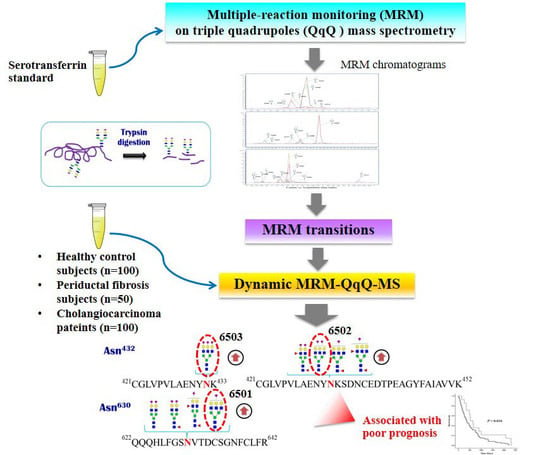
Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Serum Specimens
2.2. Tryptic Digestion In Solution
2.3. UPLC-ESI-QqQ analysis
2.4. Statistical Analysis
3. Results
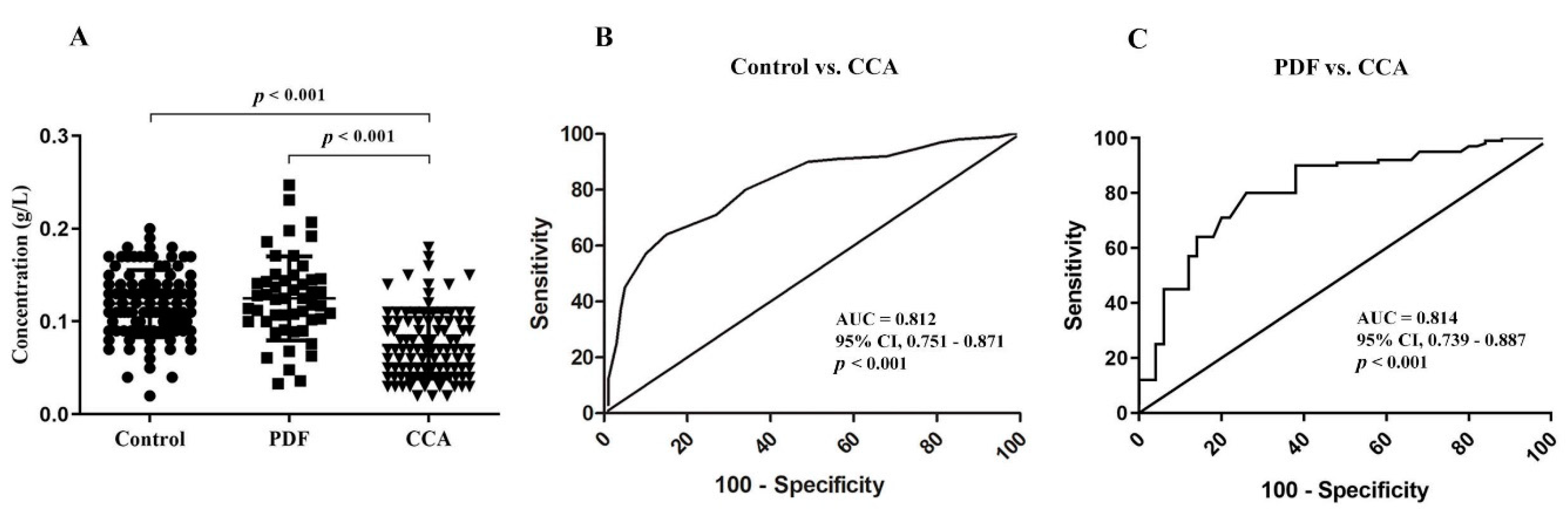
3.1. Absolute Quantiytation of Serotransferrin in Serum
3.2. Glycopeptides of Serotransferrin Standard
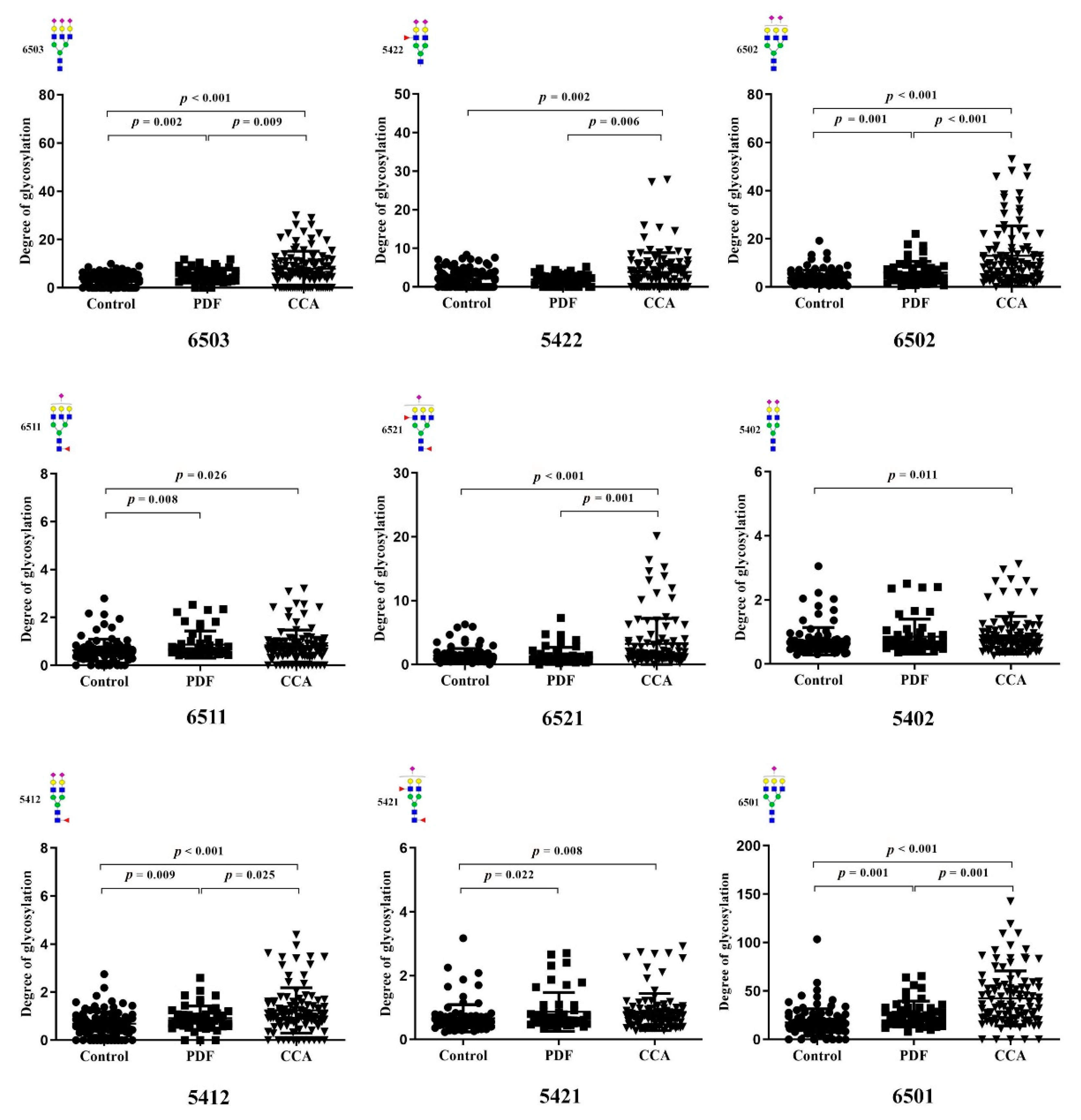
3.3. Identification of Altered Glycosylation at Asn432 and Asn630 of Serotransferrin in CCA
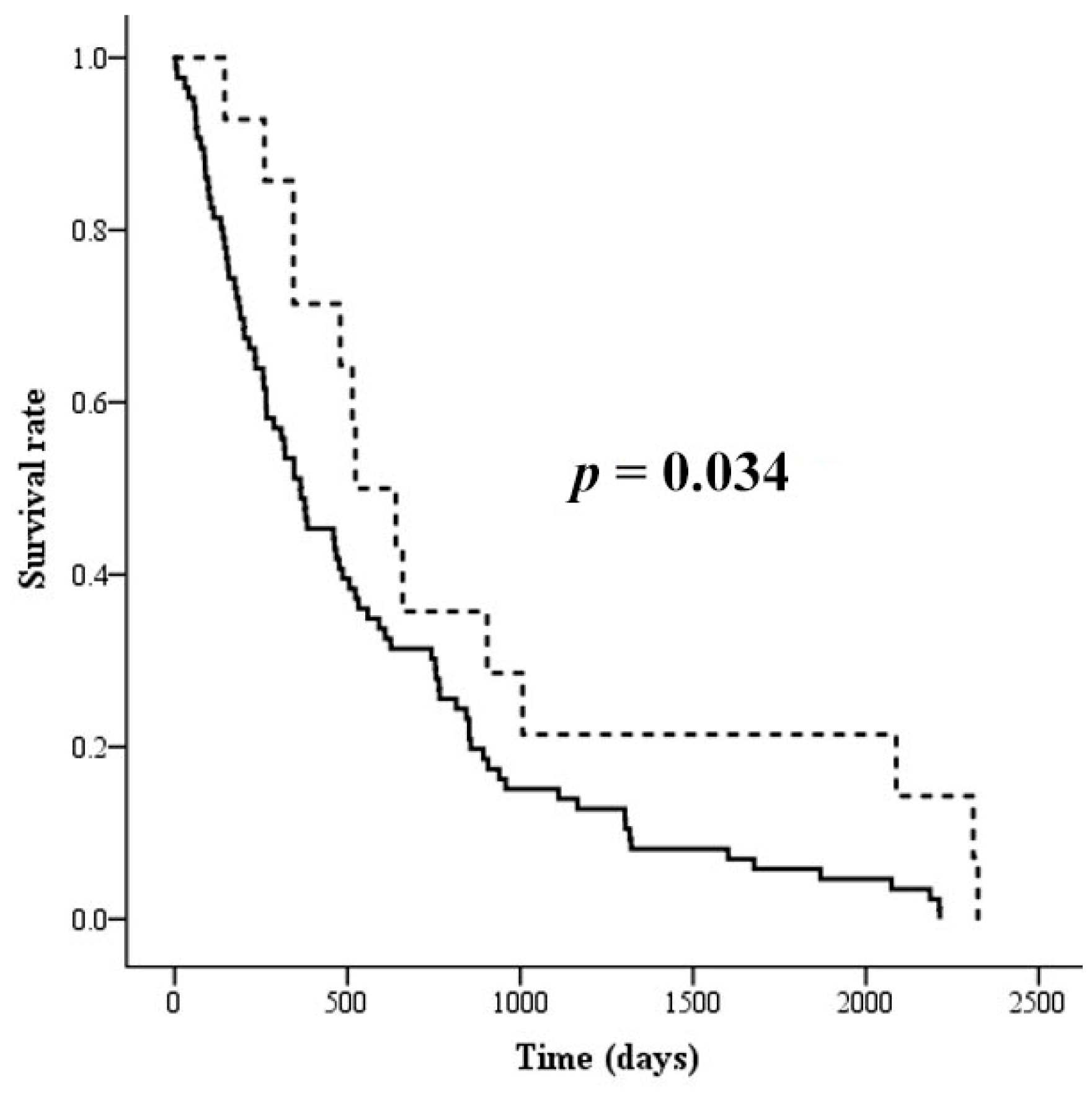
3.4. The Association of Altered Glycoforms of Serotransferrin and Clinicopathological Data of CCA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Songserm, N.; Promthet, S.; Sithithaworn, P.; Pientong, C.; Ekalaksananan, T.; Chopjitt, P.; Parkin, D.M. Risk factors for cholangiocarcinoma in high-risk area of Thailand: Role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol. 2012, 36, e89–e94. [Google Scholar] [CrossRef] [PubMed]
- Pinlaor, S.; Hiraku, Y.; Ma, N.; Yongvanit, P.; Semba, R.; Oikawa, S.; Murata, M.; Sripa, B.; Sithithaworn, P.; Kawanishi, S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: A model of inflammation-mediated carcinogenesis. Nitric Oxide 2004, 11, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pinlaor, S.; Prakobwong, S.; Boonmars, T.; Wongkham, C.; Pinlaor, P.; Hiraku, Y. Effect of praziquantel treatment on the expression of matrix metalloproteinases in relation to tissue resorption during fibrosis in hamsters with acute and chronic Opisthorchis viverrini infection. Acta Trop. 2009, 111, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Chamadol, N.; Pairojkul, C.; Khuntikeo, N.; Laopaiboon, V.; Loilome, W.; Sithithaworn, P.; Yongvanit, P. Histological confirmation of periductal fibrosis from ultrasound diagnosis in cholangiocarcinoma patients. J. Hepato Biliary Pancreat. Sci. 2014, 21, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nagakawa, T.; Konishi, I.; Ueno, K.; Kanno, M.; Akiyama, T.; Kayahara, M.; Izumi, R.; Konishi, K.; Miyazaki, I.; et al. Clinical experience of intrahepatic cholangiocarcinoma associated with hepatolithiasis. Jpn. J. Surg. 1988, 18, 47–53. [Google Scholar] [CrossRef]
- Paganuzzi, M.; Onetto, M.; Marroni, P.; Barone, D.; Conio, M.; Aste, H.; Pugliese, V. CA 19-9 and CA 50 in benign and malignant pancreatic and biliary diseases. Cancer 1988, 61, 2100–2108. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef]
- Schwarz, F.; Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 2011, 21, 576–582. [Google Scholar] [CrossRef]
- Dwek, R.A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kamiya, Y. Structural views of glycoprotein-fate determination in cells. Glycobiology 2007, 17, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Haltiwanger, R.S.; Lowe, J.B. Role of glycosylation in development. Annu. Rev. Biochem. 2004, 73, 491–537. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Dennis, J.W. N-Glycans in cancer progression. Glycobiology 2008, 18, 750–760. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Takahashi, M.; Gu, J.G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef]
- Baranov, V.S.; Schwartzman, A.L.; Gorbunova, V.N.; Gaitskhoki, V.S.; Rubtsov, N.B.; Timchenko, N.A.; Neifakh, S.A. Chromosomal localization of ceruloplasmin and transferrin genes in laboratory rats, mice and in man by hybridization with specific DNA probes. Chromosoma 1987, 96, 60–66. [Google Scholar] [CrossRef]
- Bowman, B.H.; Yang, F.M.; Adrian, G.S. Transferrin: Evolution and genetic regulation of expression. Adv. Genet. 1988, 25, 1–38. [Google Scholar]
- De Jong, G.; van Dijk, J.P.; van Eijk, H.G. The biology of transferrin. Clin. Chim. Acta 1990, 190, 1–46. [Google Scholar] [CrossRef]
- Satomi, Y.; Shimonishi, Y.; Takao, T. N-glycosylation at Asn(491) in the Asn-Xaa-Cys motif of human transferrin. FEBS Lett. 2004, 576, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y.; Shimonishi, Y.; Hase, T.; Takao, T. Site-specific carbohydrate profiling of human transferrin by nano-flow liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Abd Hamid, U.M.; Evans, R.; Arnold, J.N.; Banks, R.E.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 2007, 17, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.; Goldman, R. Glycoprotein disease markers and single protein-omics. Mol. Cell. Proteom. 2013, 12, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Misek, D.E. Glycoproteomics-based identification of cancer biomarkers. Int. J. Proteom. 2011, 2011, 601937. [Google Scholar] [CrossRef]
- Cazet, A.; Julien, S.; Bobowski, M.; Krzewinski-Recchi, M.A.; Harduin-Lepers, A.; Groux-Degroote, S.; Delannoy, P. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr. Res. 2010, 345, 1377–1383. [Google Scholar] [CrossRef]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef]
- Rachagani, S.; Torres, M.P.; Moniaux, N.; Batra, S.K. Current status of mucins in the diagnosis and therapy of cancer. Biofactors 2009, 35, 509–527. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Tamura, Y.; Suda, T. History and recent progress in evaluation of the fucosylated alpha-fetoprotein fraction. J. Gastroenterol. Hepatol. 2011, 26, 615–616. [Google Scholar] [CrossRef]
- Steinberg, W. The clinical utility of the CA 19-9 tumor-associated antigen. Am. J. Gastroenterol. 1990, 85, 350–355. [Google Scholar]
- De Leoz, M.L.; Young, L.J.; An, H.J.; Kronewitter, S.R.; Kim, J.; Miyamoto, S.; Borowsky, A.D.; Chew, H.K.; Lebrilla, C.B. High-mannose glycans are elevated during breast cancer progression. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Kronewitter, S.R.; De Leoz, M.L.; Strum, J.S.; An, H.J.; Dimapasoc, L.M.; Guerrero, A.; Miyamoto, S.; Lebrilla, C.B.; Leiserowitz, G.S. The glycolyzer: Automated glycan annotation software for high performance mass spectrometry and its application to ovarian cancer glycan biomarker discovery. Proteomics 2012, 12, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, M.A.; Smith, D.; Yang, J.; Cross, T.J.; Jackson, A.M.; Hardie, D.B.; Anderson, N.L.; Borchers, C.H. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell. Proteom. 2009, 8, 1860–1877. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, G.; Seymour, A.B. High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics. Anal. Chem. 2010, 82, 5527–5533. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Ruhaak, L.R.; Stroble, C.; Parker, E.; Huang, J.; Maverakis, E.; Lebrilla, C.B. A Method for Comprehensive Glycosite-Mapping and Direct Quantitation of Serum Glycoproteins. J. Proteome Res. 2015, 14, 5179–5192. [Google Scholar] [CrossRef] [PubMed]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lonnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Dorland, L.; Haverkamp, J.; Schut, B.L.; Vliegenthart, J.F. The structure of the asialo-carbohydrate units of human serotransferrin as proven by 360 MHz proton magnetic resonance spectroscopy. FEBS Lett. 1977, 77, 15–20. [Google Scholar] [CrossRef]
- Spik, G.; Bayard, B.; Fournet, B.; Strecker, G.; Bouquelet, S.; Montreuil, J. Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett. 1975, 50, 296–299. [Google Scholar] [CrossRef]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Kushner, I. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [CrossRef]
- Ahmed, N.; Oliva, K.T.; Barker, G.; Hoffmann, P.; Reeve, S.; Smith, I.A.; Quinn, M.A.; Rice, G.E. Proteomic tracking of serum protein isoforms as screening biomarkers of ovarian cancer. Proteomics 2005, 5, 4625–4636. [Google Scholar] [CrossRef] [PubMed]
- Tosner, J.; Krejsek, J.; Louda, B. Serum prealbumin, transferrin and alpha-1-acid glycoprotein in patients with gynecological carcinomas. Neoplasma 1988, 35, 403–411. [Google Scholar] [PubMed]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: A practical, simple and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 1999, 13, 273–279. [Google Scholar] [CrossRef]
- Charoensuk, L.; Pinlaor, P.; Wanichwecharungruang, S.; Intuyod, K.; Vaeteewoottacharn, K.; Chaidee, A.; Yongvanit, P.; Pairojkul, C.; Suwannateep, N.; Pinlaor, S. Nanoencapsulated curcumin and praziquantel treatment reduces periductal fibrosis and attenuates bile canalicular abnormalities in Opisthorchis viverrini-infected hamsters. Nanomedicine 2016, 12, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Intuyod, K.; Priprem, A.; Limphirat, W.; Charoensuk, L.; Pinlaor, P.; Pairojkul, C.; Lertrat, K.; Pinlaor, S. Anti-inflammatory and anti-periductal fibrosis effects of an anthocyanin complex in Opisthorchis viverrini-infected hamsters. Food Chem. Toxicol. 2014, 74, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Jamnongkan, W.; Thanee, M.; Yongvanit, P.; Loilome, W.; Thanan, R.; Kimawaha, P.; Boonmars, T.; Silakit, R.; Namwat, N.; Techasen, A. Antifibrotic effect of xanthohumol in combination with praziquantel is associated with altered redox status and reduced iron accumulation during liver fluke-associated cholangiocarcinogenesis. PeerJ 2018, 6, e4281. [Google Scholar] [CrossRef] [PubMed]
- Coddeville, B.; Carchon, H.; Jaeken, J.; Briand, G.; Spik, G. Determination of glycan structures and molecular masses of the glycovariants of serum transferrin from a patient with carbohydrate deficient syndrome type II. Glycoconj. J. 1998, 15, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Van Rooijen, J.J.; Jeschke, U.; Kamerling, J.P.; Vliegenthart, J.F. Expression of N-linked sialyl Le(x) determinants and O-glycans in the carbohydrate moiety of human amniotic fluid transferrin during pregnancy. Glycobiology 1998, 8, 1053–1064. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamashita, K.; Koide, N.; Endo, T.; Iwaki, Y.; Kobata, A. Altered glycosylation of serum transferrin of patients with hepatocellular carcinoma. J. Biol. Chem. 1989, 264, 2415–2423. [Google Scholar] [PubMed]
- Lesnikov, V.; Lesnikova, M.; Deeg, H.J. Pro-apoptotic and anti-apoptotic effects of transferrin and transferrin-derived glycans on hematopoietic cells and lymphocytes. Exp. Hematol. 2001, 29, 477–489. [Google Scholar] [CrossRef]
- Bhide, G.P.; Colley, K.J. Sialylation of N-glycans: Mechanism, cellular compartmentalization and function. Histochem. Cell Biol. 2017, 147, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Wongkham, S.; Boonla, C.; Kongkham, S.; Wongkham, C.; Bhudhisawasdi, V.; Sripa, B. Serum total sialic acid in cholangiocarcinoma patients: An ROC curve analysis. Clin. Biochem. 2001, 34, 537–541. [Google Scholar] [CrossRef]
- Wongkham, S.; Bhudhisawasdi, V.; Chau-in, S.; Boonla, C.; Muisuk, K.; Kongkham, S.; Wongkham, C.; Boonsiri, P.; Thuwajit, P. Clinical significance of serum total sialic acid in cholangiocarcinoma. Clin. Chim. Acta 2003, 327, 139–147. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Chiricolo, M. Sialyltransferases in cancer. Glycoconj. J. 2001, 18, 841–850. [Google Scholar] [CrossRef] [PubMed]
| Variable | Control (n = 100) | # PDF (n = 50) | $ CCA(n = 100) |
|---|---|---|---|
| Age (Years) | |||
| <56 | 57 | 29 | 41 |
| >56 | 43 | 21 | 59 |
| Gender | |||
| Male | 42 | 22 | 46 |
| Female | 58 | 28 | 54 |
| Histological grading | |||
| Papillary | - | - | 55 |
| Tubular | - | - | 45 |
| Metastasis stage | - | - | 48 |
| Non-metastasis | - | - | 52 |
| Group | $ Glycoform | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6503 | 5422 | 6502 | 6511 | 6521 | 5402 | 5412 | 5421 | 6501 | |
| Control vs. PDF | 0.608 * | 0.511 | 0.651 ** | 0.632 ** | 0.578 | 0.596 * | 0.653 ** | 0.596 | 0.653 ** |
| Control vs. CCA | 0.681 *** | 0.593 * | 0.792 *** | 0.610 ** | 0.696 *** | 0.637 ** | 0.710 *** | 0.637 ** | 0.710 *** |
| PDF vs. CCA | 0.604 * | 0.595 | 0.682 *** | 0.500 | 0.735 *** | 0.544 | 0.609 * | 0.539 | 0.685 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamnongkan, W.; Lebrilla, C.B.; Barboza, M.; Techasen, A.; Loilome, W.; Sithithaworn, P.; Khuntikeo, N.; Pairojkul, C.; Chamadol, N.; Thanan, R.; et al. Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma. Biomolecules 2019, 9, 538. https://doi.org/10.3390/biom9100538
Jamnongkan W, Lebrilla CB, Barboza M, Techasen A, Loilome W, Sithithaworn P, Khuntikeo N, Pairojkul C, Chamadol N, Thanan R, et al. Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma. Biomolecules. 2019; 9(10):538. https://doi.org/10.3390/biom9100538
Chicago/Turabian StyleJamnongkan, Wassana, Carlito B. Lebrilla, Mariana Barboza, Anchalee Techasen, Watcharin Loilome, Paiboon Sithithaworn, Narong Khuntikeo, Chawalit Pairojkul, Nittaya Chamadol, Raynoo Thanan, and et al. 2019. "Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma" Biomolecules 9, no. 10: 538. https://doi.org/10.3390/biom9100538
APA StyleJamnongkan, W., Lebrilla, C. B., Barboza, M., Techasen, A., Loilome, W., Sithithaworn, P., Khuntikeo, N., Pairojkul, C., Chamadol, N., Thanan, R., & Yongvanit, P. (2019). Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma. Biomolecules, 9(10), 538. https://doi.org/10.3390/biom9100538