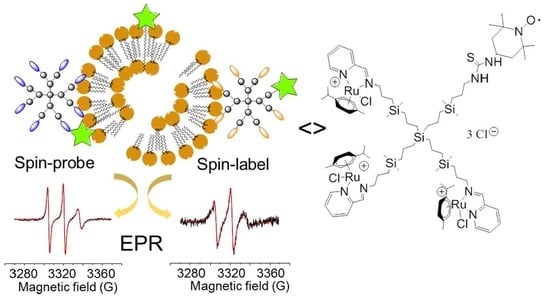
Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dendrimers and Metallodendrimers
2.2. Nuclear Magnetic Resonance
2.3. Fourier-Transform Infrared Spectroscopy
2.4. Elemental Analysis
2.5. Sample Preparation for Electron Paramagnetic Resonance Analysis
2.5.1. Dendrimer Stock Solution
2.5.2. Liposome Stock Solution
2.5.3. Micelle Stock Solution
2.5.4. Final Mixtures
2.6. Electron Paramagnetic Resonance Instrumentation
2.7. Computation and Analysis of the Electron Paramagnetic Resonance Spectra
3. Results and Discussion
3.1. Synthesis and Characterization of Heterofunctional TEMPO-Labelled Ru(II) Metallodendrimers
3.2. Electron Paramagnetic Resonance Study of TEMPO-Labelled Dendrimers in the Absence and Presence of Model Membranes
3.3. Electron Paramagnetic Resonance Study of Unlabelled Dendrimers Using CAT12 Probe in the Absence and Presence of Model Membranes: Comparison with TEMPO-Labelled Dendrimers
3.3.1. CAT12 Electron Paramagnetic Resonance Study of the Interactions between Homofunctional Gn-Py Dendrimers and Model Membranes
3.3.2. Electron Paramagnetic Resonance Study of the Interactions between Heterofunctional Gn-PyN Dendrimers and Model Membranes Using CAT12 as Spin Probe
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-installed nanocarriers toward precision therapy. Adv. Mater. 2019, e1902604. [Google Scholar] [CrossRef] [PubMed]
- Sk, U.H.; Kojima, C. Dendrimers for drug delivery of anticancer drugs. In Frontiers in Clinical Drug Research - Anti-Cancer Agents; Atta-ur-Rahman, Ed.; Bentham Science: UK, 2015; Volume 2, pp. 3–25. [Google Scholar]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Nabil, G.; Bhise, K.; Sau, S.; Atef, M.; El-Banna, H.A.; Iyer, A.K. Nano-engineered delivery systems for cancer imaging and therapy: Recent advances, future direction and patent evaluation. Drug Discov. Today 2019, 24, 462–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Mol. 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dube, T.; Chibh, S.; Kour, A.; Mishra, J.; Panda, J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 113–128. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Sánchez-Nieves, J.; Ortega, P.; Cano, J.; Gómez, R.; Mata, F.J.d.l. Poly(carbosilane) dendrimers and other silicon-containing dendrimers. In Dendrimer Chemistry: Synthetic approaches towards complex architectures; Malkoch, M., García-Gallego, S., Eds.; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Andreozzi, E.; Antonelli, A.; Cangiotti, M.; Canonico, B.; Sfara, C.; Pianetti, A.; Bruscolini, F.; Sahre, K.; Appelhans, D.; Papa, S.; et al. Interactions of nitroxide-conjugated and non-conjugated glycodendrimers with normal and cancer cells and biocompatibility studies. Bioconjug. Chem. 2017, 28, 524–538. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; El Brahmi, N.; Cangiotti, M.; Coppola, C.; Buccella, F.; Cresteil, T.; Mignani, S.; Caminade, A.M.; Costes, J.P.; Majoral, J.P. Comparative EPR studies of Cu(II)-conjugated phosphorous-dendrimers in the absence and presence of normal and cancer cells. RSC Adv. 2014, 4, 36573–36583. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Rokach, S.; Ottaviani, M.F.; Shames, A.I.; Aserin, A.; Garti, N. Behavior of PPI-G2 dendrimer in a microemulsion. J. Phys. Chem. B 2017, 121, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, M.; Ottaviani, M.F.; Aserin, A.; Garti, N. Structural effects on cosolubilization of dendrimer and propofol in water dilutable microemulsions as delivery vehicle. Colloids Surfaces A: Physicochem. Eng. Asp. 2016, 497, 257–264. [Google Scholar] [CrossRef]
- Lidich, N.; Ottaviani, M.F.; Hoffman, R.E.; Aserin, A.; Garti, N. Docosahexaenoic acid triglyceride-based microemulsions with an added dendrimer—Structural considerations. J. Colloid Interface Sci. 2016, 483, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Rokach, S.; Ottaviani, M.F.; Shames, A.I.; Nir, I.; Aserin, A.; Garti, N. W/O microemulsions as dendrimer nanocarriers: an EPR study. J. Phys. Chem. B 2012, 116, 12633–12640. [Google Scholar] [CrossRef] [PubMed]
- Bitan-Cherbakovsky, L.; Libster, D.; Ottaviani, M.F.; Aserin, A.; Garti, N. Structural behavior and interactions of dendrimer within lyotropic liquid crystals, monitored by EPR spectroscopy and rheology. J. Phys. Chem. B 2012, 116, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.F.; Favuzza, P.; Sacchi, B.; Turro, N.J.; Jockusch, S.; Tomalia, D.A. Interactions between starburst dendrimers and mixed DMPC/DMPA-Na vesicles studied by the Spin Label and the Spin Probe techniques, supported by Transmission Electron Microscopy. Langmuir 2002, 18, 2347–2357. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Matteini, P.; Brustolon, M.; Turro, N.J.; Jockusch, S.; Tomalia, D.A. Characterization of Starburst Dendrimers and Vesicle Solutions and Their Interactions by CW- and Pulsed-EPR, TEM, and Dynamic Light Scattering. J. Phys. Chem. B 1998, 102, 6029–6039. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Daddi, R.; Brustolon, M.; Turro, N.J.; Tomalia, D.A. Structural modifications of DMPC vesicles upon interaction with Poly(amidoamine) dendrimers studied by CW-Electron Paramagnetic Resonance and Electron Spin–Echo techniques. Langmuir 1999, 15, 1973–1980. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Andechaga, P.; Turro, N.J.; Tomalia, D.A. Model for the interactions between anionic dendrimers and cationic surfactants by means of the spin probe method. J. Phys. Chem. B 1997, 101, 6057–6065. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Daddi, R.; Brustolon, M.; Turro, N.J.; Tomalia, D.A. Interaction between starburst dendrimers and SDS micelles studied by continuous-wave and pulsed electron spin resonances. Appl. Magn. Reson. 1997, 13, 347–363. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Turro, N.J.; Jockusch, S.; Tomalia, D.A. Aggregational process of the positively charged surfactants CTAC and CAT16 in the presence of starburst dendrimers: an Electron Paramagnetic Resonance spectroscopic study. Colloids Surf. A 1996, 115, 9–21. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Turro, N.J.; Jockusch, S.; Tomalia, D.A. Characterization of starburst dendrimers by EPR. 3. Aggregational processes of a positively charged nitroxide surfactant. J. Phys. Chem. 1996, 100, 13675–13686. [Google Scholar] [CrossRef]
- Lin, Y.; Yokoyama, H.; Ishida, S.-I.; Tsuchihashi, N.; Ogata, T. In vivo Electron Spin Resonance analysis of nitroxide radicals injected into a rat by a flexible surface-coil-type resonator as an endoscope- or a stethoscope-like device. Magn. Reson. Mater. Phy. 1997, 5, 99–103. [Google Scholar] [CrossRef]
- Galimzyanovich Saifutdinov, R.; Ivanovna Larina, L.; Il’inichna Vakul´skaya, T.; Grigor´evich Voronkov, M. Electron Paramagnetic Resonance in Biochemistry and Medicine; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Swartz, H.M.; Williams, B.B.; Zaki, B.I.; Hartford, A.C.; Jarvis, L.A.; Chen, E.Y.; Comi, R.J.; Ernstoff, M.S.; Hou, H.; Khan, N.; et al. Clinical EPR: unique opportunities and some challenges. Acad. Radiol. 2014, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sanz del Olmo, N.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; de la Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu(II)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef]
- Maroto-Diaz, M.; Elie, B.T.; Gomez-Sal, P.; Perez-Serrano, J.; Gomez, R.; Contel, M.; de la Mata, F.J. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium(ii) complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef] [Green Version]
- Maroto-Diaz, M.; Sanz del Olmo, N.; Muñoz-Moreno, L.; Bajo, A.M.; Carmena, M.J.; Gómez, R.; García-Gallego, S.; de la Mata, F.J. In vitro and in vivo evaluation of first-generation carbosilane arene Ru(II)-metallodendrimers in advanced prostate cancer. Eur. Polym. J. 2019, 113, 229–235. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium(II) complexes: from single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Budil, D.E.; Lee, S.; Saxena, S.; Freed, J.H. Nonlinear-least-squares analysis of slow-motion EPR spectra in one and two dimensions using a modified Levenberg–Marquardt algorithm. J. Magn. Reson. 1996, 120, 155–189. [Google Scholar] [CrossRef]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Mullner, M.; de Jesus, E.; de la Mata, F.J.; Flores, J.C.; Gomez, R.; et al. Water-soluble carbosilane dendrimers: synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chemistry 2007, 13, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, C.; Cui, D.; Yan, Y.; Liu, X.; Lu, X.; Tan, X.; Lu, X.; Xu, J.; Xu, Y.; et al. Organic radical contrast agents based on polyacetylenes containing 2,2,6,6-Tetramethylpiperidine 1-Oxyl (TEMPO): Targeted Magnetic Resonance (MR)/Optical Bimodal Imaging of folate receptor expressing HeLa tumors in vitro and in vivo. Macromol. Biosci. 2015, 15, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.F.; Cossu, E.; Turro, N.J.; Tomalia, D.A. Characterization of starburst dendrimers by Electron Paramagnetic Resonance. 2. Positively charged nitroxide radicals of variable chain length used as spin probes. J. Am. Chem. Soc. 1995, 117, 4387–4398. [Google Scholar] [CrossRef]





| Entry | Sample | Components | Azz (G) | τ (ns) |
|---|---|---|---|---|
| 1 | G1-PyT (3) | Free (single) | 39.21/- | 0.014/- |
| 2 | G2-PyT (4) | Free (single) | 39.12/- | 0.017/- |
| 3 | G1-PyT (3) + CTAB | Free + Interacting | 38.72/37.68 | 0.21/0.87 |
| 4 | G2-PyT (4) + CTAB | Free + Interacting | 38.68/37.70 | 0.25/0.73 |
| 5 | G1-PyT (3) + LEC | Free + Interacting | 39.01/38.07 | 0.050/2.35 |
| 6 | G2-PyT (4) + LEC | Free + Interacting | 38.98/38.20 | 0.055/3.05 |
| 7 | G1-RuT (5) | Free + Interacting | 38.82/39.00 | 0.22/3.31 |
| 8 | G2-RuT (6) | Free + Interacting | 38.85/39.00 | 0.22/3.35 |
| 9 | G1-RuT (5) + CTAB | Free + Interacting | 38.82/37.40 | 0.22/1.90 |
| 10 | G2-RuT (6) + CTAB | Free + Interacting | 38.85/37.41 | 0.22/1.38 |
| 11 | G1-RuT (5) + LEC | Free + Interacting | 38.82/34.60 | 0.22/6.45 |
| 12 | G2-RuT (6) + LEC | Free + Interacting | 38.85/35.00 | 0.22/7.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carloni, R.; Sanz del Olmo, N.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules 2019, 9, 540. https://doi.org/10.3390/biom9100540
Carloni R, Sanz del Olmo N, Ortega P, Fattori A, Gómez R, Ottaviani MF, García-Gallego S, Cangiotti M, de la Mata FJ. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules. 2019; 9(10):540. https://doi.org/10.3390/biom9100540
Chicago/Turabian StyleCarloni, Riccardo, Natalia Sanz del Olmo, Paula Ortega, Alberto Fattori, Rafael Gómez, Maria Francesca Ottaviani, Sandra García-Gallego, Michela Cangiotti, and F. Javier de la Mata. 2019. "Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR" Biomolecules 9, no. 10: 540. https://doi.org/10.3390/biom9100540