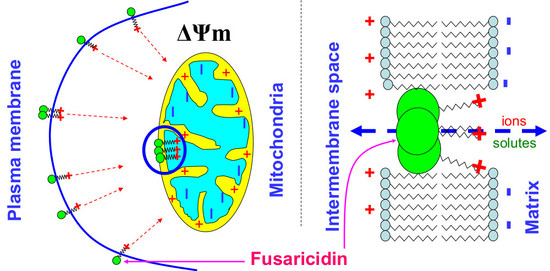
Fusaricidin-Type Compounds Create Pores in Mitochondrial and Plasma Membranes of Mammalian Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sperm Cells
2.3. Paenibacillus Polymyxa Strains
2.4. HPLC and Mass Spectrometry Analysis
2.5. Isolation and Purification of Rat Liver Mitochondria
2.6. Recording of the Permeabilization of Mitochondrial Membranes
2.7. Assessment of Membrane Potential (ΔΨm)
2.8. Measurements of the Rate of Oxygen Consumption by Isolated Mitochondria
2.9. Registration of Superoxide Anion Production
2.10. Measurement of the Release of Cytochrome C From Mitochondria
2.11. Measurement of the Size of Pores in the Mitochondrial Inner Membrane
2.12. Assay of the Toxicity of Fusaricidins to Sperm Cells
2.13. Measurement of ATP Release from Sperm Cells
2.14. Statistical Analysis
3. Results
3.1. RS10 and I/Sim Extracts Induce Mitochondrial Swelling
3.2. RS10 Extract Permeabilizes Mitochondrial Membranes in an mPTP-Independent and ΔΨm-Dependent Manner
3.3. Content of Fusaricidin-Type Compounds in RS10 and I/Sim Extracts
3.4. Mitochondrial Swelling Induced by Purified FTCs from RS10 and I/Sim Extracts
3.5. Effects of FTCs on Mitochondrial Functions and Integrity
3.6. FTCs Cause Fast Permeabilization of Plasma Membrane to Low-Molecular-Weight Solutes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ΔΨm | mitochondrial inner membrane potential |
| BSA | bovine serum albumin |
| CsA | cyclosporin A |
| EGTA | ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid |
| FCCP | carbonyl cyanide p-(trifluoromethoxy)phenyl-hydrazone |
| FTC | fusaricidin-type compound |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| IMM | inner mitochondria membrane |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1´,3,3′’-tetraethylbenzimidazolylcarbocyanine iodide |
| MCLA | 7-dihydro-2-methyl-6-(4-methoxyphenyl)imidazol[1,2-a]pyrazine-3-one |
| MDCL | MCLA-derived chemiluminescence |
| mPTP | mitochondrial permeability transition pore |
| PI | propidium iodide |
| PEG | polyethylene glycol |
| TMPD | N,N,N’N’-tetramethyl-p-phenylenediamide |
| TPP+ | tetraphenylphosphonium chloride |
Appendix A

References
- Kurusu, K.; Ohba, K.; Arai, T.; Fukushima, K. New peptide antibiotics LI-F03, F04, F05, F07, and F08, produced by Bacillus polymyxa. I. Isolation and characterization. J. Antibiot. 1987, 40, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Kaneda, M.; Fusaricidin, A. A new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Kaneda, M. Fusaricidins B, C, and D, new depsipeptide antibiotics produced by Bacillus polymyxa KT-8: Isolation, structure elucidation and biological activity. J. Antibiot. 1997, 50, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Park, S.Y.; Kim, R.; Lee, C.H.; Kim, J.F.; Park, S.H. Identification and functional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochem. Biophys. Res. Commun. 2008, 365, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Fukai, T.; Konishi, M.; Uno, J.; Kurusu, K.; Nomura, T. LI-F antibiotics, a family of antifungal cyclic depsipeptides produced by Bacillus polymyxa L-1129. Heterocycles 2000, 53, 1533–1549. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A gold mine of antibiotic candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Stawikowski, M.; Cudic, P. Lipodepsipeptide antibiotic fusaricidin and its analogues. Total solid-phase and biological activity. Adv. Exp. Med. Biol. 2009, 611, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.H.; Jensen, S.E. Paenibacillus polymyxa produces fusaricidin-type antifungal antibiotics active against Leptosphaeria maculans, the causative agent of blackleg disease of canola. Can. J. Microbiol. 2002, 48, 159–169. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Kim, Y.S.; Lee, Y.S. Biocontrol of late blight and plant growth promotion in tomato using rhizobacterial isolates. J. Microbiol. Biotechnol. 2013, 23, 897–904. [Google Scholar] [CrossRef]
- Mikkola, R.; Andersson, M.A.; Grigoriev, P.; Heinonen, M.; Salkinoja-Salonen, M.S. The toxic mode of action of cyclic lipodepsipeptide fusaricidins, produced by Paenibacillus polymyxa, toward mammalian cells. J. Appl. Microbiol. 2017, 123, 436–449. [Google Scholar] [CrossRef]
- Ekman, J.V.; Kruglov, A.; Andersson, M.A.; Mikkola, R.; Raulio, M.; Salkinoja-Salonen, M. Cereulide produced by Bacillus cereus increases the fitness of the producer organism in low-potassium environments. Microbiology 2012, 158, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinopoulos, C. Mitochondrial permeability transition pore: Back to the drawing board. Neurochem. Int. 2018, 117, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, S.; Brdiczka, D. The permeability transition pore in cell death. Apoptosis 2007, 12, 841–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemeshko, V.V.; Arias, M.; Orduz, S. Mitochondria permeabilization by a novel polycation peptide BTM-P1. J. Biol. Chem. 2005, 280, 15579–15586. [Google Scholar] [CrossRef]
- D’Agostino, D.M.; Ranzato, L.; Arrigoni, G.; Cavallari, I.; Belleudi, F.; Torrisi, M.R.; Silic-Benussi, M.; Ferro, T.; Petronilli, V.; Marin, O.; et al. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J. Biol. Chem. 2002, 277, 34424–34433. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.R.; Gudz, T.I.; Novgorodov, S.A.; Erdahl, W.L. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J. Biol. Chem. 1995, 270, 4923–4932. [Google Scholar] [CrossRef]
- Andersson, M.A.; Mikkola, R.; Rasimus, S.; Hoornstra, D.; Salin, P.; Rahkila, R.; Heikkinen, M.; Mattila, S.; Peltola, J.; Kalso, S.; et al. Boar spermatozoa as a biosensor for detecting toxic substances in indoor dust and aerosols. Toxicol. In Vitro 2010, 24, 2041–2052. [Google Scholar] [CrossRef]
- Johnson, D.; Lardy, H.A. Isolation of liver or kidney mitochondria. Methods Enzymol. 1967, 10, 94–96. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Kambayashi, Y.; Ogino, K. Reestimation of Cypridina luciferin analogs (MCLA) as a chemiluminescence probe to detect active oxygen species: Cautionary note for use of MCLA. J. Toxicol. Sci. 2003, 28, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kharechkina, E.S.; Nikiforova, A.B.; Kruglov, A.G. Pyridine nucleotides regulate the superoxide anion flash upon permeabilization of mitochondrial membranes: An MCLA-based study. Free Radic Biol Med 2018, 124, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, A.G.; Subbotina, K.B.; Saris, N.E. Redox-cycling compounds can cause the permeabilization of mitochondrial membranes by mechanisms other than ROS production. Free Radic. Biol. Med. 2008, 44, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, A.G.; Teplova, V.V.; Saris, N.E. The effect of the lipophilic cation lucigenin on mitochondria depends on the site of its reduction. Biochem. Pharmacol. 2007, 74, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Jääskeläinen, E.L.; Shaheen, R.; Pirhonen, T.; Wijnands, L.M.; Salkinoja-Salonen, M.S. Sperm bioassay for rapid detection of cereulide-producing Bacillus cereus in food and related environments. Int. J. Food Microbiol. 2004, 94, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Saks, V.; Margreiter, R.; Grimm, M. Synchronism in mitochondrial ROS flashes, membrane depolarization and calcium sparks in human carcinoma cells. Biochim. Biophys. Acta Bioenergy 2017, 1858, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.A.; Nikulin, M.; Köljalg, U.; Andersson, M.C.; Rainey, F.; Reijula, K.; Hintikka, E.L.; Salkinoja-Salonen, M. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 1997, 63, 387–393. [Google Scholar] [Green Version]
- Lai, B.; Agarwal, R.; Nelson, L.D.; Swaminathan, S.; London, E. Low pH-induced pore formation by the T domain of botulinum toxin type A is dependent upon NaCl concentration. J. Membr. Biol. 2010, 236, 191–201. [Google Scholar] [CrossRef]
- Lonchamp, E.; Dupont, J.L.; Wioland, L.; Courjaret, R.; Mbebi-Liegeois, C.; Jover, E.; Doussau, F.; Popoff, M.R.; Bossu, J.L.; de Barry, J.; et al. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS ONE 2010. [Google Scholar] [CrossRef]
- Liu, S.I.; Cheng, H.H.; Huang, C.J.; Chang, H.C.; Chen, W.C.; Chen, I.S.; Hsu, S.S.; Chang, H.T.; Huang, J.K.; Chen, J.S.; et al. Melittin-induced [Ca2+]i increases and subsequent death in canine renal tubular cells. Hum. Exp. Toxicol. 2008, 27, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Ahmad, A.; Asthana, N.; Azmi, S.; Srivastava, R.M.; Srivastava, S.; Verma, R.; Vishwakarma, A.L.; Ghosh, J.K. Cell-selective lysis by novel analogues of melittin against human red blood cells and Escherichia coli. Biochemistry 2010, 49, 7920–7929. [Google Scholar] [CrossRef] [PubMed]
- Weidema, A.F.; Kropacheva, T.N.; Raap, J.; Ypey, D.L. Membrane permeabilization of a mammalian neuroendocrine cell type (PC12) by the channel-forming peptides zervamicin, alamethicin, and gramicidin. Chem. Biodivers. 2007, 4, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Kourie, J.I.; Shorthouse, A.A. Properties of cytotoxic peptide-formed ion channels. Am. J. Physiol. Cell Physiol. 2000, 278, 1063–1087. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.O., Jr.; Richards, F.M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature 1982, 300, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Chugh, J.K.; Wallace, B.A. Peptaibols: Models for ion channels. Biochem. Soc. Trans. 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Broekemeier, K.M.; Iben, J.R.; LeVan, E.G.; Crouser, E.D.; Pfeiffer, D.R. Pore formation and uncoupling initiate a Ca2+-independent degradation of mitochondrial phospholipids. Biochemistry 2002, 41, 7771–7780. [Google Scholar] [CrossRef]
- Petrosillo, G.; Ruggiero, F.M.; Pistolese, M.; Paradies, G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: Role of cardiolipin. J. Biol. Chem. 2004, 279, 53103–53108. [Google Scholar] [CrossRef]
- Korge, P.; John, S.A.; Calmettes, G.; Weiss, J.N. Reactive oxygen species production induced by pore opening in cardiac mitochondria: The role of complex II. J. Biol. Chem. 2017, 292, 9896–9905. [Google Scholar] [CrossRef] [Green Version]










| Number of Toxic HPLC Fraction | Compound * | Mass Ion [M + H]+ m/z |
|---|---|---|
| 1 | Fusaricidin C | 947.7 |
| Fusaricidin D | 961.6 | |
| 2 | Fusaricidin A | 883.7 |
| Fusaricidin B | 897.6 | |
| 3 | LI-F05a | 897.6 |
| LI-F06b/LI-F05b | 911.6 | |
| LI-F07b | 945.5 | |
| LI-F07a | 931.6 | |
| 4 | LI-F06b/LI-F05b and LI-F07b | 911.6 and 945.6 |
| LI-F08b | 925.6 | |
| Fusaricidin/LI-F ** | 960.5 |
| 1-h Incubation | Motility | ΔΨm (JC1 Staining) | PM Permeability (PI Staining) |
|---|---|---|---|
| Control | ≥60% | ≥98% | ≤5% |
| Ethanol (0.5%) | ≥60% | ≥98% | ≤5% |
| RS10 (µg/mL) 50 | 1–2% | 1–2% | >90% |
| 25 | ~10% | 5–10% | ~60% |
| 12.5 | ~40% | 30–40% | 30–40% |
| 6.25 | ~50% | 40–50% | ≤20% |
| A+B (9.35 µg/mL) | 1–2% | 1–2% | ~90% |
| LI-F05a (5.06 µg/mL) | 5–10% | 5–10% | ≥70% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikkola, R.; Andersson, M.; Kharechkina, E.; Kruglova, S.; Kruglov, A. Fusaricidin-Type Compounds Create Pores in Mitochondrial and Plasma Membranes of Mammalian Cells. Biomolecules 2019, 9, 433. https://doi.org/10.3390/biom9090433
Mikkola R, Andersson M, Kharechkina E, Kruglova S, Kruglov A. Fusaricidin-Type Compounds Create Pores in Mitochondrial and Plasma Membranes of Mammalian Cells. Biomolecules. 2019; 9(9):433. https://doi.org/10.3390/biom9090433
Chicago/Turabian StyleMikkola, Raimo, Maria Andersson, Ekaterina Kharechkina, Svetlana Kruglova, and Alexey Kruglov. 2019. "Fusaricidin-Type Compounds Create Pores in Mitochondrial and Plasma Membranes of Mammalian Cells" Biomolecules 9, no. 9: 433. https://doi.org/10.3390/biom9090433