Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Microarray
2.2. Immunohistochemistry
2.3. Immunohistochemistry-Scoring
2.4. The Cancer Genome Atlas Dataset Analysis
2.5. Cell Culture
2.6. Preparation of Tobacco Extract
2.7. MTT Assay
2.8. Reverse Transcriptase-Polymerase Chain Reaction
2.9. Akt1/2 Gene Silencing
2.10. Cell Cycle Analysis
2.11. Clonogenic Assay
2.12. Migration Assay
2.13. Flow Cytometric Assessment of Cell Viability
2.14. Western Blot Analysis
2.15. Statistical Analysis
3. Results
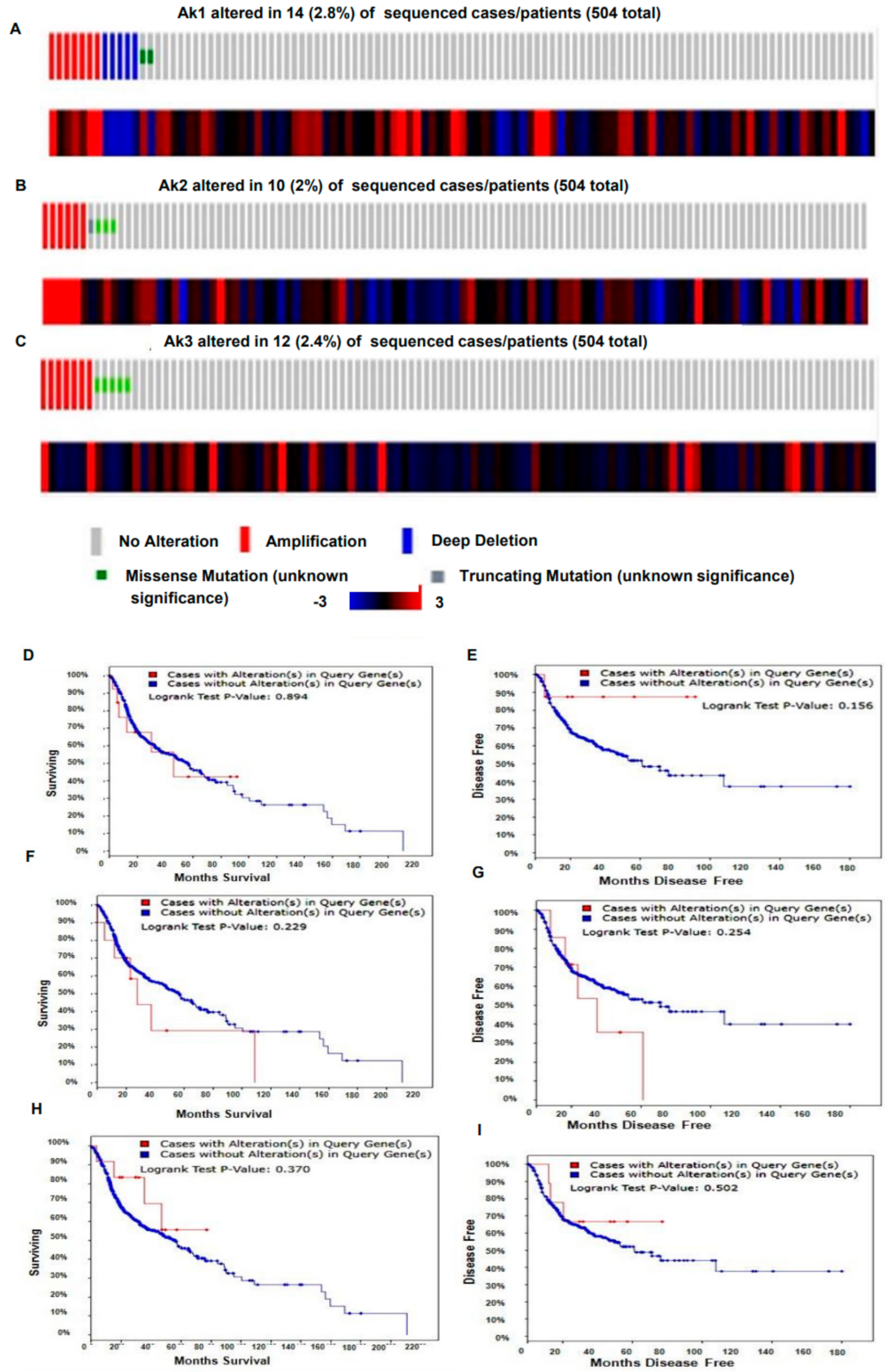
3.1. Overexpression of Akt1 and 2 Isoforms in Oral Cancer Tissues
3.2. Genetic Alteration of Akt1 and 2 Isoforms Was Associated with Poor Overall Survival and Disease-Free Survival
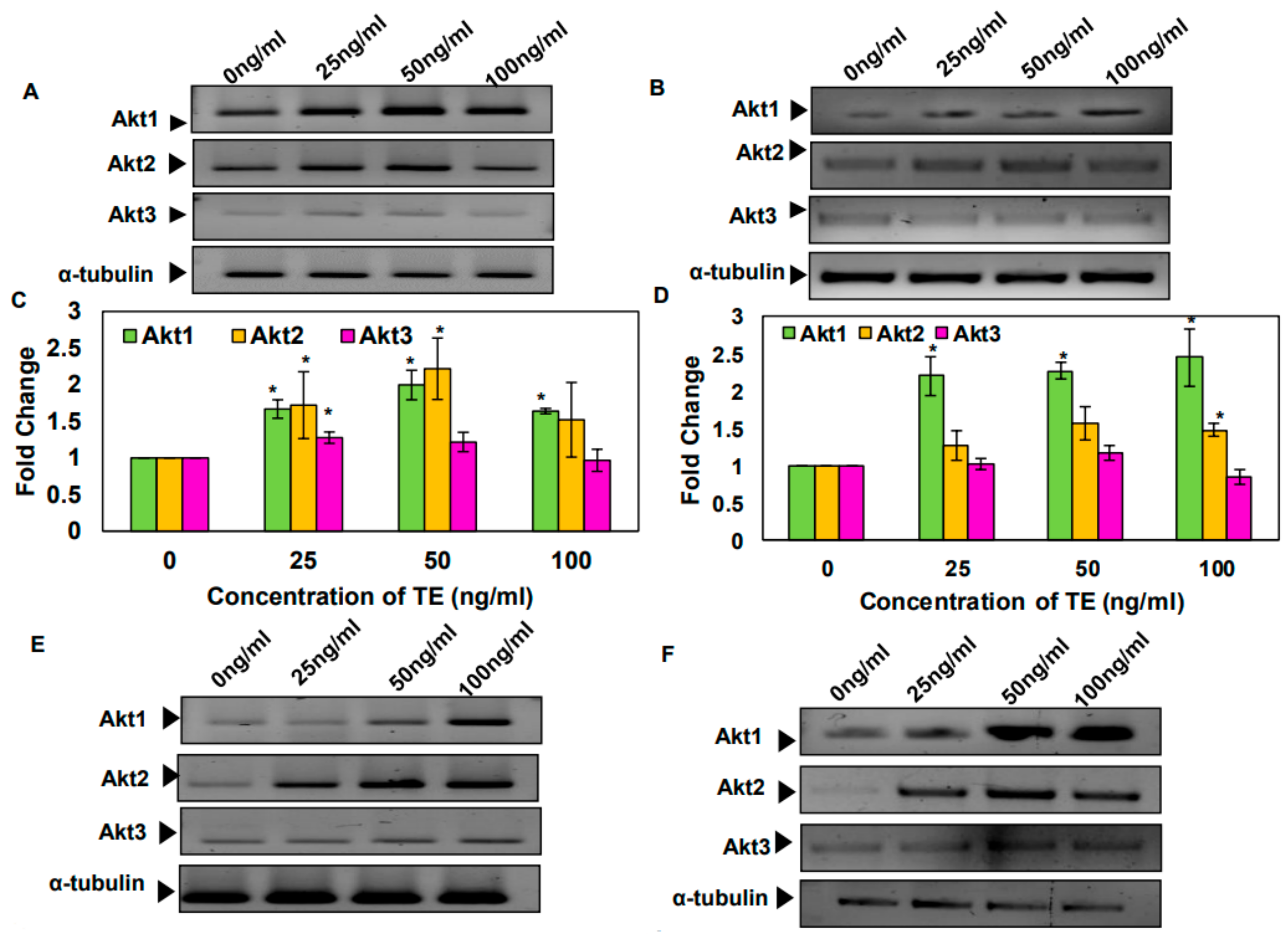
3.3. Tobacco and Its Components Increase the mRNA Levels of Akt1 and 2 Isoforms in SAS and KB Cells
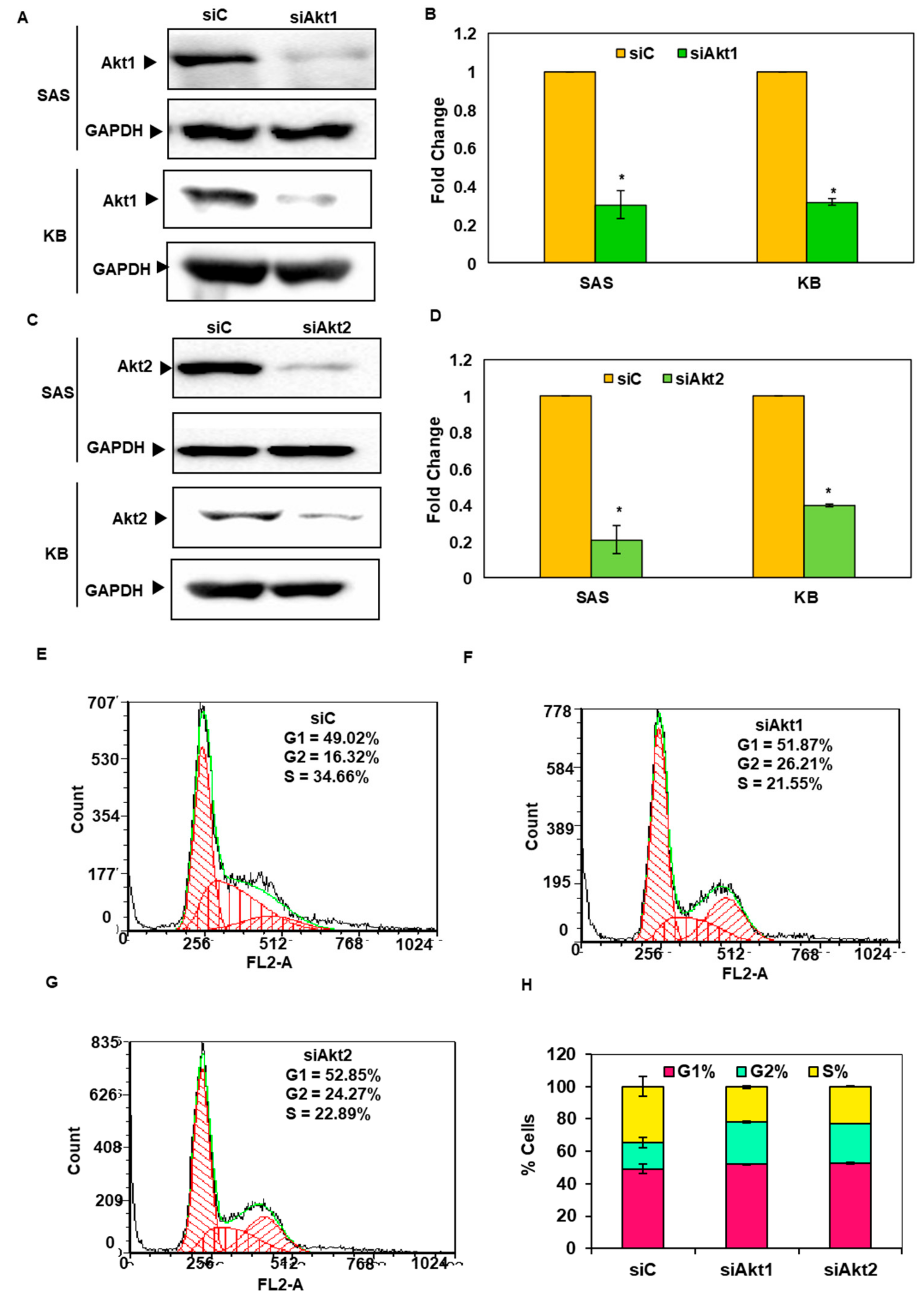
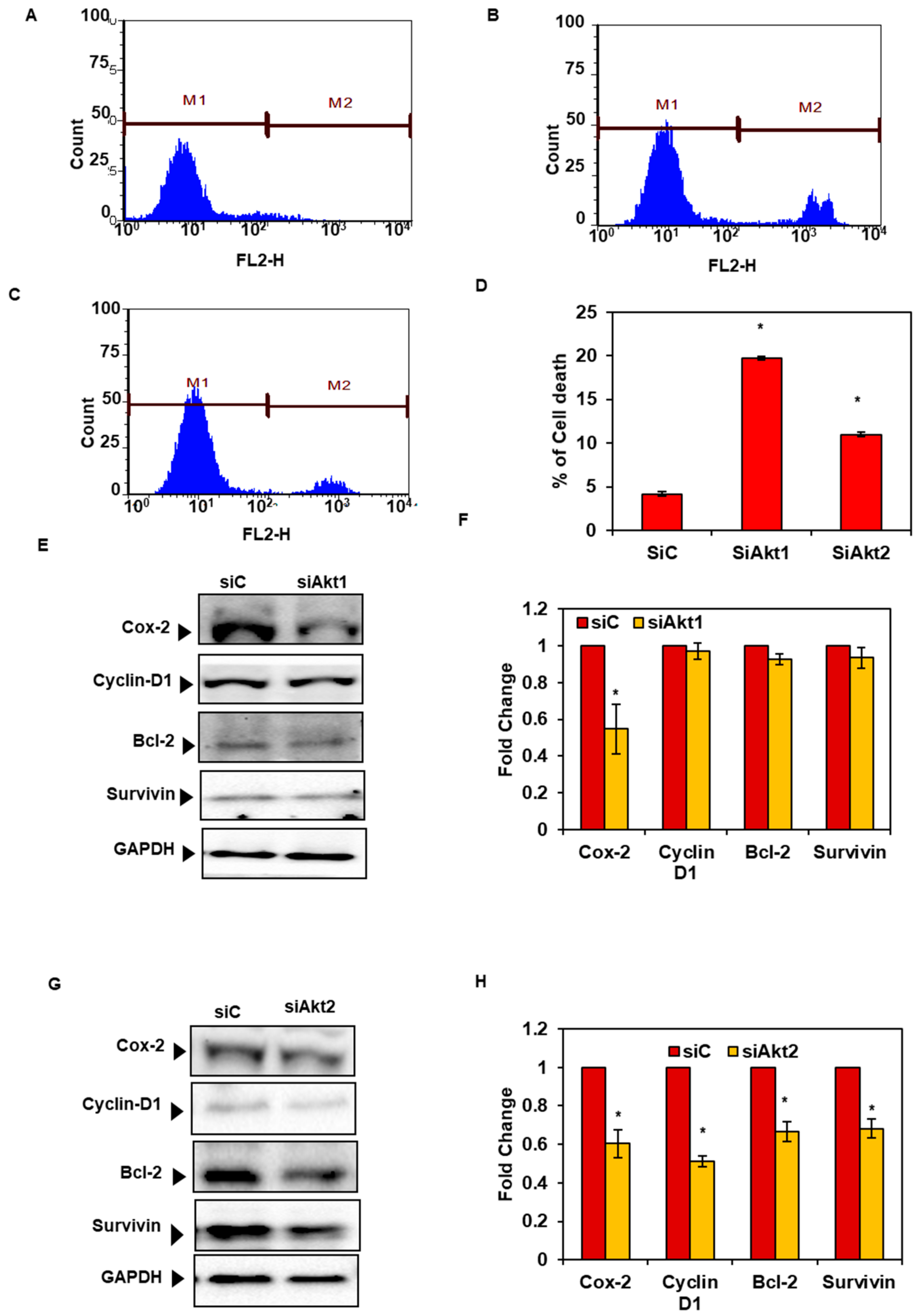
3.4. Silencing of Akt1 and 2 Isoforms Led to Cell Cycle Arrest in G2/M Phase
3.5. Silencing of Akt1 and Akt2 Isoforms Decreases the Tobacco-Induced Clonogenicity of SAS Cells
3.6. Silencing of Akt1 and Akt2 Isoforms Decreases the Tobacco-Induced Migration of SAS Cells
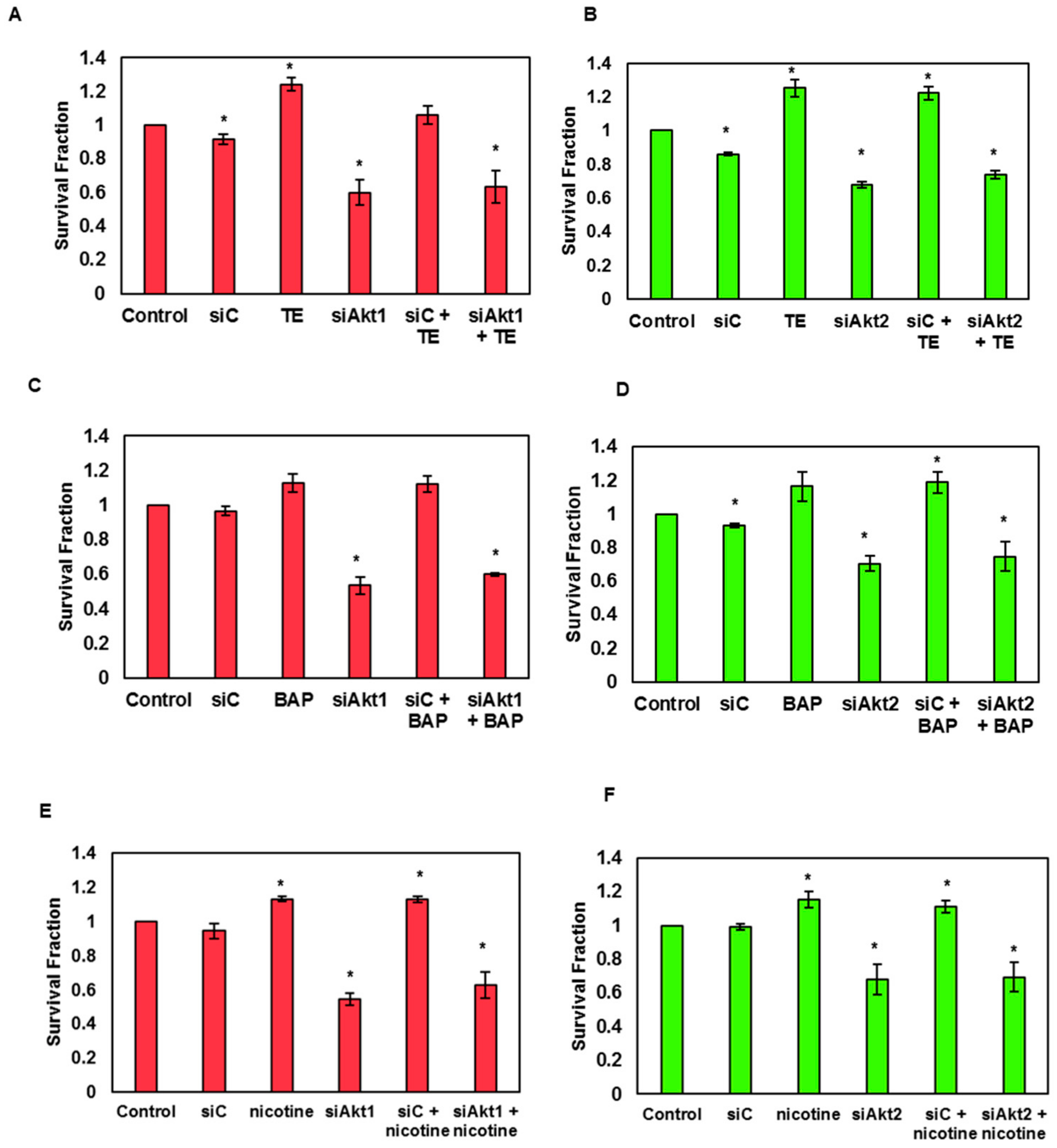
3.7. Silencing of Akt1 and 2 Isoforms Decreased Cell Survival and Expression of Proteins Associated with Cell Survival and Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Gorsky, M. Epidemiologic and Demographic Update in Oral Cancer: California and National Data—1973 to 1985. J. Am. Dent. Assoc. 1990, 120, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- De Stefani, E.; Victora, C.G.; Castelletto, R.; Castellsagué, X.; Muñoz, N.; Rolón, P.A.; Quintana, M.J. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int. J. Cancer 1999, 82, 657–664. [Google Scholar]
- Schlecht, N.F. Prognostic value of human papillomavirus in the survival of head and neck cancer patients: An overview of the evidence. Oncol. Rep. 2005, 14, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, N.; Tyagi, B.B.; Raina, V. Cancer incidences in rural Delhi—2004–05. Asian Pac. J. Cancer Prev. 2010, 11, 73–77. [Google Scholar]
- Bell, R.B.; Kademani, D.; Homer, L.; Dierks, E.J.; Potter, B.E. Tongue Cancer: Is There a Difference in Survival Compared With Other Subsites in the Oral Cavity? J. Oral Maxillofac. Surg. 2007, 65, 229–236. [Google Scholar] [CrossRef]
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef]
- Mydlarz, W.K.; Hennessey, P.T.; Califano, J.A. Advances and Perspectives in the Molecular Diagnosis of Head and Neck Cancer. Expert Opin. Med. Diagn. 2010, 4, 53–65. [Google Scholar] [CrossRef]
- West, K.A.; Clark, A.S.; Linnoila, I.R.; Yang, X.; Harris, C.; Belinsky, S.; Dennis, P.A.; Brognard, J.; Swain, S.M. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Investig. 2003, 111, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Neasta, J.; Ben Hamida, S.; Yowell, Q.V.; Carnicella, S.; Ron, D. AKT Signaling Pathway in the Nucleus Accumbens Mediates Excessive Alcohol Drinking Behaviors. Boil. Psychiatry 2011, 70, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Sterk, R.T.; DeHaro, S.A.; Ozbun., M.A. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J. Virol. 2013, 87, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT Kinases in Cancer: Implications for Therapeutic Targeting. Advan. Cancer Res. 2005, 94, 29–86. [Google Scholar]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase–AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. γ-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Ophiopogonin D modulates multiple oncogenic signaling pathways, leading to suppression of proliferation and chemosensitization of human lung cancer cells. Phytomedicine 2018, 40, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Nam, D.; Um, J.-Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.H.; Lee, S.G.; Chinnathambi, A.; Alharbi, S.A.; Yang, W.M.; Um, J.Y.; et al. Isorhynchophylline, a Potent Plant Alkaloid, Induces Apoptotic and Anti-Metastatic Effects in Human Hepatocellular Carcinoma Cells through the Modulation of Diverse Cell Signaling Cascades. Int. J. Mol. Sci. 2017, 18, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis 2011, 16, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sung, B.; Kunnumakkara, A.B.; Chaturvedi, M.M.; Aggarwal, B.B. SH-5, an AKT inhibitor potentiates apoptosis and inhibits invasion through the suppression of anti-apoptotic, proliferative and metastatic gene products regulated by IκBα kinase activation. Biochem. Pharmacol. 2008, 76, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Lorenzato, A.; Biolatti, M.; Delogu, G.; Capobianco, G.; Farace, C.; Dessole, S.; Cossu, A.G.M.; Tanda, F.; Madeddu, R.; Olivero, M.; et al. AKT activation drives the nuclear localization of CSE1L and a pro-oncogenic transcriptional activation in ovarian cancer cells. Exp. Cell Res. 2013, 319, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Rychahou, P.G.; Kang, J.; Gulhati, P.; Doan, H.Q.; Chen, L.A.; Xiao, S.-Y.; Chung, D.H.; Evers, B.M. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 20315–20320. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Ruggeri, B.; Klein, W.M.; Sonoda, G.; Altomare, D.A.; Watson, D.K.; Testa, J.R. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA 1996, 93, 3636–3641. [Google Scholar] [CrossRef]
- Iacovides, D.C.; Johnson, A.B.; Wang, N.; Boddapati, S.; Korkola, J.; Gray, J.W. Identification and Quantification of AKT Isoforms and Phosphoforms in Breast Cancer Using a Novel Nanofluidic Immunoassay. Mol. Cell. Proteom. 2013, 12, 3210–3220. [Google Scholar] [CrossRef]
- Qiu, Z.-X.; Zhang, K.; Qiu, X.-S.; Zhou, M.; Li, W.-M. The Prognostic Value of Phosphorylated AKT Expression in Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e81451. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Golla, R.; Kunnumakkara, A.; Kotoky, J. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-P.; Kok, L.-F.; Wang, P.-H.; Wu, T.S.; Tyan, Y.-S.; Cheng, Y.-W.; Lee, M.-Y.; Yang, S.-F. Scoring of p16INK4a immunohistochemistry based on independent nuclear staining alone can sufficiently distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. Mod. Pathol. 2009, 22, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Schicketanz, K.-H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef]
- De Matos, L.L.; Stabenow, E.; Tavares, M.R.; Ferraz, A.R.; Capelozzi, V.L.; Pinhal, M.A.D.S. Immunohistochemistry quantification by a digital computer-assisted method compared to semiquantitative analysis. Clinics 2006, 61, 417–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, C.-P.; Lee, M.-Y.; Tzeng, S.-L.; Yao, C.-C.; Wang, P.-H.; Cheng, Y.-W.; Chen, S.-L.; Wu, T.S.; Tyan, Y.-S.; Kok, L.-F. Nuclear Receptor Interaction Protein (NRIP) expression assay using human tissue microarray and immunohistochemistry technology confirming nuclear localization. J. Exp. Clin. Cancer Res. 2008, 27, 25. [Google Scholar] [CrossRef]
- Kamoi, S.; Aljuboury, M.I.; Akin, M.-R.; Silverberg, S.G. Immunohistochemical Staining in the Distinction Between Primary Endometrial and Endocervical Adenocarcinomas: Another Viewpoint. Int. J. Gynecol. Pathol. 2002, 21, 217–223. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; Cerami, E.; Sander, C.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Iamaroon, A.; Krisanaprakornkit, S. Overexpression and activation of Akt2 protein in oral squamous cell carcinoma. Oral Oncol. 2009, 45, e175–e179. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Fernandez-Hernando, C.; Cirino, G.; Sessa, W.C. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. USA 2009, 106, 14552–14557. [Google Scholar] [CrossRef]
- Yu, H.; Littlewood, T.; Bennett, M. Akt isoforms in vascular disease. Vasc. Pharmacol. 2015, 71, 57–64. [Google Scholar] [CrossRef]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7, 44244. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, K.; Okazaki, Y.; Tonogi, M.; Tanaka, Y.; Yamane, G.-Y. Activation of PI3K-AKT Pathway in Oral Epithelial Dysplasia and Early Cancer of Tongue. Bull. Tokyo Dent. Coll. 2009, 50, 125–133. [Google Scholar] [CrossRef]
- Massarelli, E.; Liu, D.D.; Lee, J.J.; El-Naggar, A.K.; Muzio, L.L.; Staibano, S.; De Placido, S.; Myers, J.N.; Papadimitrakopoulou, V.A. Akt activation correlates with adverse outcome in tongue cancer. Cancer 2005, 104, 2430–2436. [Google Scholar] [CrossRef]
- Tumino, R.; Vicario, G. Head and neck cancers: Oral cavity, pharynx, and larynx. Epidemiol. Prev. 2004, 28, 28–33. [Google Scholar]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Singh, A.; Padmavathi, G.; Kunnumakkara, A.B. Isoform-specific Role of Akt Kinase in Cancer and its Selective Targeting by Potential Anticancer Natural Agents. Nat. Prod. J. 2017. Ahead of print. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, W.; Fu, X.; Sun, M.; Wei, Q.; Li, D.; Chen, H.; Xiang, J.; Li, H.; Zhang, Y.; et al. Phosphorylated AKT1 is associated with poor prognosis in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 2893. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chu, L.; Zhao, K.; Chen, H.; Xiang, J.; Zhang, Y.; Li, H.; Zhao, W.; Sun, M.; Wei, Q.; et al. A nomogram based on phosphorylated AKT1 for predicting locoregional recurrence in patients with oesophageal squamous cell carcinoma. J. Cancer 2017, 8, 3755–3763. [Google Scholar] [CrossRef][Green Version]
- Miao, X.; Song, Y.; Lv, T.; Zhan, P.; Lv, Y.; Yuan, D. Expression and prognostic value of AKT2 in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2011, 14, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, J.; Castillo, S.; Brognard, J.; Granville, C.A.; Zhang, C.; Gills, J.J.; Sayyah, J.; Dennis, P.A. Tobacco components stimulate Akt-dependent proliferation and NFκB-dependent survival in lung cancer cells. Carcinogenesis 2005, 26, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Sobus, S.L.; Warren, G.W. The biologic effects of cigarette smoke on cancer cells. Cancer 2014, 120, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Stopper, H.; Schmitt, E.; Gregor, C.; Mueller, S.O.; Fischer, W.H. Increased cell proliferation is associated with genomic instability: Elevated micronuclei frequencies in estradiol-treated human ovarian cancer cells. Mutagenesis 2003, 18, 243–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burdick, A.D.; Davis, J.W.; Liu, K.J.; Hudson, L.G.; Shi, H.; Monske, M.L.; Burchiel, S.W. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003, 63, 7825–7833. [Google Scholar]
- Kometani, T.; Yoshino, I.; Miura, N.; Okazaki, H.; Ohba, T.; Takenaka, T.; Shoji, F.; Yano, T.; Maehara, Y. Benzo[a]pyrene promotes proliferation of human lung cancer cells by accelerating the epidermal growth factor receptor signaling pathway. Cancer Lett. 2009, 278, 27–33. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, L.; Chen, Y.; He, W.; Yang, J.; Geng, C.; Liu, T.; Chen, H.; Li, Y. Benzo[a]pyrene promotes gastric cancer cell proliferation and metastasis likely through the Aryl hydrocarbon receptor and ERK-dependent induction of MMP9 and c-myc. Int. J. Oncol. 2016, 49, 2055–2063. [Google Scholar] [CrossRef]
- Grando, S.A. Connections of nicotine to cancer. Nat. Rev. Cancer 2014, 14, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Guo, H.-Y.; Lee, S.-K.; Jeon, B.-H.; Jun, C.-D.; Lee, S.-K.; Park, M.-H.; Kim, E.-C. Effects of nicotine on proliferation, cell cycle, and differentiation in immortalized and malignant oral keratinocytes. J. Oral Pathol. Med. 2005, 34, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, X.; Jin, H.; Liu, G. Nicotine may promote tongue squamous cell carcinoma progression by activating the Wnt/β-catenin and Wnt/PCP signaling pathways. Oncol. Lett. 2017, 13, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.C.; Maier, C.R.; Hobbs, E.A.; Ashmore, A.R.; Linnoila, R.I.; Dennis, P.A. Akt1 deletion prevents lung tumorigenesis by mutant K-ras. Oncogene 2011, 30, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.; Zhou, X.; Maier, C.R.; Patterson, A.D.; Ding, X.; Dennis, P.A. A Cyp2a polymorphism predicts susceptibility to NNK-induced lung tumorigenesis in mice. Carcinogenesis 2011, 32, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Brait, M.; Munari, E.; Lebron, C.; Noordhuis, M.G.; Begum, S.; Michailidi, C.; Gonzalez-Roibon, N.; Maldonado, L.; Sen, T.; Guerrero-Preston, R.; et al. Genome-wide methylation profiling and the PI3K-AKT pathway analysis associated with smoking in urothelial cell carcinoma. Cell Cycle 2013, 12, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.S.; Skeen, J.; Majewski, N.; Di Cristofano, A.; Pandolfi, P.P.; Feliciano, C.S.; Gartel, A.; Hay, N. Activation of Akt/Protein Kinase B Overcomes a G2/M Cell Cycle Checkpoint Induced by DNA Damage. Mol. Cell. Boil. 2002, 22, 7831–7841. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, D.S.; Lee, J.H.; Lee, B.S.; Lee, S.H.; Jung, H.L.; Sung, K.W.; Kim, H.T.; Yoo, K.H.; Koo, H.H. Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci. 2011, 102, 1822–1828. [Google Scholar] [CrossRef]
- DiPaola, R.S. To arrest or not to G(2)-M Cell-cycle arrest: Commentary re: A. K. Tyagi et al., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519, 2002. Clin. Cancer. Res. 2002, 8, 3311–3314. [Google Scholar]
- Barkley, L.R.; Santocanale, C.; Barkley, L. MicroRNA-29a regulates the benzo[a]pyrene dihydrodiol epoxide-induced DNA damage response through Cdc7 kinase in lung cancer cells. Oncogenesis 2013, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-M.; Gweon, E.-J.; Chung, K.-W.; Kim, K.-H.; Cho, H.-S.; Kim, S.-J. Gallotannin regulates apoptosis and COX-2 expression via Akt and p38kinase pathway in human lung cancer cell line, A549. Anim. Cells Syst. 2012, 16, 366–375. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Cheriyan, V.T.; Thulasidasan, A.K.T.; Bava, S.V.; Vinod, B.S.; Prabhu, P.R.; Varghese, R.; Bevin, A.; Venugopal, S.; Anto, R.J. Nicotine-induced survival signaling in lung cancer cells is dependent on their p53 status while its down-regulation by curcumin is independent. Mol. Cancer 2010, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Joy, A.; Kapoor, M.; Georges, J.; Butler, L.; Chang, Y.; Li, C.; Crouch, A.; Smirnov, I.; Nakada, M.; Hepler, J.; et al. The role of AKT isoforms in glioblastoma: AKT3 delays tumor progression. J. Neurooncol. 2016, 130, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mure, H.; Matsuzaki, K.; Kitazato, K.T.; Mizobuchi, Y.; Kuwayama, K.; Kageji, T.; Nagahiro, S. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro. Oncol. 2010, 12, 221–232. [Google Scholar] [CrossRef]
- Endersby, R.; Zhu, X.; Hay, N.; Ellison, D.W.; Baker, S.J. Non-redundant functions for Akt isoforms in astrocyte growth and gliomagenesis in an orthotopic transplantation model. Cancer Res. 2011, 71, 4106–4116. [Google Scholar] [CrossRef]
- Zitzmann, K.; Vlotides, G.; Brand, S.; Lahm, H.; Spottl, G.; Göke, B.; Auernhammer, C.J. Perifosine-mediated Akt inhibition in neuroendocrine tumor cells: Role of specific Akt isoforms. Endocr. Relat. Cancer 2012, 19, 423–434. [Google Scholar] [CrossRef]
- Watkins, A. The Role of Akt Isoforms in BCR-ABL Induced Chronic Myeloid Leukemia. Ph.D. Thesis, Thomas Jefferson University, Philadelphia, PA, USA, 2012. [Google Scholar]
- Hung, C.-S.; Peng, Y.-J.; Wei, P.-L.; Lee, C.-H.; Su, H.-Y.; Ho, Y.-S.; Lin, S.-Y.; Wu, C.-H.; Chang, Y.-J. The α9 Nicotinic Acetylcholine Receptor is the Key Mediator in Nicotine-enhanced Cancer Metastasis in Breast Cancer Cells. J. Exp. Clin. Med. 2011, 3, 283–292. [Google Scholar] [CrossRef]
- Wei, P.-L.; Kuo, L.-J.; Huang, M.-T.; Ting, W.-C.; Ho, Y.-S.; Wang, W.; An, J.; Chang, Y.-J. Nicotine Enhances Colon Cancer Cell Migration by Induction of Fibronectin. Ann. Surg. Oncol. 2011, 18, 1782–1790. [Google Scholar] [CrossRef]
- Di Cello, F.; Flowers, V.L.; Li, H.; Vecchio-Pagan, B.; Gordon, B.; Harbom, K.; Shin, J.; Beaty, R.; Wang, W.; Brayton, C.; et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Mol. Cancer. 2013, 12, 90. [Google Scholar] [CrossRef]
- Warren, G.W.; Singh, A.K.; Gw, W.; Ak, S. Nicotine and lung cancer. J. Carcinog. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, L.; Jin, H.; Xia, Y.; Wang, L.; He, W.; Li, W.; Chen, H. Benzopyrene promotes lung cancer A549 cell migration and invasion through up-regulating cytokine IL8 and chemokines CCL2 and CCL3 expression. Exp. Boil. Med. 2016, 241, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Szabo, E. Fifty Years of Tobacco Carcinogenesis Research: From Mechanisms to Early Detection and Prevention of Lung Cancer. Cancer Prev. Res. 2014, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bulj, Z.; Duchi, S.; Bevilacqua, A.; Gherardi, A.; Dozza, B.; Piccinini, F.; Adalgisa Mariani, G.; Lucarelli, E.; Giannini, S.; Donati, D.; et al. Protein kinase B/AKT isoform 2 drives migration of human mesenchymal stem cells. Int. J. Oncol. 2013, 42, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 2005, 280, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Calamito, M.; Juntilla, M.M.; Thomas, M.; Northrup, D.L.; Rathmell, J.; Birnbaum, M.J.; Koretzky, G.; Allman, D. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood 2010, 115, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Haggblad Sahlberg, S.; Mortensen, A.C.; Haglof, J.; Engskog, M.K.; Arvidsson, T.; Pettersson, C.; Glimelius, B.; Stenerlow, B.; Nestor, M. Different functions of AKT1 and AKT2 in molecular pathways, cell migration and metabolism in colon cancer cells. Int. J. Oncol. 2017, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gately, S. The Contributions of Cyclooxygenase-2 to Tumor Angiogenesis. Cancer Metastasis Rev. 2000, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- St-Germain, M.-È; Gagnon, V.; Parent, S.; Asselin, E. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-κB/IκB pathway. Mol. Cancer 2004, 3, 7. [Google Scholar] [CrossRef]
- Uddin, S.; Ahmed, M.; Hussain, A.; Assad, L.; Bavi, P.; Munkarah, A.; Al-Dayel, F.; Al-Kuraya, K.S.; Al-Dayel, F.; Al-Kuraya, K.S. Cyclooxygenase-2 inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian cancer. Int. J. Cancer 2010, 126, 382–394. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.-W.; Lin, S.-C.; Chang, K.-W.; Chi, C.-W.; Chang, C.-S.; Liu, T.-Y. Elevated expression of cyclooxygenase (COX)-2 in oral squamous cell carcinoma–Evidence for COX-2 induction by areca quid ingredients in oral keratinocytes. J. Oral Pathol. Med. 2003, 32, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Pannone, G.; Sanguedolce, F.; De Maria, S.; Farina, E.; Muzio, L.L.; Serpico, R.; Emanuelli, M.; Rubini, C.; De Rosa, G.; Staibano, S.; et al. Cyclooxygenase Isozymes in Oral Squamous Cell Carcinoma: A Real-Time RT-PCR Study with Clinic Pathological Correlations. Int. J. Immunopathol. Pharmacol. 2007, 20, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Søland, T.M.; Husvik, C.; Koppang, H.S.; Boysen, M.; Sandvik, L.; Clausen, O.P.F.; Christoffersen, T.; Bryne, M. A study of phosphorylated ERK1/2 and COX-2 in early stage (T1-T2) oral squamous cell carcinomas. J. Oral Pathol. Med. 2008, 37, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, K.; Ebrahimi, M.; Wahlin, Y.B.; Nylander, K.; Boldrup, L. Increased levels of COX-2 in oral lichen planus supports an autoimmune cause of the disease. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1415–1419. [Google Scholar] [CrossRef]
- Rakesh, N.; Iyengar, A.; Majumdar, K.; Vidya, G.S.; Shantha Kumar, S.S. Quantitative Evaluation of Tumour--Associated Tissue Eosinophilia and Cyclo-oxegenase-2 Gene in Oral Cancer Patients with Assessment of Long Term Outcomes. Pathol. Oncol. Res. 2016, 22, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.S.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-κB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef]
- Salimi, M.; Esfahani, M.; Habibzadeh, N.; Aslani, H.R.; Amanzadeh, A.; Esfandiary, M.; Sedaghati, B.; Bidgoli, S.A.; Ghahremani, M.H. Change in Nicotine-Induced VEGF, PGE2 AND COX-2 Expression Following COX Inhibition in Human Oral Squamous Cancer. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 349–356. [Google Scholar] [CrossRef]
- Moazeni-Roodi, A.; Allameh, A.; Harirchi, I.; Motiee-Langroudi, M.; Garajei, A. Studies on the Contribution of Cox-2 Expression in the Progression of Oral Squamous Cell Carcinoma and H-Ras Activation. Pathol. Oncol. Res. 2017, 23, 355–360. [Google Scholar] [CrossRef]
- Sappayatosok, K.; Maneerat, Y.; Swasdison, S.; Viriyavejakul, P.; Dhanuthai, K.; Zwang, J.; Chaisri, U. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in oral squamous cell carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 319–324. [Google Scholar]
- Hong, Z.-H.; Shao, F.; Zhu, G.-G.; Su, T. Expression of NF-κB and COX-2 in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 2010, 19, 335–338. [Google Scholar] [PubMed]
- Morita, Y.; Hata, K.; Nakanishi, M.; Nishisho, T.; Yura, Y.; Yoneda, T. Cyclooxygenase-2 promotes tumor lymphangiogenesis and lymph node metastasis in oral squamous cell carcinoma. Int. J. Oncol. 2012, 41, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wu, M.W.; Yang, A.K.; Zhang, W.D.; Sun, J.; Liu, T.R.; Chen, Y.F. COX-2 Gene increases tongue cancer cell proliferation and invasion through VEGF-C pathway. Med. Oncol. 2011, 28 (Supp. 1), S360–S366. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Cui, J. COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma. Asian Pac. J. Trop. Med. 2013, 6, 640–643. [Google Scholar] [CrossRef]
- Kono, M.; Watanabe, M.; Abukawa, H.; Hasegawa, O.; Satomi, T.; Chikazu, D. Cyclo-Oxygenase–2 Expression Is Associated with Vascular Endothelial Growth Factor C Expression and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2013, 71, 1694–1702. [Google Scholar] [CrossRef]
- Morita, Y.; Morita, N.; Hata, K.; Nakanishi, M.; Kimoto, N.; Omata, T.; Nakamura, Y.; Yoneda, T. Cyclooxygenase-2 expression is associated with vascular endothelial growth factor-c and lymph node metastasis in human oral tongue cancer. Oral Sur. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Segawa, E.; Sakurai, K.; Kishimoto, H.; Takaoka, K.; Noguchi, K.; Hashitani, S.; Hirota, S.; Urade, M. Expression of cyclooxygenase-2 and DNA topoisomerase II α in precancerous and cancerous lesions of the oral mucosa. Oral Oncol. 2008, 44, 664–671. [Google Scholar] [CrossRef]
- Prado, S.M.D.; Cedrún, J.L.L.; Rey, R.L.; Villaamil, V.M.; Álvarez García, A.; Ayerbes, M.V.; Aparicio, L.A. Evaluation of COX-2, EGFR, and p53 as biomarkers of non-dysplastic oral leukoplakias. Exp. Mol. Pathol. 2010, 89, 197–203. [Google Scholar] [CrossRef][Green Version]
- Pontes, H.A.; Pontes, F.S.; Fonseca, F.P.; de Carvalho, P.L.; Pereira, E.M.; de Abreu, M.C.; de Freitas Silva, B.S.; dos Santos Pinto, D., Jr. Nuclear factor κB and cyclooxygenase-2 immunoexpression in oral dysplasia and oral squamous cell carcinoma. Ann. Diagn. Pathol. 2013, 17, 45–50. [Google Scholar] [CrossRef]
- Byatnal, A.A.; Byatnal, A.; Sen, S.; Guddattu, V.; Solomon, M.C. Cyclooxygenase-2—An Imperative Prognostic Biomarker in Oral Squamous Cell Carcinoma—An Immunohistochemical Study. Pathol. Oncol. Res. 2015, 21, 1123–1131. [Google Scholar] [CrossRef]
- Mittal, M.; Kapoor, V.; Mohanti, B.K.; Das, S.N. Functional variants of COX-2 and risk of tobacco-related oral squamous cell carcinoma in high-risk Asian Indians. Oral Oncol. 2010, 46, 622–626. [Google Scholar] [CrossRef]
- Li, D.; Hao, S.-H.; Sun, Y.; Hu, C.-M.; Ma, Z.-H.; Wang, Z.M.; Liu, J.; Liu, H.B.; Ye, M.; Zhang, Y.F.; et al. Functional Polymorphisms inCOX-2Gene Are Correlated with the Risk of Oral Cancer. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 Mediated Inflammation in Oral Squamous Cell Carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The Role of Cyclooxygenase-2 in Cell Proliferation and Cell Death in Human Malignancies. Int. J. Cell Boil. 2010, 2010, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bai, L.; Wang, X.; Xu, S.; Belinsky, S.A.; Lin, Y. Acquired activation of the Akt/cyclooxygenase-2/Mcl-1 pathway renders lung cancer cells resistant to apoptosis. Mol. Pharmacol. 2010, 77, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Hammadi, N.K.; Mester, J.; Gaben, A.-M. Akt2 knock-down reveals its contribution to human lung cancer cell proliferation, growth, motility, invasion and endothelial cell tube formation. Sci. Rep. 2015, 5, 12759. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and Abnormal Functions. Endocrinol. 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A.; Blalock, W. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leuk. 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Grabinski, N.; Bartkowiak, K.; Grupp, K.; Brandt, B.; Pantel, K.; Jücker, M. Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell. Signal. 2011, 23, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Jabbour, A.M.; Sandow, J.J.; Riffkin, C.D.; Masouras, D.; Daunt, C.P.; Salmanidis, M.; Brumatti, G.; Hemmings, B.A.; Guthridge, M.A.; et al. Akt1 is the principal Akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013, 20, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequence | Amplification Length |
|---|---|---|
| Akt1 | 5′-CAC CAT GAG CGA CGT GGC TAT-3′ | 450 bp |
| 5′-CCA GCA GCT TCA GGT ACT CA-3′ | ||
| Akt2 | 5′-TTG CCA AGG ATG AAG TCG CT-3′ | 934 bp |
| 5′-AAC CAC CCA GCG GTG ATG G-3′ | ||
| Akt3 | 5′-ATA ATC AGA TGT CTC CAG TG-3′ | 604 bp |
| 5′-CTT GAG ATC ACG GTA CAC A-3′ | ||
| α-tubulin β-actin | 5′-TAT CGA GCG CCC AAC CTA CAC T-3′ | 683 bp 564 bp |
| 5′-CCT CAC CCT CTC CTT CAA CAG AAT C-3′ 5′- CTG GGA CGA CAT GGA GAA AA -3′ 5′- AAG GAA GGC TGG AAG AGT GC -3’ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, N.K.; Monisha, J.; Padmavathi, G.; Lalhruaitluanga, H.; Kumar, N.S.; Singh, A.K.; Bordoloi, D.; Baruah, M.N.; Ahmed, G.N.; Longkumar, I.; et al. Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules 2019, 9, 253. https://doi.org/10.3390/biom9070253
Roy NK, Monisha J, Padmavathi G, Lalhruaitluanga H, Kumar NS, Singh AK, Bordoloi D, Baruah MN, Ahmed GN, Longkumar I, et al. Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules. 2019; 9(7):253. https://doi.org/10.3390/biom9070253
Chicago/Turabian StyleRoy, Nand Kishor, Javadi Monisha, Ganesan Padmavathi, H. Lalhruaitluanga, Nachimuthu Senthil Kumar, Anuj Kumar Singh, Devivasha Bordoloi, Munindra Narayan Baruah, Gazi Naseem Ahmed, Imliwati Longkumar, and et al. 2019. "Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma" Biomolecules 9, no. 7: 253. https://doi.org/10.3390/biom9070253
APA StyleRoy, N. K., Monisha, J., Padmavathi, G., Lalhruaitluanga, H., Kumar, N. S., Singh, A. K., Bordoloi, D., Baruah, M. N., Ahmed, G. N., Longkumar, I., Arfuso, F., Kumar, A. P., & Kunnumakkara, A. B. (2019). Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules, 9(7), 253. https://doi.org/10.3390/biom9070253







