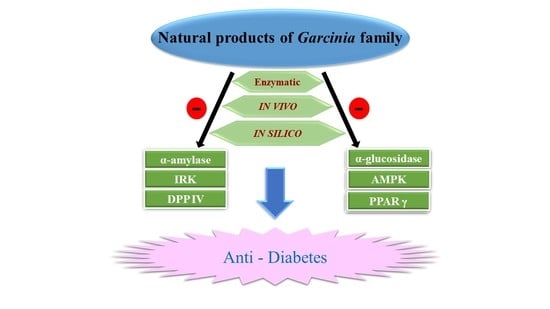
The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia linii with Comprehensive Review of the Garcinia Family
Abstract
:1. Introduction
1.1. Impact of Diabetes
1.2. Therapy Agent of Diabetes
1.3. Distribution of Garcinia Plants and Recent Discovery of Anti-Diabetic Agents with Garcinia Plants
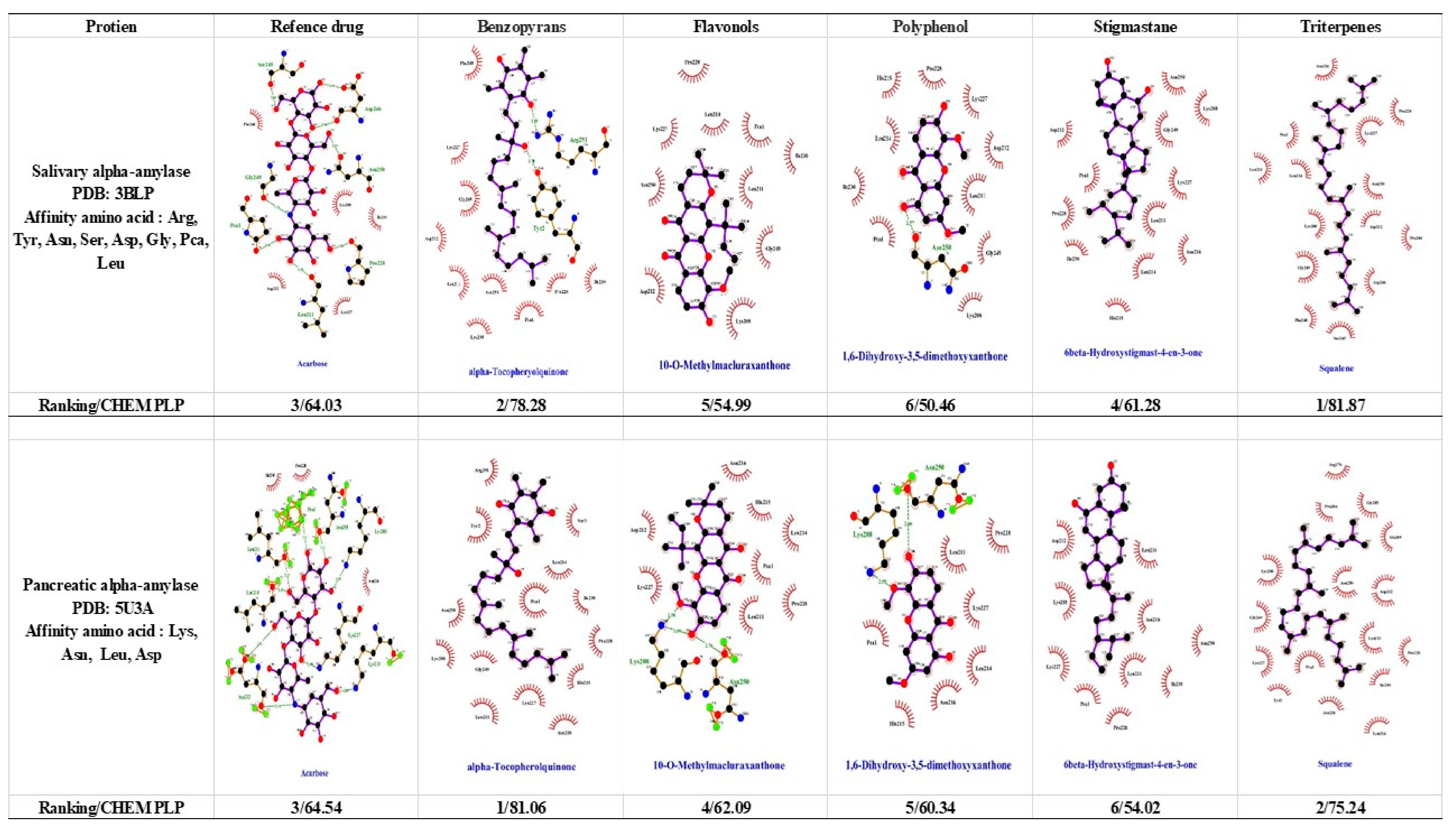
1.4. α-Amylase and α-Glucosidase
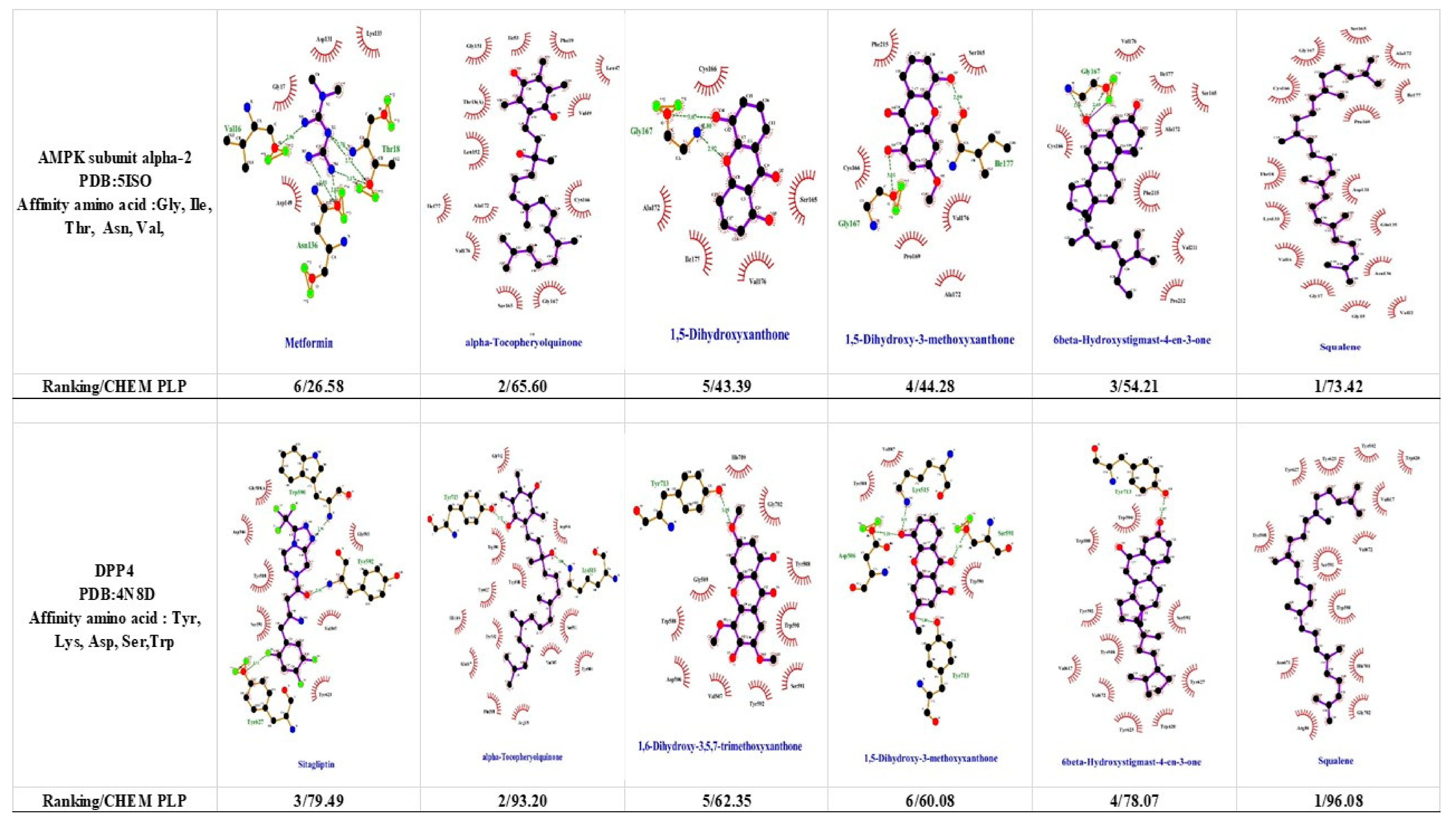
1.5. 5′-Adenosine Monophosphate-Activated Protein Kinase (AMPK)
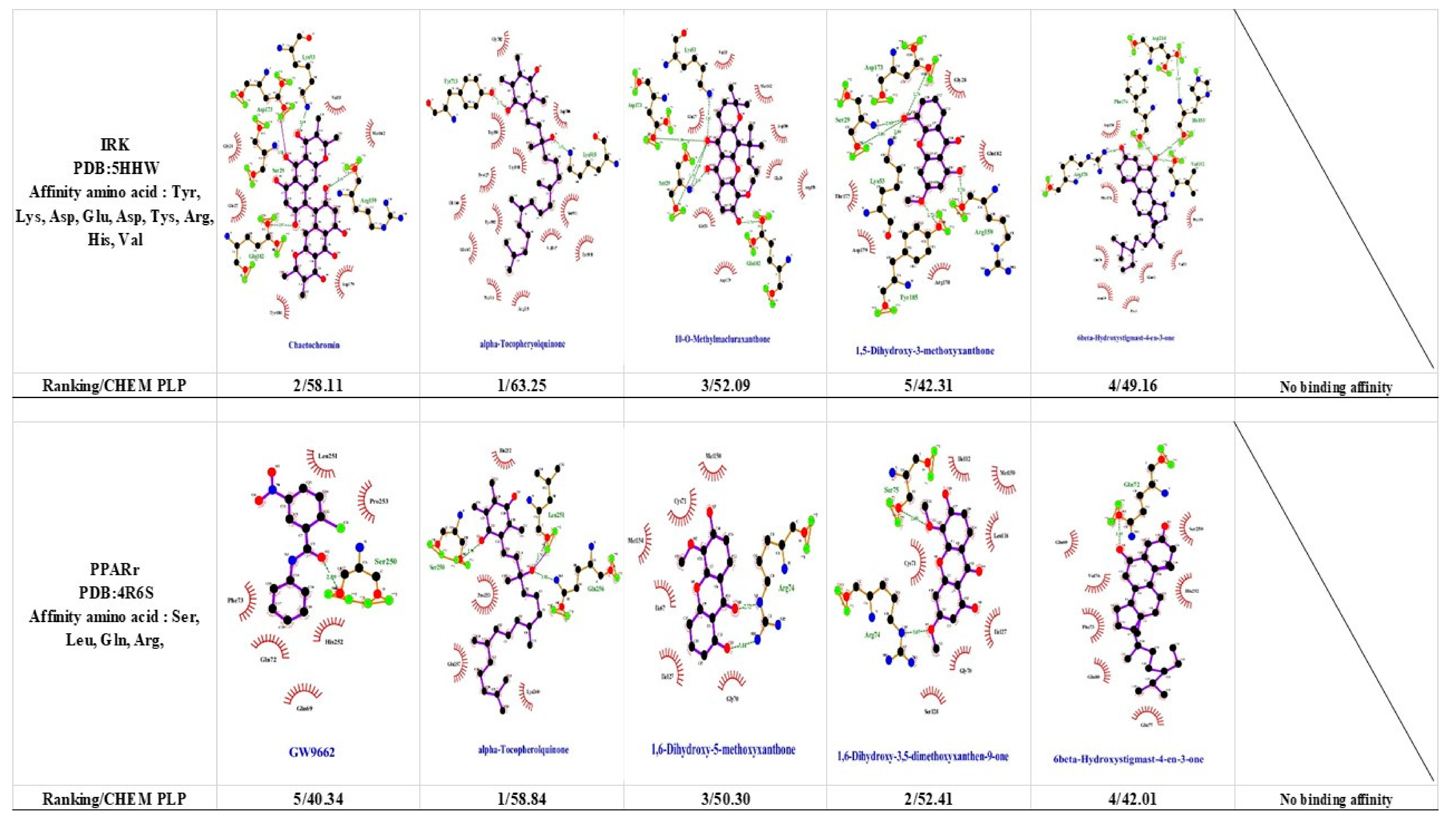
1.6. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ)
1.7. Dipeptidyl-Peptidase 4 (DPP-4) and Glucagon-Like Peptide 1 (GLP-1)
1.8. Insulin Receptor Kinase (IRK)
2. Conclusions and Future Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hay, S.I.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Atlas, D.; International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Gregg, B. Metformin; a review of its history and future: From lilac to longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mendler, M.; Kopf, S.; Groener, J.B.; Riedinger, C.; Fleming, T.H.; Nawroth, P.P.; Okun, J.G. Urine levels of 5-aminoimidazole-4-carboxamide riboside (AICAR) in patients with type 2 diabetes. Acta Diabetol. 2018, 55, 585–592. [Google Scholar] [CrossRef]
- Scott, L.J. Sitagliptin: A Review in Type 2 Diabetes. Drugs 2017, 77, 209–224. [Google Scholar] [CrossRef]
- Abou Daya, K.; Daya, H.A.; Eddine, M.N.; Nahhas, G.; Nuwayri-Salti, N. Effects of rosiglitazone (PPAR gamma agonist) on the myocardium in non-hypertensive diabetic rats (PPAR gamma). J. Diabetes 2015, 7, 85–94. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-gamma Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of intraduodenal hydroxycitrate on glucose absorption, incretin release, and glycemia in response to intraduodenal glucose infusion in health and type 2 diabetes: A randomised controlled trial. Nutrition 2016, 32, 553–559. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xiao, C.; Chen, D.; Xiao, Y.; Mei, Z. Rapid screening and identification of alpha-amylase inhibitors from Garcinia xanthochymus using enzyme-immobilized magnetic nanoparticles coupled with HPLC and MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 960, 166–173. [Google Scholar] [CrossRef]
- Adaramoye, O.A. Antidiabetic effect of kolaviron, a biflavonoid complex isolated from Garcinia kola seeds, in Wistar rats. Afr. Health Sci. 2012, 12, 498–506. [Google Scholar] [CrossRef]
- Karim, N.; Rahman, A.; Chanudom, L.; Thongsom, M.; Tangpong, J. Mangosteen Vinegar Rind from Garcinia mangostana Prevents High-Fat Diet and Streptozotocin-Induced Type II Diabetes Nephropathy and Apoptosis. J. Food Sci. 2019, 84, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.; Huang, D. Assay-guided fractionation study of alpha-amylase inhibitors from Garcinia mangostana pericarp. J. Agric. Food Chem. 2007, 55, 9805–9810. [Google Scholar] [PubMed]
- Ali, M.Y.; Paul, S.; Tanvir, E.M.; Hossen, M.S.; Rumpa, N.N.; Saha, M.; Bhoumik, N.C.; Islam, M.A.; Hossain, M.S.; Alam, N.; et al. Antihyperglycemic, Antidiabetic, and Antioxidant Effects of Garcinia pedunculata in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 2979760. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.; Amiroudine, M.Z.; Rezali, M.F.; Taher, M. Friedelin and lanosterol from Garcinia prainiana stimulated glucose uptake and adipocytes differentiation in 3T3-L1 adipocytes. Nat. Prod. Res. 2013, 27, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kainuma, M.; Baba, K.; Oshiro, N.; Kimura, N.; Chan, E.W. Garcinia subelliptica Merr. (Fukugi): A multipurpose coastal tree with promising medicinal properties. J. Intercult. Ethnopharmacol. 2017, 6, 121–127. [Google Scholar]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- Tousian Shandiz, H.; Razavi, B.M.; Hosseinzadeh, H. Review of Garcinia mangostana and its Xanthones in Metabolic Syndrome and Related Complications. Phytother. Res. 2017, 31, 1173–1182. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Kamal, M.A.; Gan, S.H. Updates on Managing Type 2 Diabetes Mellitus with Natural Products: Towards Antidiabetic Drug Development. Curr. Med. Chem. 2018, 25, 5395–5431. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, Y.T.; Lin, L.G. New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct. 2015, 6, 383–393. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Trinh, B.T.D.; Tran, T.B.; Nguyen, L.T.; Jager, A.K.; Nguyen, L.D. Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 2017, 27, 3301–3304. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Laksmitawati, D.R.; Wargasetia, T.L.; Afifah, E.; Amalia, A.; Arinta, Y.; Rizal, R.; Suciati, T. Mangosteen peel extract (Garcinia mangostana L. ) as protective agent in glucose-induced mesangial cell as in vitro model of diabetic glomerulosclerosis. Iran. J. Basic Med. Sci. 2018, 21, 972–977. [Google Scholar] [PubMed]
- Li, Y.; Zhao, P.; Chen, Y.; Fu, Y.; Shi, K.; Liu, L.; Liu, H.; Xiong, M.; Liu, Q.H.; Yang, G.; et al. Depsidone and xanthones from Garcinia xanthochymus with hypoglycemic activity and the mechanism of promoting glucose uptake in L6 myotubes. Bioorg. Med. Chem. 2017, 25, 6605–6613. [Google Scholar] [CrossRef] [PubMed]
- Maia-Landim, A.; Ramirez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC Complement. Altern. Med. 2018, 18, 26. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, alpha-glucosidase, alpha-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef]
- Chen, J.J.; Chen, I.S.; Duh, C.Y. Cytotoxic xanthones and biphenyls from the root of Garcinia linii. Planta Med. 2004, 70, 1195–1200. [Google Scholar] [CrossRef]
- Chen, J.J.; Peng, C.F.; Huang, H.Y.; Chen, I.S. Benzopyrans, biphenyls and xanthones from the root of Garcinia linii and their activity against Mycobacterium tuberculosis. Planta Med. 2006, 72, 473–477. [Google Scholar] [CrossRef]
- Pantidos, N.; Boath, A.; Lund, V.; Conner, S.; McDougall, G.J. Phenolic-rich extracts from the edible seaweed, ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. J. Funct. Foods 2014, 10, 201–209. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Olivier, S.; Foretz, M.; Viollet, B. Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 2018, 153, 147–158. [Google Scholar] [CrossRef]
- Scott, J.W.; Ling, N.; Issa, S.M.; Dite, T.A.; O’Brien, M.T.; Chen, Z.P.; Galic, S.; Langendorf, C.G.; Steinberg, G.R.; Kemp, B.E.; et al. Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem. Biol. 2014, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hashi, K.; Irie, N.; Sakai, T.; Okuda, K.; Nagasawa, H.; Hirata, Y.; Kiuchi, K. Elucidation of a novel phenformin derivative on glucose-deprived stress responses in HT-29 cells. Mol. Cell. Biochem. 2016, 419, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija-Asimi, Z.; Pearson, E.R.; Semiz, S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet. Med. 2016, 33, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, S.; Farhangi, M.; Modarres, S. The effect of different dietary inclusion levels of rutin (a flavonoid) on some liver enzyme activities and oxidative stress indices in rainbow trout, Oncorhynchus mykiss (Walbaum) exposed to Oxytetracycline. Aquac. Res. 2017, 48, 4356–4362. [Google Scholar] [CrossRef]
- Meena, K.P.; Vijayakumar, M.R.; Dwibedy, P.S. Catechin-loaded Eudragit microparticles for the management of diabetes: Formulation, characterization and in vivo evaluation of antidiabetic efficacy. J. Microencapsul. 2017, 34, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Che Hassan, N.K.N.; Taher, M.; Susanti, D. Phytochemical constituents and pharmacological properties of Garcinia xanthochymus—A review. Biomed. Pharmacother. 2018, 106, 1378–1389. [Google Scholar] [CrossRef]
- Wang, Q.; Imam, M.U.; Yida, Z.; Wang, F. Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) as a Target for Concurrent Management of Diabetes and Obesity-Related Cancer. Curr. Pharm. Des. 2017, 23, 3677–3688. [Google Scholar] [CrossRef]
- Janani, C.; Kumari, B.D.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Polvani, S.; Tarocchi, M.; Tempesti, S.; Bencini, L.; Galli, A. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J. Gastroenterol. 2016, 22, 2441–2459. [Google Scholar] [CrossRef]
- Siersbaek, R.; Nielsen, R.; Mandrup, S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, I.; Candeias, E.; Santos, M.S.; de Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Insulin as a Bridge between Type 2 Diabetes and Alzheimer Disease - How Anti-Diabetics Could be a Solution for Dementia. Front. Endocrinol. 2014, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Elaidy, S.M.; Hussain, M.A.; El-Kherbetawy, M.K. Time-dependent therapeutic roles of nitazoxanide on high-fat diet/streptozotocin-induced diabetes in rats: Effects on hepatic peroxisome proliferator-activated receptor-gamma receptors. Can. J. Physiol. Pharmacol. 2018, 96, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kang, S.; Gong, D.; Oh, S.H.; Park, E.Y.; Oak, M.H.; Yi, E. Combination of Garcinia cambogia Extract and Pear Pomace Extract Additively Suppresses Adipogenesis and Enhances Lipolysis in 3T3-L1 Cells. Pharmacogn. Mag. 2018, 14, 220–226. [Google Scholar]
- Bohm, A.; Wagner, R.; Machicao, F.; Holst, J.J.; Gallwitz, B.; Stefan, N.; Fritsche, A.; Haring, H.U.; Staiger, H. DPP4 gene variation affects GLP-1 secretion, insulin secretion, and glucose tolerance in humans with high body adiposity. PLoS ONE 2017, 12, e0181880. [Google Scholar] [CrossRef]
- Nahon, K.J.; Doornink, F.; Straat, M.E.; Botani, K.; Martinez-Tellez, B.; Abreu-Vieira, G.; van Klinken, J.B.; Voortman, G.J.; Friesema, E.C.H.; Ruiz, J.R.; et al. Effect of sitagliptin on energy metabolism and brown adipose tissue in overweight individuals with prediabetes: A randomised placebo-controlled trial. Diabetologia 2018, 61, 2386–2397. [Google Scholar] [CrossRef]
- Araujo, F.; Shrestha, N.; Gomes, M.J.; Herranz-Blanco, B.; Liu, D.; Hirvonen, J.J.; Granja, P.L.; Santos, H.A.; Sarmento, B. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale 2016, 8, 10706–10713. [Google Scholar] [CrossRef] [Green Version]
- Andel, M.; Skrha, P.; Kraml, P.; Potockova, J.; Hoffmanova, I.; Silhova, E.; Fontana, J.; Richterova, A.; Gadiredi, M.; Busek, P.; et al. Annual monitoring of side effects of administering sitagliptin in patients with type 2 diabetes mellitus. Vnitr. Lék. 2016, 62, 455–461. [Google Scholar]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.K.; Lin, S.R.; Kankala, R.K.; Lee, C.H.; Weng, C.F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef]
- Hayamizu, K.; Hirakawa, H.; Oikawa, D.; Nakanishi, T.; Takagi, T.; Tachibana, T.; Furuse, M. Effect of Garcinia cambogia extract on serum leptin and insulin in mice. Fitoterapia 2003, 74, 267–273. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Shih, H.-Y.; Chia, Y.-C.; Lee, C.-H.; Ashida, H.; Lai, Y.-K.; Weng, C.-F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. In Glucose Transport [Working Title]; 2018; Available online: https://www.intechopen.com/online-first/role-of-pi3k-akt-pathway-in-insulin-mediated-glucose-uptake (accessed on 23 October 2019). [CrossRef]
- Hsu, C.Y.; Sulake, R.S.; Huang, P.K.; Shih, H.Y.; Sie, H.W.; Lai, Y.K.; Chen, C.; Weng, C.F. Synthetic (+)-antroquinonol exhibits dual actions against insulin resistance by triggering AMP kinase and inhibiting dipeptidyl peptidase IV activities. Br. J. Pharmacol. 2015, 172, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martin-Cabrejas, M.A.; de Mejia, E.G. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Zakaria, T.M.F.S.T.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 135. [Google Scholar]
- Asghar, M.; Monjok, E.; Kouamou, G.; Ohia, S.E.; Bagchi, D.; Lokhandwala, M.F. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol. Cell. Biochem. 2007, 304, 93–99. [Google Scholar] [CrossRef]
- Talpur, N.; Echard, B.W.; Yasmin, T.; Bagchi, D.; Preuss, H.G. Effects of niacin-bound chromium, Maitake mushroom fraction SX and (-)-hydroxycitric acid on the metabolic syndrome in aged diabetic Zucker fatty rats. Mol. Cell. Biochem. 2003, 252, 369–377. [Google Scholar] [CrossRef]

| Species. | Molecular Targets | Basic Findings |
|---|---|---|
| G. cambogia | α-Glucosidase, PPARγ, DPP4 | Small intestinal exposure to HCA resulted in a modest reduction in glycemia of healthy individuals [20]. Mixture (GE containing HCA as an active ingredient, PE, anti-adipogenic activity) reduced the expression of adipogenesis-related factors C/EBP-α, PPARγ, and FAS [46]. Insulin resistance did not develop in HCA-SX-supplemented rats via lowered fasting plasma insulin and glucose [58,59]. |
| G. xanthochymus | α-Amylase, α-Glucosidase, AMPK, IRK | Activated PI3K/PKB/Akt signaling pathway and AMPK signaling pathway, resulting in the translocation of GLUT4 in L6 myotubes without affecting the expression of GLUT4 [18]. Identification of α-amylase inhibitor from G. xanthochymus [21]. |
| G. kola | α-Amylase, IRK | KV offered significant anti-diabetic relief via reduction of FBG, α-amylase and HbA1c [22]. |
| G. mangostana | α-Amylase, IRK | MVR from G. mangostana fruit pericarp had an α-amylase inhibitor and enhanced insulin sensitivity [23,24] GME significantly reduced the blood glucose level in normoglycemic rats and STZ-induced diabetic rats [57]. |
| G. pedunculata | IRK | Elevated insulin levels of rats [25]. |
| G. prainiana | IRK | Increased insulin sensitivity of 3T3-L1 adipocytes [26]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-H.; Tsai, M.-J.; Fu, Y.-S.; Weng, C.-F. The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia linii with Comprehensive Review of the Garcinia Family. Biomolecules 2019, 9, 641. https://doi.org/10.3390/biom9110641
Chen T-H, Tsai M-J, Fu Y-S, Weng C-F. The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia linii with Comprehensive Review of the Garcinia Family. Biomolecules. 2019; 9(11):641. https://doi.org/10.3390/biom9110641
Chicago/Turabian StyleChen, Ting-Hsu, May-Jywan Tsai, Yaw-Syan Fu, and Ching-Feng Weng. 2019. "The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia linii with Comprehensive Review of the Garcinia Family" Biomolecules 9, no. 11: 641. https://doi.org/10.3390/biom9110641