, S.E. Macrophyte decline in Danish lakes and streams over the past 100 years. J. Ecol. 2000, 88, 1030–1040. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, X.; Yang, X.; Odgaard, B.V.; Jeppesen, E. Hydrologic and anthropogenic influences on aquatic macrophyte development in a large, shallow lake in China. Freshw. Biol. 2019, 64, 799–812. [Google Scholar] [CrossRef]
- Dong, X.; Kattel, G.; Jeppesen, E. Subfossil cladocerans as quantitative indicators of past ecological conditions in Yangtze River Basin lakes, China. Sci. Total Environ. 2020, 728, 138794. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.; Juggins, S.; Battarbee, R. Matching diatom assemblages in lake sediment cores and modern surface sediment samples: The implications for lake conservation and restoration with special reference to acidified systems. Hydrobiologia 1997, 344, 27–40. [Google Scholar] [CrossRef]
- Villamagna, A.; Murphy, B. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Bennion, H.; Battarbee, R.W.; Sayer, C.D.; Simpson, G.L.; Davidson, T.A. Defining reference conditions and restoration targets for lake ecosystems using palaeolimnology: A synthesis. J. Paleolimnol. 2011, 45, 533–544. [Google Scholar] [CrossRef]
- Salgado, J.; Sayer, C.; Carvalho, L.; Davidson, T.; Gunn, I. Assessing aquatic macrophyte community change through the integration of palaeolimnological and historical data at Loch Leven, Scotland. J. Paleolimnol. 2010, 43, 191–204. [Google Scholar] [CrossRef]



| Index | Summer 2004 | Autumn 2007 | Autumn 2011 | Summer 2017 |
|---|---|---|---|---|
| pH | 9.14 | 8.62 | 8.05 | 8.90 |
| TN (mg/L) | 3.34 | 3.51 | 1.55 | 1.47 |
| TP (mg/L) | 0.14 | 0.167 | 0.113 | 0.093 |
| Water quality grade 1 | Ⅴ | Ⅴ | Ⅳ | Ⅳ |
| Family | Species | Life Span | Nutrient Requirement | Sensitivity to Environment |
|---|---|---|---|---|
| Najadaceae | Najas minor All. | Annual | Nutrient-poor | Sensitive |
| Hydrocharitaceae | Vallisneria natans (Lour.) Hara | Perennial | Meso-to-eutrophic | Medium tolerant |
| Hydrocharitaceae | Vallisneria denseserrulata (Makino) Makino | Perennial | Meso-to-eutrophic | Medium tolerant |
| Characeae | Chara sp. | Annual | Nutrient-poor | Sensitive |
| Hydrocharitaceae | Hydrilla verticillata (Linn. f.) Royle | Perennial | Meso-to-eutrophic | Nontolerant |
| Potamogetonaceae | Potamogeton maackianus A. Bennett | Perennial | Meso-to-eutrophic | Nontolerant |
| Potamogetonaceae | Potamogeton crispus L. | Perennial | Meso-to-eutrophic | Tolerant |
| Ceratophyllaceae Gray | Ceratophyllum demersum L. | Perennial | Meso-to-eutrophic | Tolerant |
| Haloragidaceae | Myriophyllum spicatum L. | Perennial | Middle-eutrophic | Tolerant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Li, L.; Dong, X.; Li, Y.; Kattel, G. Plant Macrofossils Reveal Aquatic Macrophyte Successions of a Typical Shallow Lake (Huanggai Lake, China) in the Past Century. Plants 2022, 11, 1413. https://doi.org/10.3390/plants11111413
Cheng Q, Li L, Dong X, Li Y, Kattel G. Plant Macrofossils Reveal Aquatic Macrophyte Successions of a Typical Shallow Lake (Huanggai Lake, China) in the Past Century. Plants. 2022; 11(11):1413. https://doi.org/10.3390/plants11111413
Chicago/Turabian StyleCheng, Qijuan, Liangfang Li, Xuhui Dong, Yan Li, and Giri Kattel. 2022. "Plant Macrofossils Reveal Aquatic Macrophyte Successions of a Typical Shallow Lake (Huanggai Lake, China) in the Past Century" Plants 11, no. 11: 1413. https://doi.org/10.3390/plants11111413






