Diversity of Amyloid Motifs in NLR Signaling in Fungi
Abstract
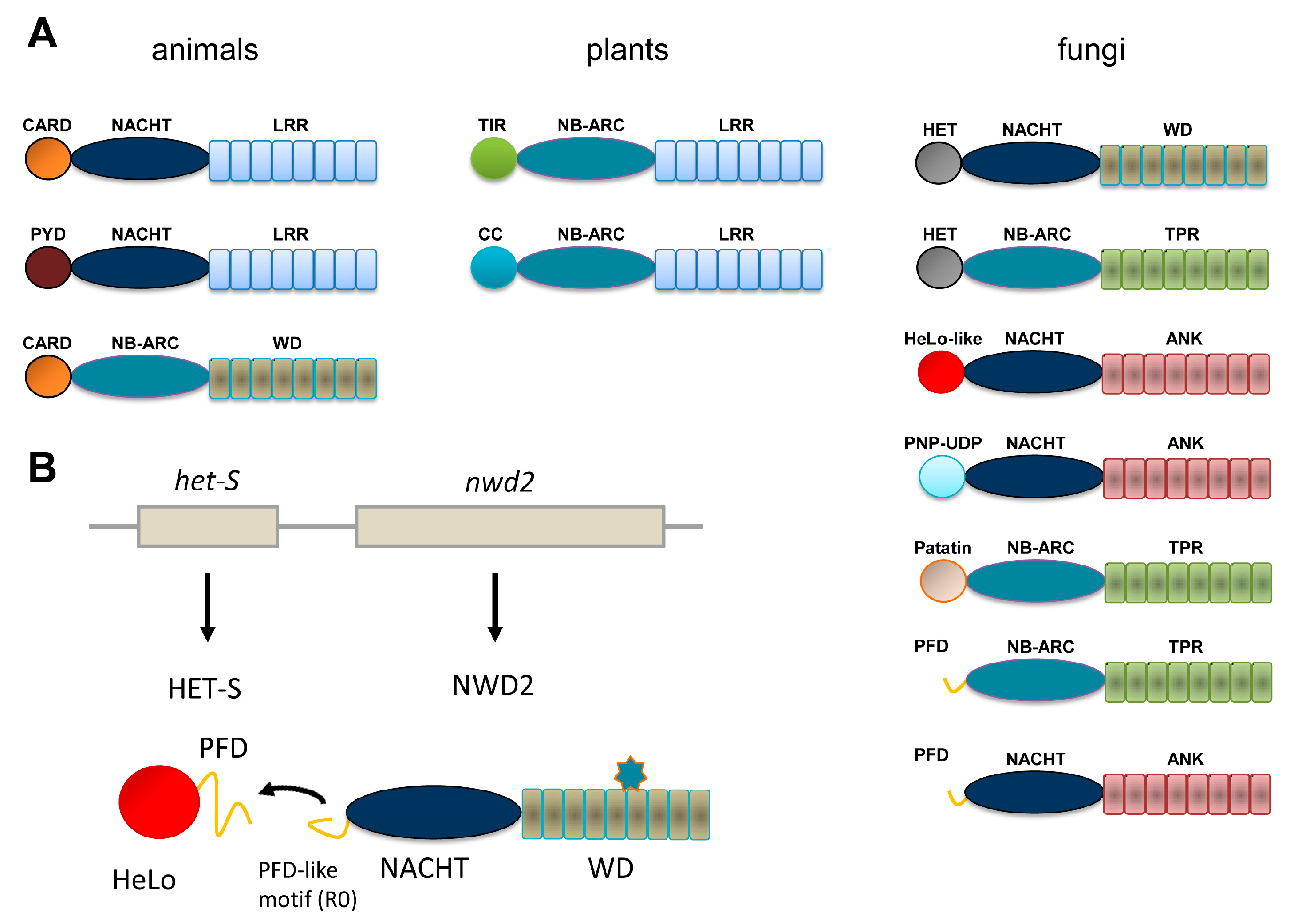
:1. NLR in Animals and Plants and NLR-Like Receptors in Fungi
2. The HET-s/HET-S System
3. The NWD2/HET-S Paradigm
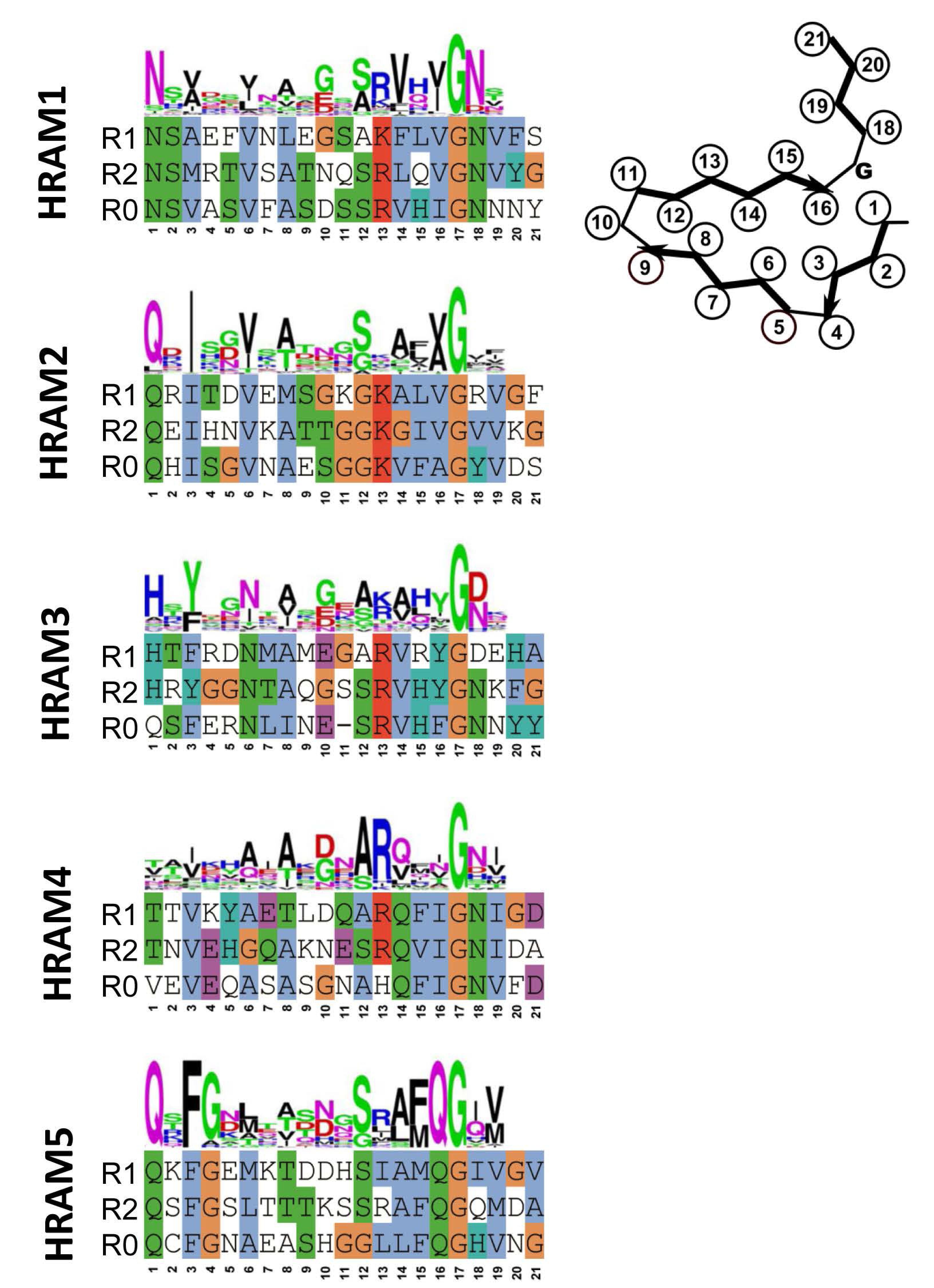
4. Additional Het-s-Related Amyloid Motifs
5. PP and Sigma, Two Other Types of NLR-Associated Amyloid Signaling Motifs
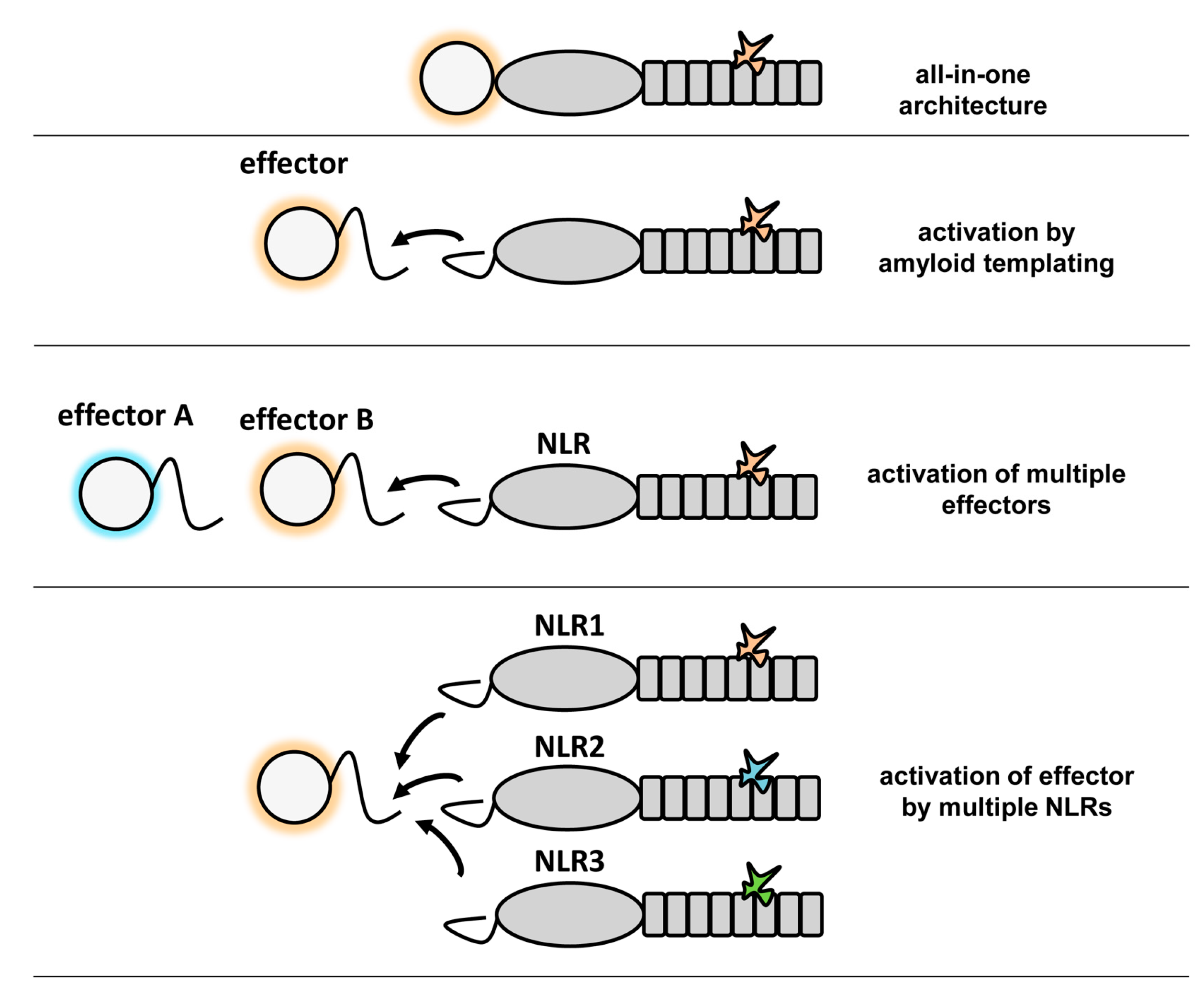
6. Potential Advantages of Amyloid Signaling
7. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, J.D.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Kufer, T.A.; Schulze-Lefert, P. NLR functions in plant and animal immune systems: So far and yet so close. Nat. Immunol. 2011, 12, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Diebolder, C.A.; Halff, E.F.; Koster, A.J.; Huizinga, E.G.; Koning, R.I. Cryoelectron tomography of the NAIP5/NLRC4 inflammasome: Implications for NLR activation. Structure 2015, 23, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Halff, E.F.; Diebolder, C.A.; Versteeg, M.; Schouten, A.; Brondijk, T.H.; Huizinga, E.G. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J. Biol. Chem. 2012, 287, 38460–38472. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, Z.; Ma, Y.; Furzer, O.J.; Huh, S.U.; Cevik, V.; Jones, J.D.; Sarris, P.F. Pathogen perception by NLRS in plants and animals: Parallel worlds. Bioessays 2016, 38, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Dyrka, W.; Lamacchia, M.; Durrens, P.; Kobe, B.; Daskalov, A.; Paoletti, M.; Sherman, D.J.; Saupe, S.J. Diversity and variability of NOD-like receptors in fungi. Genome Biol. Evol. 2014, 6, 3137–3158. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Paoletti, M.; Ness, F.; Saupe, S.J. Genomic clustering and homology between HET-S and the NWD2 stand protein in various fungal genomes. PLoS ONE 2012, 7, e34854. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Dyrka, W.; Saupe, S.J. Theme and variations: Evolutionary diversification of the HET-S functional amyloid motif. Sci. Rep. 2015, 5, 12494. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Habenstein, B.; Martinez, D.; Debets, A.J.; Sabate, R.; Loquet, A.; Saupe, S.J. Signal transduction by a fungal NOD-like receptor based on propagation of a prion amyloid fold. PLoS Biol. 2015, 13, e1002059. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Habenstein, B.; Sabate, R.; Berbon, M.; Martinez, D.; Chaignepain, S.; Coulary-Salin, B.; Hofmann, K.; Loquet, A.; Saupe, S.J. Identification of a novel cell death-inducing domain reveals that fungal amyloid-controlled programmed cell death is related to necroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Pinan-Lucarre, B.; Paoletti, M.; Clave, C. Cell death by incompatibility in the fungus Podospora. Semin. Cancer Biol. 2007, 17, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Saupe, S.J. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin. Cell Dev. Biol. 2011, 22, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Wasmer, C.; Lange, A.; Van Melckebeke, H.; Siemer, A.B.; Riek, R.; Meier, B.H. Amyloid fibrils of the HET-s(218–289) prion form a βsolenoid with a triangular hydrophobic core. Science 2008, 319, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Stubbs, G. Fungal prion HET-s as a model for structural complexity and self-propagation in prions. Proc. Natl. Acad. Sci. USA 2014, 111, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Gantner, M.; Walti, M.A.; Schmidlin, T.; Chi, C.N.; Wasmer, C.; Schutz, A.; Ceschin, J.; Clave, C.; Cescau, S.; et al. Contribution of specific residues of the β-solenoid fold to HET-s prion function, amyloid structure and stability. PLoS Pathog. 2014, 10, e1004158. [Google Scholar] [CrossRef] [PubMed]
- Balguerie, A.; Dos Reis, S.; Ritter, C.; Chaignepain, S.; Coulary-Salin, B.; Forge, V.; Bathany, K.; Lascu, I.; Schmitter, J.M.; Riek, R.; et al. Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J. 2003, 22, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, J.; Buhtz, C.; Ritter, C.; Kwiatkowski, W.; Choe, S.; Maddelein, M.L.; Ness, F.; Cescau, S.; Soragni, A.; Leitz, D.; et al. The mechanism of prion inhibition by HET-S. Mol. Cell 2010, 38, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Seuring, C.; Riek, R.; Saupe, S.J.; Liebman, S.W. Localization of HET-S to the cell periphery, not to [Het-s] aggregates, is associated with [Het-s]–HET-S toxicity. Mol. Cell. Biol. 2012, 32, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Seuring, C.; Greenwald, J.; Wasmer, C.; Wepf, R.; Saupe, S.J.; Meier, B.H.; Riek, R. The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol. 2012, 10, e1001451. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, A.; Saupe, S.J. As a toxin dies a prion comes to life: A tentative natural history of the [Het-s] prion. Prion 2015, 9, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, J.; Xu, H.; Liu, S.; Jiang, Q.X.; Halfmann, R.; Chen, Z.J. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 2014, 156, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I.; et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kajava, A.V.; Klopffleisch, K.; Chen, S.; Hofmann, K. Evolutionary link between metazoan RHIM motif and prion-forming domain of fungal heterokaryon incompatibility factor HET-s/HET-s. Sci. Rep. 2014, 4, 7436. [Google Scholar] [CrossRef] [PubMed]
- Graziani, S.; Silar, P.; Daboussi, M.J. Bistability and hysteresis of the ‘Secteur’ differentiation are controlled by a two-gene locus in Nectria haematococca. BMC Biol. 2004, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. Higher-order assemblies in a new paradigm of signal transduction. Cell 2013, 153, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xu, H.; Chen, Z.J. Prion-like polymerization in immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Sun, L.; Zheng, H.; Skaug, B.; Jiang, Q.X.; Chen, Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011, 146, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schroder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Dawe, A.L.; Churbanov, A.; Smith, M.L.; Milgroom, M.G.; Nuss, D.L. Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 2012, 190, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Saupe, S.; Turcq, B.; Begueret, J. A gene responsible for vegetative incompatibility in the fungus Podospora anserina encodes a protein with a GTP-binding motif and Gβ homologous domain. Gene 1995, 162, 135–139. [Google Scholar] [CrossRef]
- Chevanne, D.; Bastiaans, E.; Debets, A.; Saupe, S.J.; Clave, C.; Paoletti, M. Identification of the het-r vegetative incompatibility gene of Podospora anserina as a member of the fast evolving HNWD gene family. Curr. Genet. 2009, 55, 93–102. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loquet, A.; Saupe, S.J. Diversity of Amyloid Motifs in NLR Signaling in Fungi. Biomolecules 2017, 7, 38. https://doi.org/10.3390/biom7020038
Loquet A, Saupe SJ. Diversity of Amyloid Motifs in NLR Signaling in Fungi. Biomolecules. 2017; 7(2):38. https://doi.org/10.3390/biom7020038
Chicago/Turabian StyleLoquet, Antoine, and Sven J. Saupe. 2017. "Diversity of Amyloid Motifs in NLR Signaling in Fungi" Biomolecules 7, no. 2: 38. https://doi.org/10.3390/biom7020038





