Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell Proliferation Assay Using Real-time Cell Analysis (RTCA)
2.5. Multicellular Tumor Spheroid Assay
2.6. Annexin V/Propidium iodide (PI) Apoptosis Assay
2.7. Caspase-3/7 Activity Assay
2.8. γ-H2AX Immunofluorescence Staining
2.9. Real-Time Cell Migration Assay
2.10. Gene Expression Analysis by Quantitative Reverse Transcription PCR (RT-qPCR)
2.11. Quantification of Vascular Endothelial Growth Factor-A (VEGF-A) Secretion
2.12. Zebrafish Embryo Angiogenesis Assay
2.13. Statistical Analysis
3. Results and Discussion
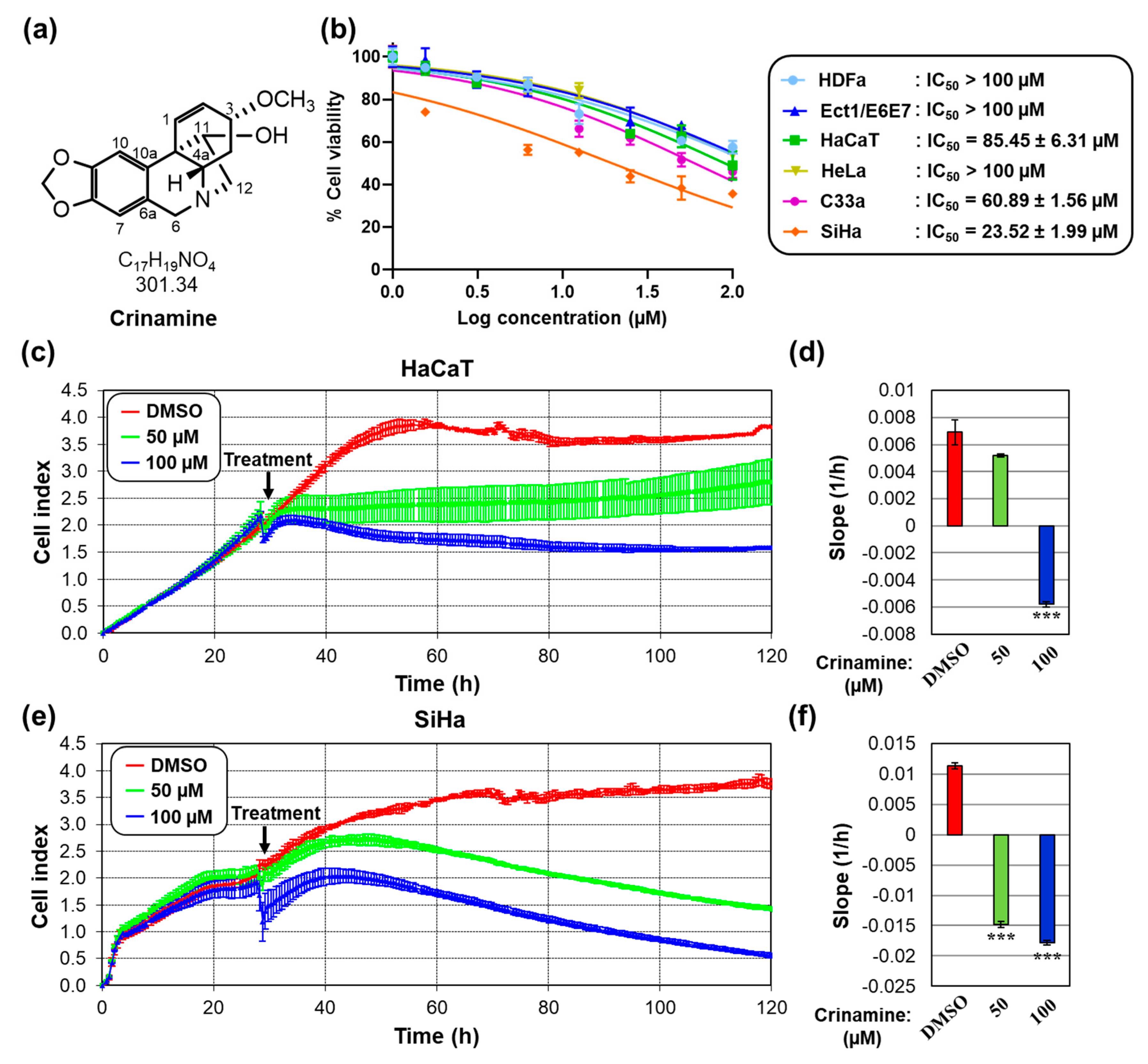
3.1. Crinamine Is More Cytotoxic to Cervical Cancer Cells Compared to Normal Cells
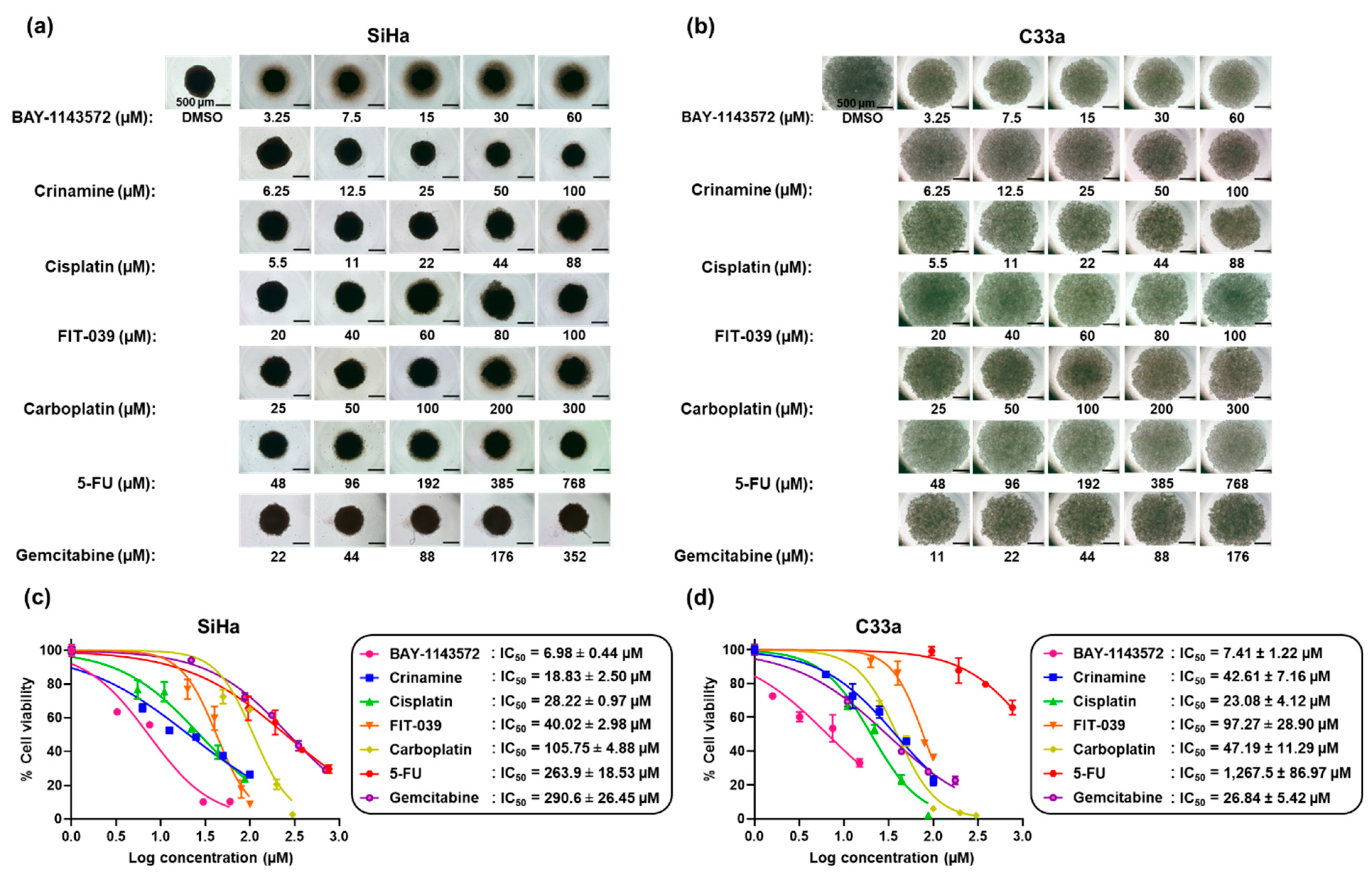
3.2. Crinamine Inhibits Tumor Spheroid Growth with Higher Potency than Existing Chemotherapeutic Drugs
3.3. Crinamine Induces Apoptosis in Cervical Cancer SiHa Cells without Activating DNA Damage
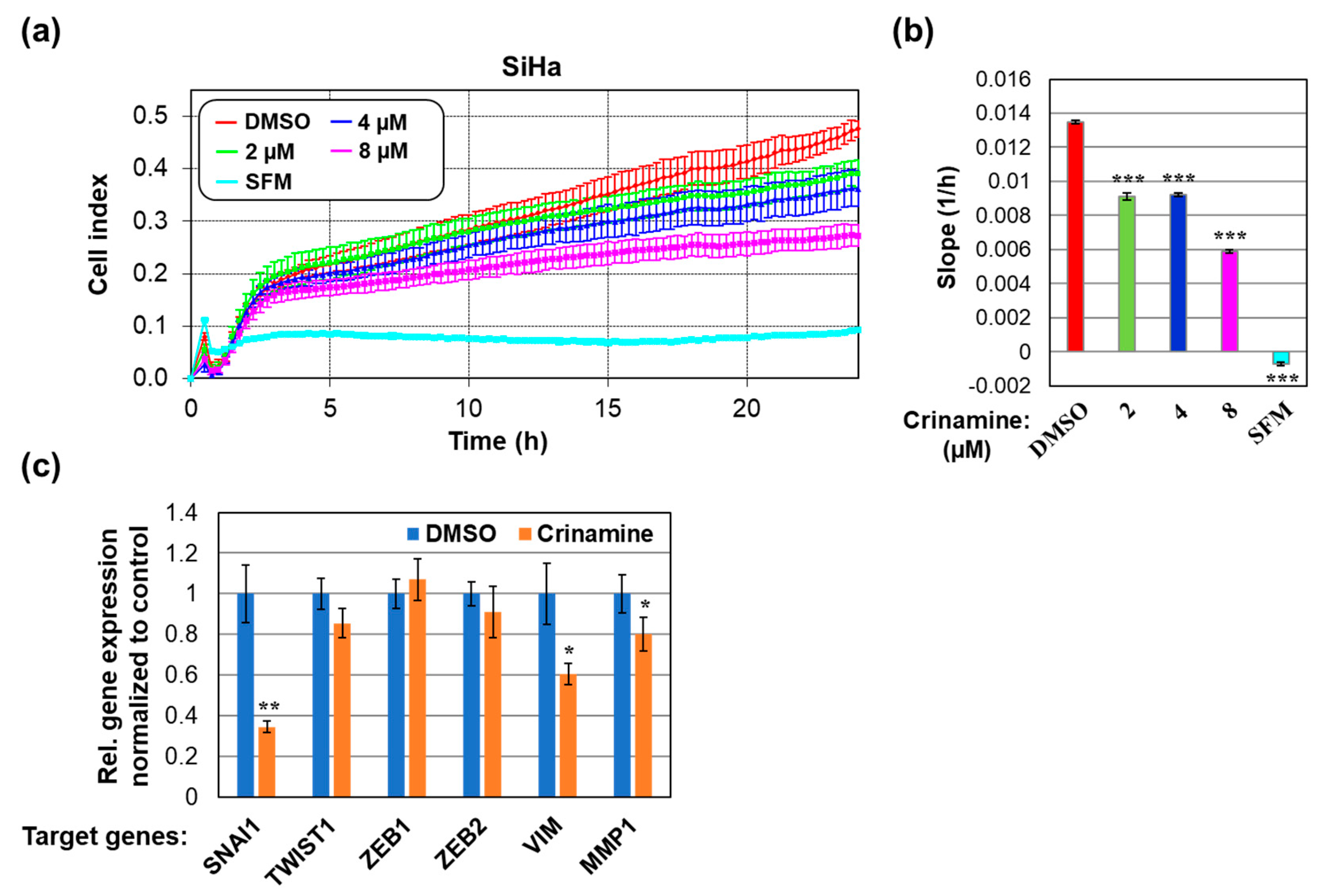
3.4. Crinamine Inhibits SiHa Cells Migration by Downregulating Expression of SNAI1 and VIM
3.5. Crinamine Inhibits VEGF-A Expression and In Vivo Angiogenesis in Zebrafish Embryos
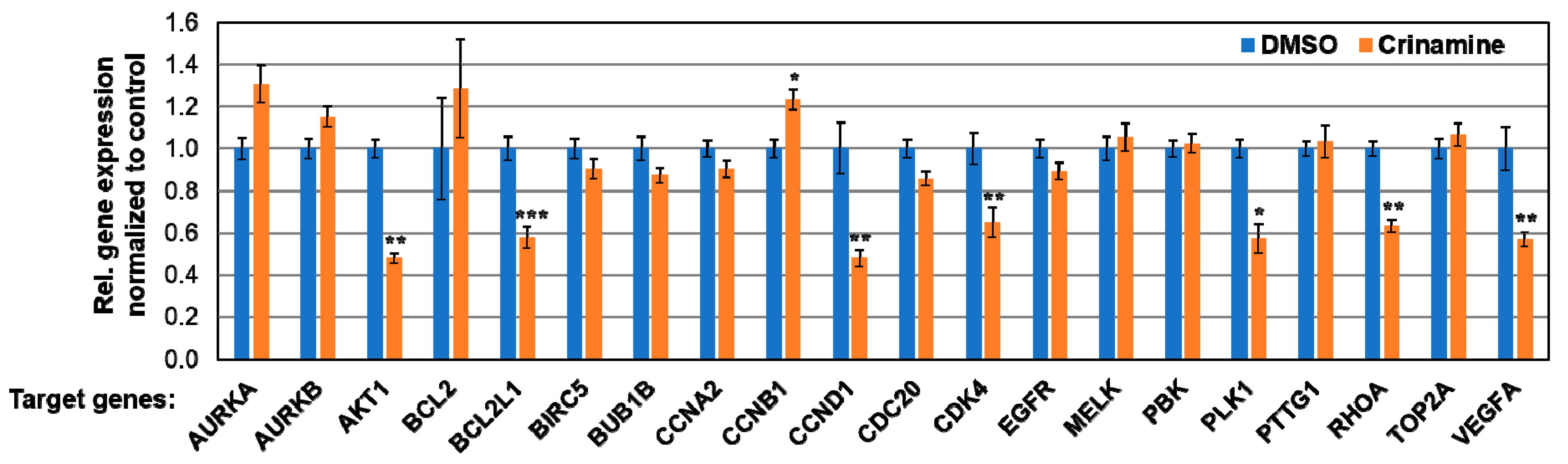
3.6. Crinamine Inhibits mRNA Expression of AKT1, BCL2L1, CCND1, CDK4, PLK1, RHOA, and VEGF-A in SiHa Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Small, W.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenrueang, T.; Promthet, S.; Kamsa-Ard, S.; Pengsaa, P. Cervical cancer in Khon Kaen, Thailand: Analysis of 1990-2014 incidence data and prediction of future trends. Asian Pac. J. Cancer Prev. 2019, 20, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.; Siriarechakul, S.; et al. National and subnational population-based incidence of cancer in Thailand: Assessing cancers with the highest burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975-2016; National Cancer Institute: Bethesda, MD, USA, 2019.
- Monk, B.J.; Sill, M.W.; McMeekin, D.S.; Cohn, D.E.; Ramondetta, L.M.; Boardman, C.H.; Benda, J.; Cella, D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2009, 27, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant. Sci. 2015. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical cancer: An overview of pathophysiology and management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2013, 30, 849–868. [Google Scholar] [CrossRef]
- He, M.; Qu, C.; Gao, O.; Hu, X.; Hong, X. Biological and pharmacological activities of Amaryllidaceae alkaloids. RSC Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Takla, S.S.; Shawky, E.; Hammoda, H.M.; Darwish, F.A. Green techniques in comparison to conventional ones in the extraction of Amaryllidaceae alkaloids: Best solvents selection and parameters optimization. J. Chromatogr. A 2018, 1567, 99–110. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Sharmin, R.; Uddin, M.N.; Rana, M.S.; Ahmed, N.U. Antibacterial, antioxidant and cytotoxic properties of Crinum asiaticum bulb extract. Bangla. J. Microbiol. 2012, 28, 1–5. [Google Scholar] [CrossRef]
- Endo, Y.; Sugiura, Y.; Funasaki, M.; Kagechika, H.; Ishibashi, M.; Ohsaki, A. Two new alkaloids from Crinum asiaticum var. japonicum. J. Nat. Med. 2019, 73, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Smitinand, T. Thai Plant Names, rev. ed.; Office of the Forest Herbarium, Department of Natural Park, Wildlife and Plant Conservation: Bangkok, Thailand, 2014; p. 160.
- Sun, Q.; Shen, Y.-H.; Tian, J.-M.; Tang, J.; Su, J.; Liu, R.-H.; Li, H.-L.; Xu, X.-K.; Zhang, W.-D. Chemical constituents of Crinum asiaticum L. var. sinicum Baker and their cytotoxic activities. Chem. Biodivers. 2009, 6, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Angerhofer, C.K.; Chai, H.; Pezzuto, J.M.; Cordell, G.A.; Ruangrungsi, N. Cytotoxic and antimalarial alkaloids from the bulbs of Crinum amabile. J. Nat. Prod. 1993, 56, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Nair, J.J.; Codina, C.; Bastida, J.; Pandey, S.; Gerasimoff, J.; Griffin, C. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry 2007, 68, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, O.B.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. New crinine-type alkaloids with inhibitory effect on induction of inducible nitric oxide synthase from Crinum yemense. J. Nat. Prod. 2004, 67, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, E.J.; Park, M.H.; Badarch, U.; Woldemichael, G.M.; Beutler, J.A. Crinamine from Crinum asiaticum var. japonicum inhibits hypoxia inducible factor-1 activity but not activity of hypoxia inducible factor-2. Biol. Pharm. Bull. 2006, 29, 2140–2142. [Google Scholar] [CrossRef] [PubMed]
- Piboonprai, K.; Khumkhrong, P.; Khongkow, M.; Yata, T.; Ruangrungsi, N.; Chansriniyom, C.; Iempridee, T. Anticancer activity of arborinine from Glycosmis parva leaf extract in human cervical cancer cells. Biochem. Biophys. Res. Commun. 2018, 500, 866–872. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. [Google Scholar] [CrossRef]
- Aldo, P.; Marusov, G.; Svancara, D.; David, J.; Mor, G. Simple Plex(TM): A novel multi-analyte, automated microfluidic immunoassay platform for the detection of human and mouse cytokines and chemokines. Am. J. Reprod. Immunol. 2016, 75, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Nugitrangson, P.; Puthong, S.; Iempridee, T.; Pimtong, W.; Pornpakakul, S.; Chanchao, C. In vitro and in vivo characterization of the anticancer activity of Thai stingless bee (Tetragonula laeviceps) cerumen. Exp. Biol. Med. 2016, 241, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Sutherland, R. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B. Multicellular resistance: A paradigm for clinical resistance? Crit. Rev. Oncol. Hematol. 2000, 36, 193–207. [Google Scholar] [CrossRef]
- Dufau, I.; Frongia, C.; Sicard, F.; Dedieu, L.; Cordelier, P.; Ausseil, F.; Ducommun, B.; Valette, A. Multicellular tumor spheroid model to evaluate spatio-temporal dynamics effect of chemotherapeutics: Application to the gemcitabine/CHK1 inhibitor combination in pancreatic cancer. BMC Cancer 2012. [Google Scholar] [CrossRef] [PubMed]
- Lücking, U.; Scholz, A.; Lienau, P.; Siemeister, G.; Kosemund, D.; Bohlmann, R.; Briem, H.; Terebesi, I.; Meyer, K.; Prelle, K.; et al. Identification of Atuveciclib (BAY 1143572), the first highly selective, clinical PTEFb/CDK9 inhibitor for the treatment of cancer. ChemMedChem 2017, 12, 1776–1793. [Google Scholar] [CrossRef]
- Ajiro, M.; Sakai, H.; Onogi, H.; Yamamoto, M.; Sumi, E.; Sawada, T.; Nomura, T.; Kabashima, K.; Hosoya, T.; Hagiwara, M. CDK9 inhibitor FIT-039 suppresses viral oncogenes E6 and E7 and has a therapeutic effect on HPV-induced neoplasia. Clin. Cancer Res. 2018, 24, 4518–4528. [Google Scholar] [CrossRef]
- Kurvinen, K.; Syrjänen, K.; Syrjänen, S. p53 and bcl-2 proteins as prognostic markers in human papillomavirus-associated cervical lesions. J. Clin. Oncol. 1996, 14, 2120–2130. [Google Scholar] [CrossRef]
- Dimitrakakis, C.; Kymionis, G.; Diakomanolis, E.; Papaspyrou, I.; Rodolakis, A.; Arzimanoglou, I.; Leandros, E.; Michalas, S. The possible role of p53 and bcl-2 expression in cervical carcinomas and their premalignant lesions. Gynecol. Oncol. 2000, 77, 129–136. [Google Scholar] [CrossRef]
- Tuohetimulati, G.; Zhu, M.; Chen, J.; Niyazi, M. Expressions and clinical significance of Bcl-2, Bcl-xL and c-IAP1 protein in cervical cancer. Int. J. Clin. Exp. Med. 2018, 11, 12361–12367. [Google Scholar]
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longoria, T.C.; Tewari, K.S. Pharmacologic management of advanced cervical cancer: Antiangiogenesis therapy and immunotherapeutic considerations. Drugs 2015, 75, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, X.; Zang, D.; Lan, X.; Liao, S.; Yang, C.; Zhang, P.; Wu, J.; Li, X.; Liu, N.; et al. Platinum-containing compound platinum pyrithione is stronger and safer than cisplatin in cancer therapy. Biochem. Pharmacol. 2016, 116, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, C.; Karnik, A.; McNulty, J.; Pandey, S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol. Cancer Ther. 2011, 10, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, N.; Pearson, H.B.; Burrows, A. Investigating Metastasis Using In Vitro Platforms. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Qureshi, R.; Arora, H.; Rizvi, M.A. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Letters 2015, 356, 321–331. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.; Datta, P. Regulation of EMT in colorectal cancer: A culprit in metastasis. Cancers 2017, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The role of Snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The role of vimentin intermediate filaments in the progression of lung cancer. Am. J. Respir. Cell Mol. Biol. 2013, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Du, Y.; Beckford, J.; Alachkar, H. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J. Transl. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Tewari, K.S. Targeting angiogenesis in advanced cervical cancer. Ther. Adv. Med. Oncol. 2014, 6, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, S.I.; Lee, M.; Kim, H.S.; Kim, J.W.; Park, N.H.; Song, Y.S. Bevacizumab efficacy and recurrence pattern of persistent and metastatic cervical cancer. In Vivo 2019, 33, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). The Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lei, Y.; Cong, C.; Tan, D.; Zhou, X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol. Med. Rep. 2019, 19, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, B.I.; Steine, S.J.; Sandvei, R.; Molven, A.; Laerum, O.D. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: Frequent PIK3CA amplification and AKT phosphorylation. Int. J. Cancer 2006, 118, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.H.; Yu, M.M.Y.; Lo, K.W.K.; Yim, S.F.; Chung, T.K.H.; Wong, Y.F. Alteration of cyclin D1 and CDK4 gene in carcinoma of uterine cervix. Cancer Lett. 2001, 166, 199–206. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, T.; Assani, G.; Ling, H.; Zhou, Q.; Zeng, Y.; Zhou, F.; Zhou, Y. Ribociclib, a selective cyclin D kinase 4/6 inhibitor, inhibits proliferation and induces apoptosis of human cervical cancer in vitro and in vivo. Biomed. Pharmacother. 2019. [Google Scholar] [CrossRef]
- Yang, X.; Chen, G.; Li, W.; Peng, C.; Zhu, Y.; Yang, X.; Li, T.; Cao, C.; Pei, H. Cervical cancer growth is regulated by a c-ABL–PLK1 signaling axis. Cancer Res. 2017, 77, 1142–1154. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Liu, G. Overexpression of RhoA promotes the proliferation and migration of cervical cancer cells. Biosci. Biotechnol. Biochem. 2014, 78, 1895–1901. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumkhrong, P.; Piboonprai, K.; Chaichompoo, W.; Pimtong, W.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Japrung, D.; Asawapirom, U.; Suksamrarn, A.; et al. Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells. Biomolecules 2019, 9, 494. https://doi.org/10.3390/biom9090494
Khumkhrong P, Piboonprai K, Chaichompoo W, Pimtong W, Khongkow M, Namdee K, Jantimaporn A, Japrung D, Asawapirom U, Suksamrarn A, et al. Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells. Biomolecules. 2019; 9(9):494. https://doi.org/10.3390/biom9090494
Chicago/Turabian StyleKhumkhrong, Phattharachanok, Kitiya Piboonprai, Waraluck Chaichompoo, Wittaya Pimtong, Mattaka Khongkow, Katawut Namdee, Angkana Jantimaporn, Deanpen Japrung, Udom Asawapirom, Apichart Suksamrarn, and et al. 2019. "Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells" Biomolecules 9, no. 9: 494. https://doi.org/10.3390/biom9090494




