A Panel of Recombinant Mucins Carrying a Repertoire of Sialylated O-Glycans Based on Different Core Chains for Studies of Glycan Binding Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Engineering of Stable CHO-K1 Cells Expressing PSGL-1/mIgG2b Carrying a Repertoire of Different Sialylated O-Glycans
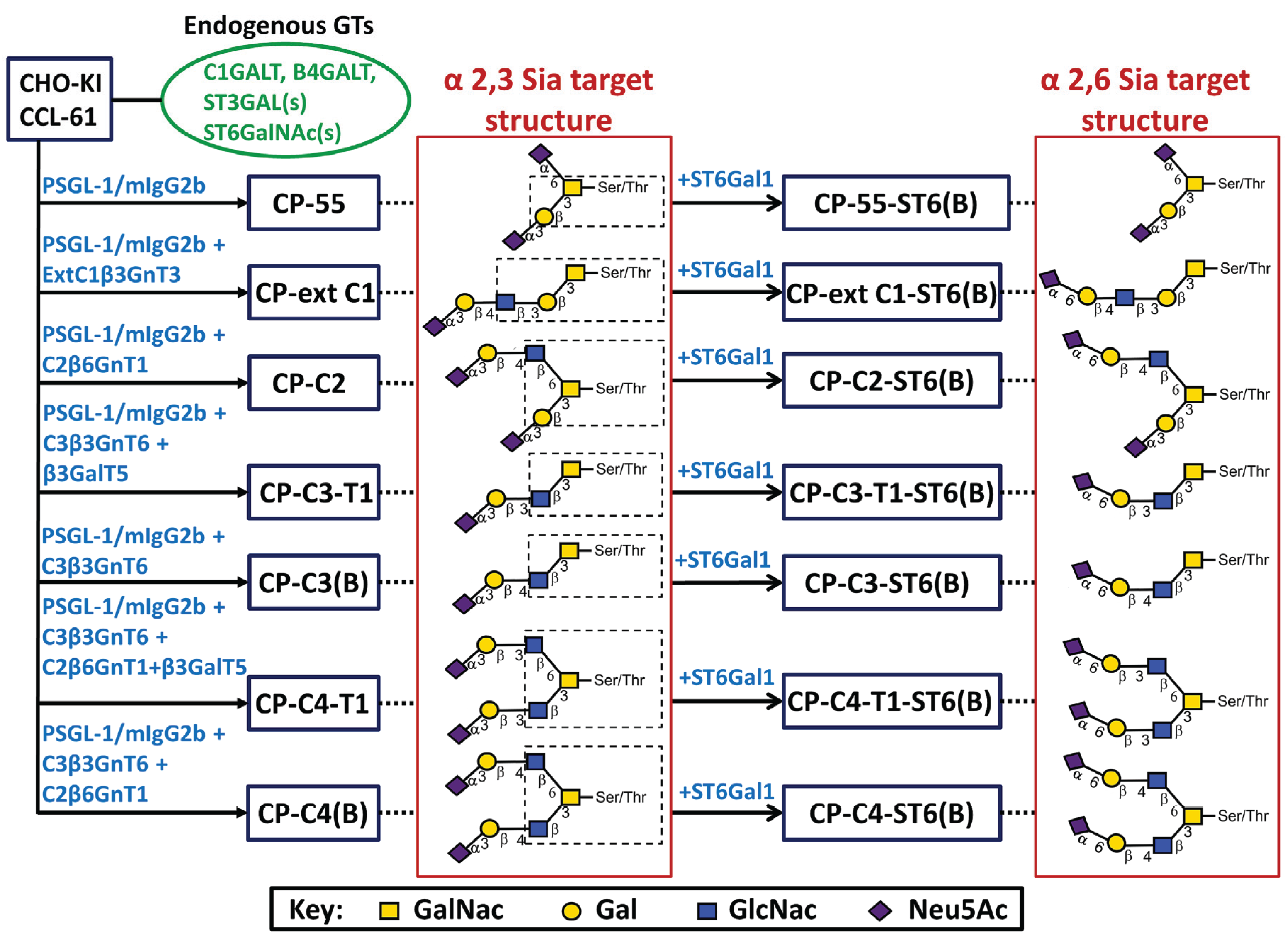
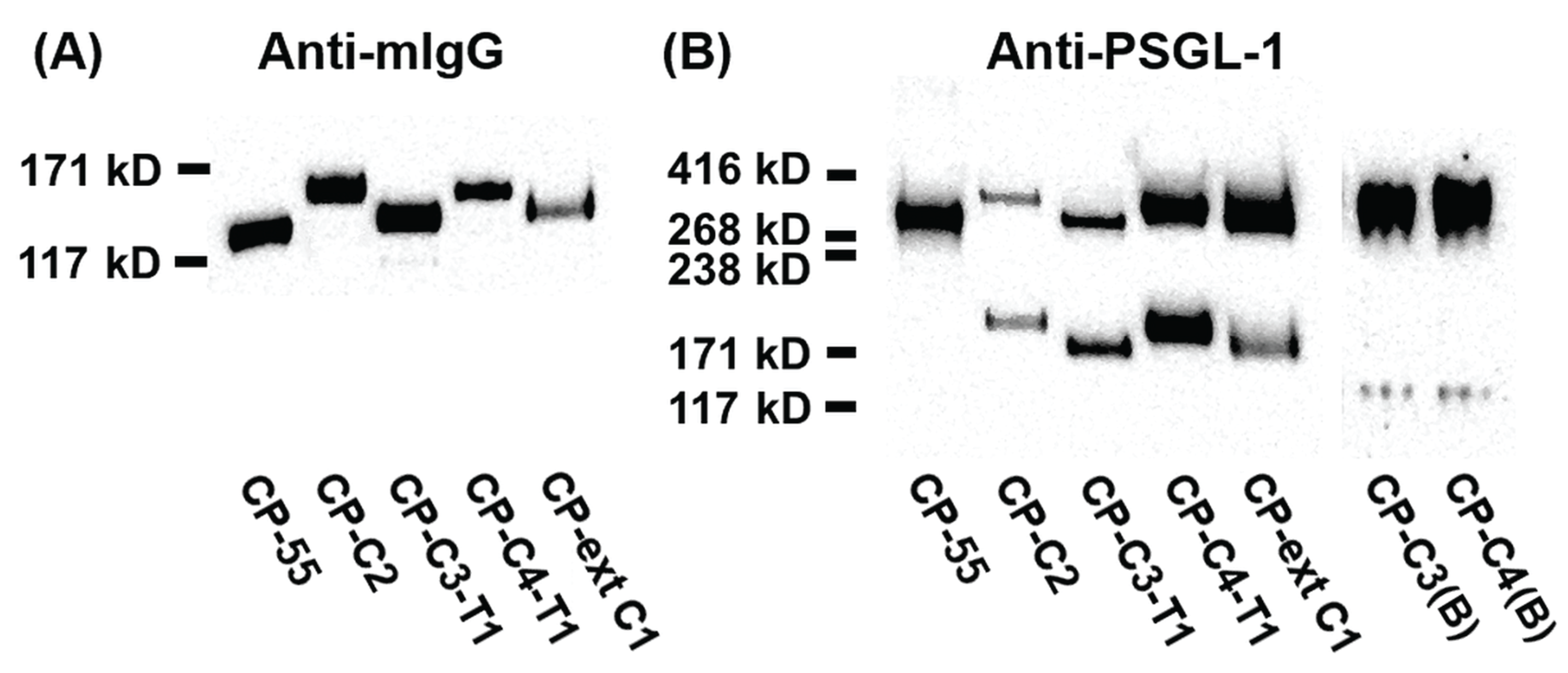
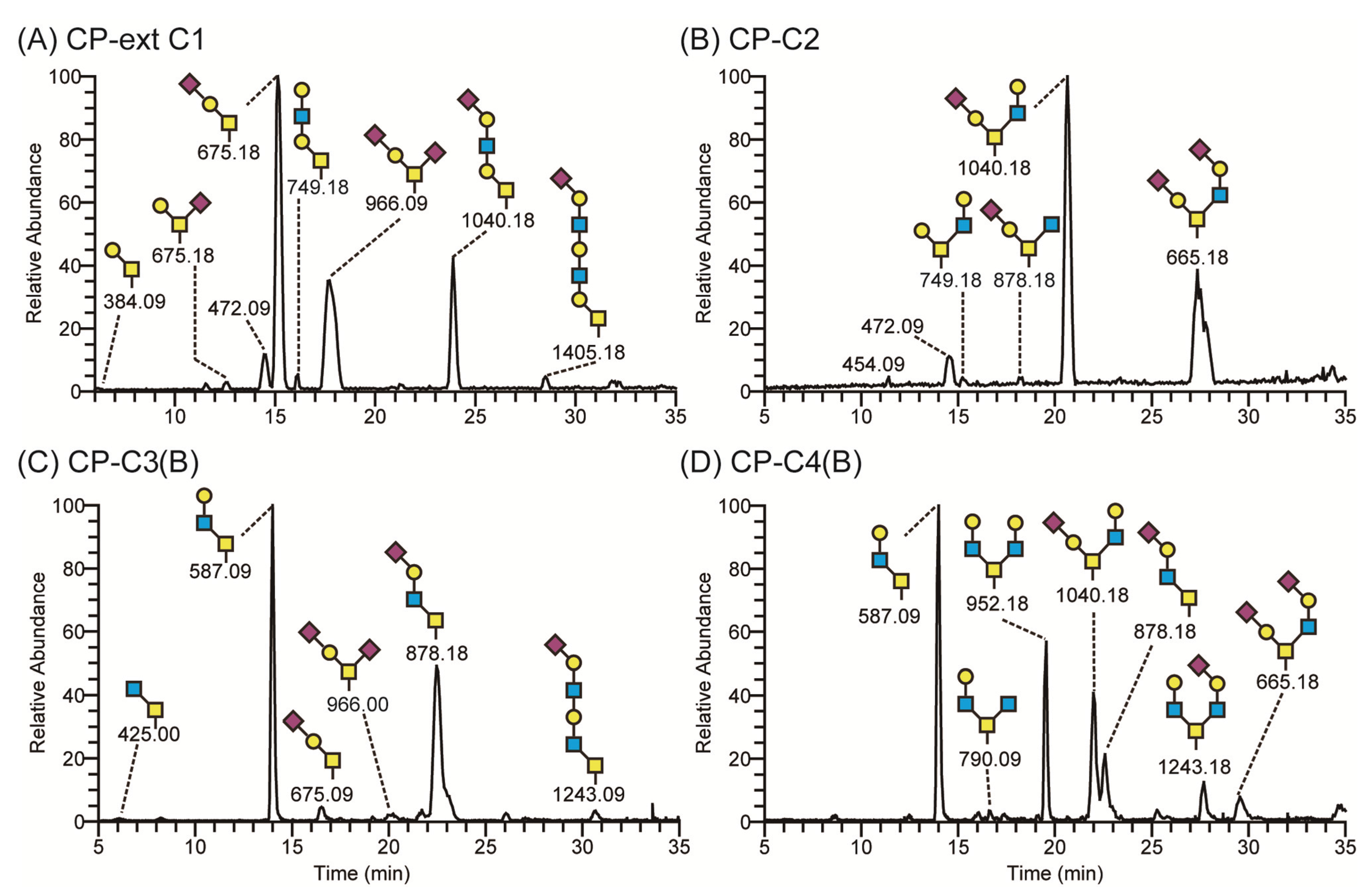
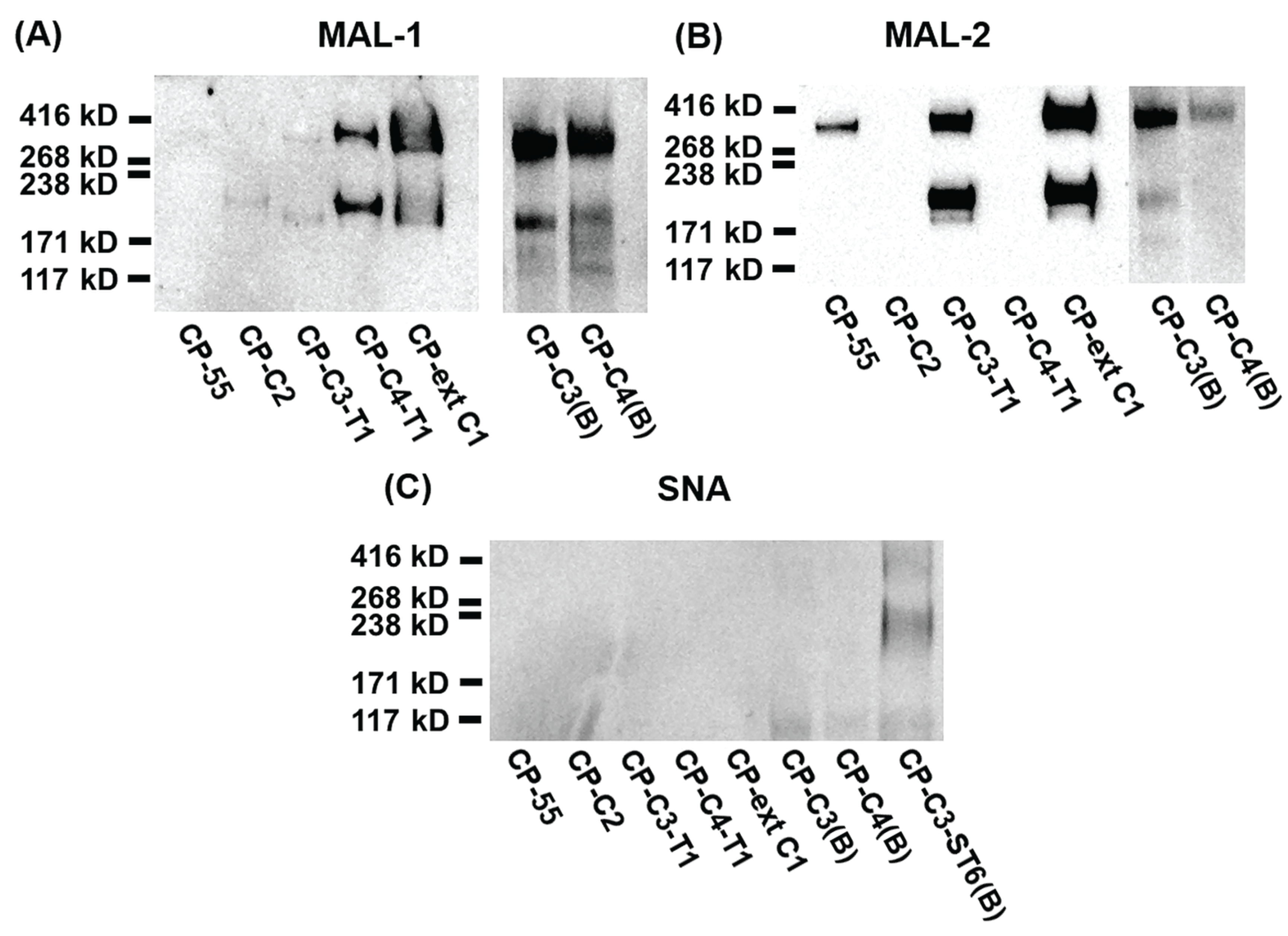
2.2. Expression and Characterization of PSGL-1/ mIgG2b Carrying the Major O-Glycan Core Structures
| Mass | Compsition | Putative structures | Core | exC1 | C2 | C3B | C4B | C3T1 | C4T1 | ST6B | exC1ST6B | C2ST6B | C3ST6B | C4ST6B | C3T1ST6B | C4T1ST6B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 384 | H1N1 | Galβ1-3GalNAcol | 1 | 0.5 | — | — | — | 2.0 | — | 5.2 | 6.6 | 5.5 | — | — | — | 3.9 | |
| 425 | N2 | GlcNAcβ1-3GalNAcol | 3 | — | — | 2.2 | 2.1 | — | — | — | — | — | 1.0 | — | — | — | |
| 587-1 | H1N2 | Galβ1-3(GlcNAcβ1-6)GalNAcol | 2 | — | — | — | 0.7 | — | 0.2 | — | — | — | — | — | — | 1.7 | |
| 587-2 | H1N2 | Galβ1-4GlcNAcβ1-3GalNAcol | 3 | — | — | 29.1 | 17.1 | — | 1.6 | — | — | — | 11.4 | 12.8 | 4.0 | 1.0 | |
| 587-3 | H1N2 | Galβ1-3GlcNAcβ1-3GalNAcol | 3 | — | — | — | — | 0.7 | 1.2 | — | — | — | 1.9 | — | 29.0 | 3.8 | |
| 667 | H1N2S1 | Galβ1-4(6S)GlcNAcβ1-3GalNAcol | 3 | — | — | 1.2 | 2.1 | — | — | — | — | — | — | — | — | — | |
| 675-1 | Na1H1N1 | Galβ1-3(NeuAcα2-6)GalNAcol | 1 | 1.0 | — | 1.2 | 0.2 | 1.9 | — | 4.1 | 3.6 | 2.3 | — | — | — | 1.1 | |
| 675-2 | Na1H1N1 | NeuAcα2-3Galβ1-3GalNAcol | 1 | 37.0 | 1.5 | 5.2 | 1.3 | 40.9 | 3.6 | 47.1 | 33.2 | 20.5 | 6.2 | 5.9 | 14.5 | 7.8 | |
| 691 | Ng1H1N1 | NeuGcα2-3Galβ1-3GalNAcol | 1 | 1.5 | — | — | — | 1.4 | — | 1.5 | 1.2 | 0.8 | — | — | — | — | |
| 749-1 | H2N2 | Galβ1-3(Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | 1.3 | 1.3 | 1.3 | — | 2.5 | — | — | 1.8 | — | 2.7 | — | 8.2 | |
| 749-2 | H2N2 | Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | 1.1 | — | — | — | — | — | — | 4.3 | — | 1.9 | — | — | — | |
| 790-1 | H1N3 | GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 0.5 | — | 0.1 | — | — | — | — | 2.8 | — | 3.2 | |
| 790-2 | H1N3 | Galβ1-4GlcNAcβ1-3(GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 1.1 | — | — | — | — | — | — | — | — | — | |
| 829 | H2N2S1 | Galβ1-3[Galβ1-4(6S)GlcNAcβ1-6]GalNAcol | 2 | — | — | — | — | — | 0.3 | — | — | — | — | — | — | 2.1 | |
| 878-1 | Na1H1N2 | NeuAcα2-6Galβ1-4GlcNAcβ1-3GalNAcol | 3 | — | — | — | — | — | — | — | — | — | 0.7 | — | — | — | |
| 878-2 | Na1H1N2 | NeuAcα2-3Galβ1-3(GlcNAcβ1-6)GalNAcol | 2 | — | 1.7 | — | 0.8 | — | 2.8 | — | — | — | 3.0 | 7.3 | — | 1.8 | |
| 878-3 | Na1H1N2 | NeuAcα2-3Galβ1-4GlcNAcβ1-3GalNAcol | 3 | 0.7 | — | 43.6 | 7.6 | — | 1.7 | — | — | — | 20.3 | — | — | — | |
| 878-4 | Na1H1N2 | NeuAcα2-3Galβ1-3GlcNAcβ1-3GalNAcol | 3 | — | — | — | — | 1.7 | — | — | — | — | — | 11.4 | 39.7 | 1.9 | |
| 894 | Ng1H1N2 | NeuGcα2-3Galβ1-3GlcNAcβ1-3GalNAcol | 3 | — | — | 3.0 | — | — | — | — | — | — | — | — | — | — | |
| 952-1 | H2N3 | Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 11.0 | — | 1.2 | — | — | — | — | 6.9 | — | — | |
| 952-1 | H2N3 | Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3GalNAcol | 3 | — | — | 1.7 | 0.6 | — | — | — | — | — | — | — | — | — | |
| 966 | Na2H1N1 | NeuAcα2-3Galβ1-3(NeuAcα2-6)GalNAcol | 1 | 33.8 | — | 5.7 | — | 49.4 | 0.5 | 40.6 | 20.8 | 17.6 | 4.1 | — | 12.8 | 3.1 | |
| 982 | Na1Ng1H1N1 | NeuGcα2-3Galβ1-3(NeuAcα2-6)GalNAcol | 1 | 1.8 | — | — | — | 1.9 | — | 1.5 | — | 1.4 | — | — | — | — | |
| 1040-1 | Na1H2N2 | NeuAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | — | — | — | — | — | — | — | 4.0 | — | — | — | — | — | |
| 1040-2 | Na1H2N2 | NeuAcα2-3Galβ1-3(Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | 0.2 | 39.1 | 0.3 | 15.1 | 1.6 | 16.7 | — | — | 12.3 | 17.6 | — | — | 32.2 | |
| 1040-3 | Na1H2N2 | NeuAcα2-3Galβ1-3(Galβ1-3GlcNAcβ1-6)GalNAcol | 2 | — | — | — | — | — | — | — | — | — | — | 1.8 | — | — | |
| 1040-4 | Na1H2N2 | Galβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | — | — | 1.5 | — | — | — | — | — | — | — | — | — | |
| 1040-5 | Na1H2N2 | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | 17.1 | — | — | — | — | — | — | 9.9 | — | — | — | — | 5.1 | |
| 1056 | Ng1H2N2 | NeuGcα2-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | 1.0 | — | — | — | — | — | — | — | 1.1 | — | — | — | — | |
| 1081 | Na1H1N3 | GlcNAcβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 1.1 | — | — | — | — | — | — | — | — | — | |
| 1114 | H3N3 | Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | 0.4 | — | — | — | — | — | — | 4.5 | — | — | — | — | — | |
| 1120-1 | Na1H2N2S1 | NeuAcα2-3Galβ1-3[Galβ1-4(6S)GlcNAcβ1-6]GalNAcol | 2 | — | — | — | 1.2 | — | 6.8 | — | — | 4.5 | 7.2 | — | — | — | |
| 1120-2 | Na1H2N2S1 | NeuAcα2-3Galβ1-4(6S)GlcNAcβ1-3Galβ1-3GalNAcol | 1 | — | — | — | — | — | — | — | — | — | — | 2.9 | — | 1.2 | |
| 1243-1 | Na1H2N3 | NeuAcα2-3Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 2.1 | — | — | — | — | — | — | — | — | — | |
| 1243-2 | Na1H2N3 | Galβ1-4GlcNAcβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 6.5 | — | 0.7 | — | — | — | — | — | — | — | |
| 1243-3 | Na1H2N3 | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3GalNAcol | 3 | — | — | 4.7 | 1.1 | — | — | — | — | — | — | — | — | — | |
| 1331-1 | Na2H2N2 | NeuAcα2-3Galβ1-3(NeuAcα2-6Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | — | — | — | — | — | — | — | 14.3 | 13.7 | — | — | — | |
| 1331-2 | Na2H2N2 | NeuAcα2-3Galβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | 54.4 | — | 8.6 | 0.5 | 53.8 | — | — | — | — | 45.5 | — | 18.0 | |
| 1347 | Na1Ng1H2N2 | NeuGcα2-3Galβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | — | — | 1.9 | — | 1.3 | — | — | — | — | — | — | — | |
| 1405-1 | Na1H3N3 | NeuAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | — | — | — | — | — | — | — | — | — | 3.9 | — | — | 2.1 | |
| 1405-2 | Na1H3N3 | NeuAcα2-3Galβ1-3(Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | 0.7 | — | 2.2 | — | 0.8 | — | — | — | — | — | — | — | |
| 1405-3 | Na1H3N3 | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 1 | 3.4 | — | — | — | — | — | — | 11.9 | — | — | — | — | — | |
| 1411 | Na2H2N2S1 | NeuAcα2-3Galβ1-3[NeuAcα2-3Galβ1-4(6S)GlcNAcβ1-6]GalNAcol | 2 | — | — | — | 3.5 | — | 3.7 | — | — | 13.3 | — | — | — | — | |
| 1534 | Na2H2N3 | NeuAcα2-3Galβ1-4GlcNAcβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 6.2 | — | — | — | — | — | — | — | — | — | |
| 1550 | Na1Ng1H2N3 | NeuAcα2-3Galβ1-4GlcNAcβ1-3(NeuAGcα2-3Galβ1-4GlcNAcβ1-6)GalNAcol | 4 | — | — | — | 1.0 | — | — | — | — | — | — | — | — | — | |
| 1608 | Na1H3N4 | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3GalNAcol | 1 | — | — | 0.7 | — | — | — | — | — | — | — | — | — | — | |
| 1614 | Na2H2N3S1 | NeuAcα2-3Galβ1-4GlcNAcβ1-3[NeuAGcα2-3Galβ1-4(6S)GlcNAcβ1-6]GalNAcol | 3 | — | — | — | 1.0 | — | — | — | — | — | — | — | — | — | |
| 1696-1 | Na2H3N3 | NeuAcα2-3Galβ1-3(NeuAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6)GalNAcol | 2 | — | — | — | — | — | — | — | — | 4.6 | 7.1 | — | — | — | |
| 1696-2 | Na2H3N3 | NeuAcα2-3Galβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6)GalNAcol | 3 | — | 1.2 | — | — | — | 0.5 | — | — | — | — | — | — | 1.8 | |
| 1771 | Na1H4N4 | NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcol | 3 | 0.6 | — | — | — | — | — | — | — | — | — | — | — | — | |
| 1899 | Na2H3N4 | NeuAcα2-3Galβ1-4GlcNAcβ1-3(NeuAcα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6)GalNAcol | 3 | — | — | — | 0.7 | — | — | — | — | — | — | — | — | — | |
2.3. Expression and Characterization of PSGL-1/mIgG2b Carrying O-Glycans Extended with Type 1 Outer Core Chains
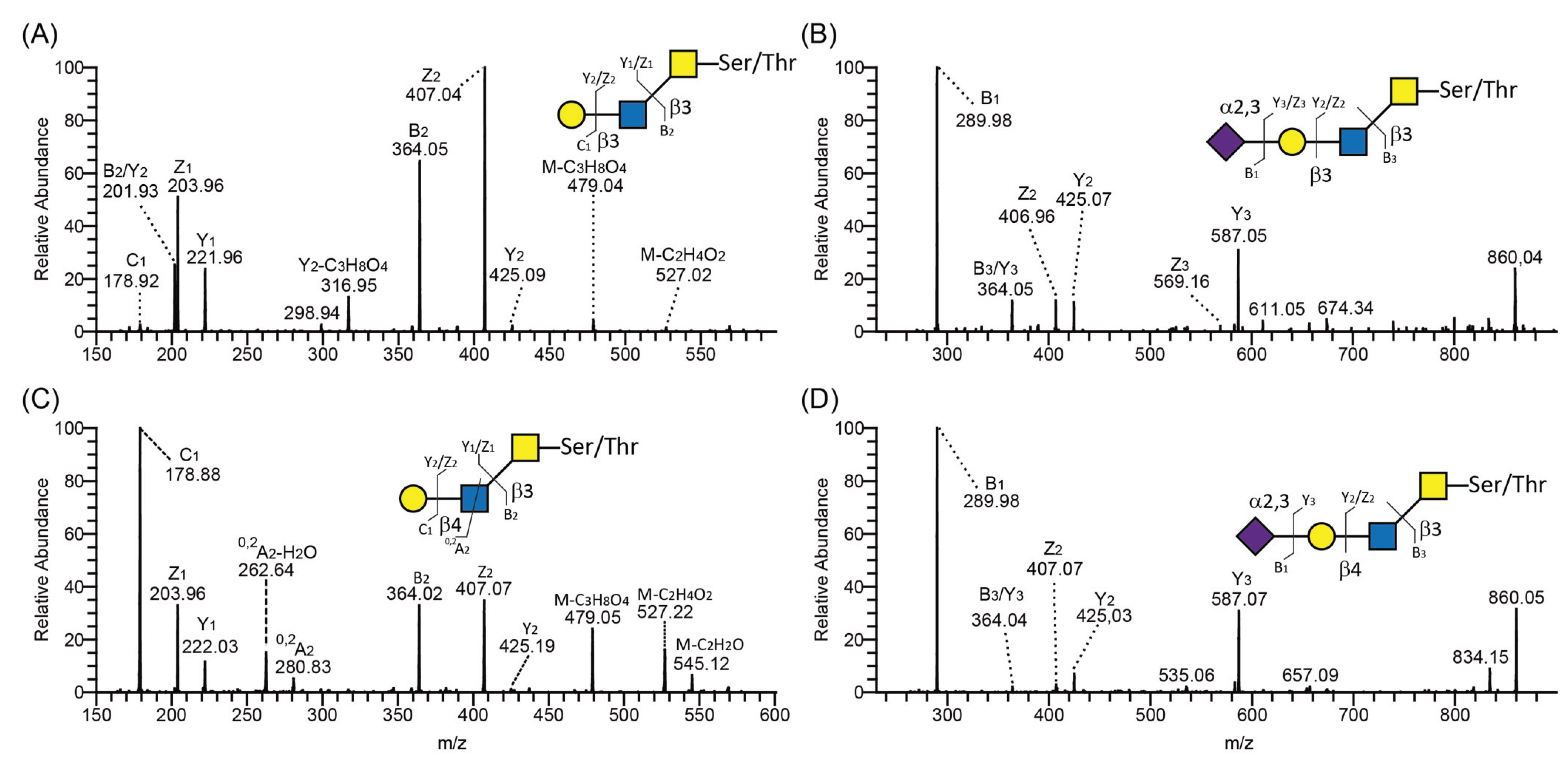
2.4. Expression and Characterization of PSGL-1/ mIgG2b Carrying α2,3- and α2,6-Sialylated O-Glycan Core Structures
3. Experimental Section
3.1. Cell Culture and Expression Vectors
| Expression vector | cDNA | Resistance gene | Source | Reference |
|---|---|---|---|---|
| EF1α/PSGL-1/EK/mIgG2b | PSGL-1/mIgG2b fusion gene | Puromycin acetyltransferase (puromycin resistance) | HL-60 cDNA library | [36] |
| EF1α/C2 β6GnT1 | β1,6-N-acetylglucosaminyltransferase 1 (GCNT1) | Neomycin phosphotransferase (G418 resistance) | HL-60 cDNA library | [25] |
| CMV/C3 β3GnT6 | β1,3-N-acetylglucosaminyltransferase 6 (B3GNT6) | Hygromycin resistance | Human stomach cDNA library | [23] |
| CMV/β3GalT5 | β1,3-galactosyltransferase 5 (B3GALT5) | Guanosine phosphoribosyl transferase (mycophenolic acid, xanthine and hypoxanthine resistance) | Human placental cDNA library | [23] |
| CMV/extended C1 β3GnT3 | β1,3-N-acetylglucosaminyltransferase 3 (B3GNT3) | ShBle (zeocin) resistance | HT-29 cDNA library | [18] |
| CMV/ST6Gal 1 | β-galactoside α 2,6 sialyltransferase 1 (ST6GAL1) | Blasticidin S deaminase (blasticidin resistance | Human placental cDNA library | [18] |
3.2. Transfection and Clonal Selection of Glyco-Engineered CHO Cells
3.3. Production and Purification of PSGL-1/mIgG2b Produced in Glyco-Engineered CHO Cells
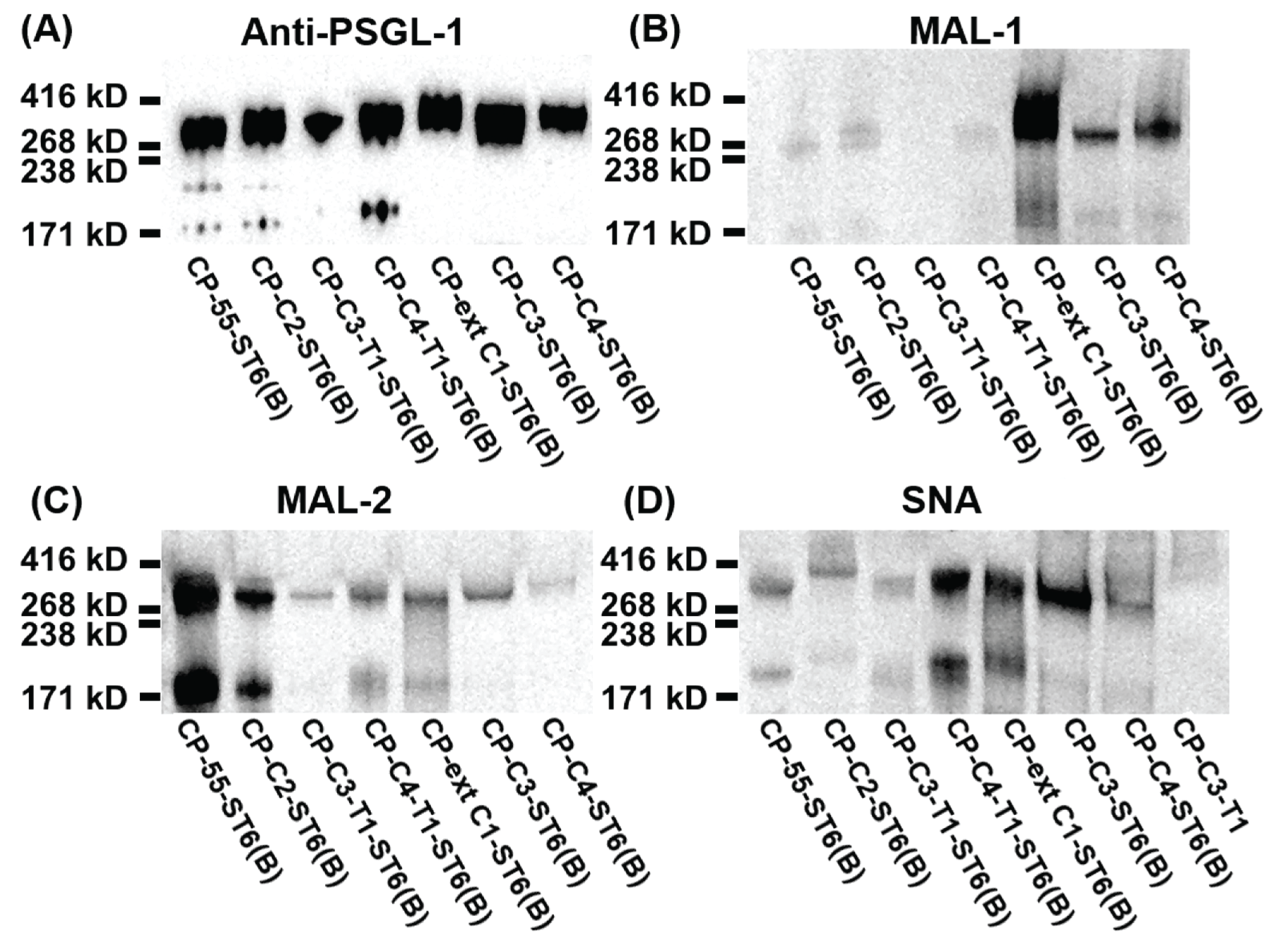
3.4. SDS-PAGE and Western Blotting
3.5. LC-MS of O-Glycans Released from Recombinant Proteins
4. Conclusions
- A number of stable CHO cell lines secreting a mucin-type fusion protein, PSGL-1/mIgG2b, carrying O-glycans with different O-glycan core saccharides (core 2, 98.0%; core 3, 86.2%; core 4, 31.2%; and ext core 1, 23.5%) have been generated.
- Stable expression of human B3GALT5 in the CP-C3-T1 and CP-C4-T1 clones extended the O-glycans with a type 1 outer chain.
- Endogenous ST3GAL(s) activity in CHO cells supports terminal α2,3 sialylation on all O-glycan core structures and on both type 1 and type 2 outer chains, while ST6GALT1 could only α2,6-sialylate the type 2 and not the type1 chain.
- The panel of recombinant mucin-like proteins described here, carrying a repertoire of sialylated O-glycans based on different core saccharides, will be an important tool for determining the fine O-glycan binding specificity of sialic acid-specific microbial adhesins and lectins.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van den Steen, P.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef] [PubMed]
- Elhammer, A.; Kornfeld, S. Purification and characterization of UDP-N-acetylgalactosamine: Polypeptide N-acetylgalactosaminyltransferase from bovine colostrum and murine lymphoma BW5147 cells. J. Biol. Chem. 1986, 261, 5249–5255. [Google Scholar] [PubMed]
- Hang, H.C.; Bertozzi, C.R. The chemistry and biology of mucin-type O-linked glycosylation. Bioorg. Med. Chem. 2005, 13, 5021–5034. [Google Scholar] [CrossRef] [PubMed]
- Hounsell, E.F.; Davies, M.J.; Renouf, D.V. O-linked protein glycosylation structure and function. Glycoconj. J. 1996, 13, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Ten Hagen, K.G. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 2009, 26, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Amado, M.; Almeida, R.; Schwientek, T.; Clausen, H. Identification and characterization of large galactosyltransferase gene families: Galactosyltransferases for all functions. Biochim. Biophys. Acta 1999, 1473, 35–53. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Argueso, P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul. Surf. 2010, 8, 8–17. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997, 11, 248–255. [Google Scholar] [PubMed]
- Gambaryan, A.; Yamnikova, S.; Lvov, D.; Tuzikov, A.; Chinarev, A.; Pazynina, G.; Webster, R.; Matrosovich, M.; Bovin, N. Receptor specificity of influenza viruses from birds and mammals: New data on involvement of the inner fragments of the carbohydrate chain. Virology 2005, 334, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Srinivasan, A.; Raman, R.; Viswanathan, K.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 2008, 26, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Blixt, O.; Glaser, L.; Taubenberger, J.K.; Palese, P.; Paulson, J.C.; Wilson, I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006, 355, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, P.; Cheriyan, M.; Hurtado-Ziola, N.; Brinkman-van der Linden, E.C.M.; Anderson, D.; McClure, H.; Varki, A.; Varki, N.M. Human-specific Regulation of α2-6-linked Sialic Acids. J. Biol. Chem. 2003, 278, 48245–48250. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Chandrasekaran, A.; Srinivasan, A.; Raman, R.; Sasisekharan, V.; Sasisekharan, R. Glycans as receptors for influenza pathogenesis. Glycoconj. J. 2010, 27, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokhbeh, M.R.; Hazra, S.; Alexander, D.A.; Khan, A.; McAllister, M.; Suuronen, E.J.; Griffith, M.; Dimock, K. Enterovirus 70 binds to different glycoconjugates containing α2,3-linked sialic acid on different cell lines. J. Virol. 2005, 79, 7087–7094. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J. Detection and functions of mammalian lectins—With emphasis on membrane lectins. Biochim. Biophys. Acta 1991, 1071, 1–18. [Google Scholar] [CrossRef]
- Kletter, D.; Singh, S.; Bern, M.; Haab, B.B. Global comparisons of lectin-glycan interactions using a database of analyzed glycan array data. Mol. Cell. Proteomics 2013, 12, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, C.; Cherian, R.M.; Karlsson, N.G.; Holgersson, J. O-glycan repertoires on a mucin-type reporter protein expressed in CHO cell pools transiently transfected with O-glycan core enzyme cDNAs. J. Biotechnol. 2015, 199, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. Structure and function of the selectin ligand PSGL-1. Braz. J. Med. Biol. Res. 1999, 32, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qian, Y.; Holgersson, J. Removal of xenoreactive human anti-pig antibodies by absorption on recombinant mucin-containing glycoproteins carrying the Gal alpha1,3Gal epitope. Transplantation 1997, 63, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Sako, D.; Chang, X.J.; Barone, K.M.; Vachino, G.; White, H.M.; Shaw, G.; Veldman, G.M.; Bean, K.M.; Ahern, T.J.; Furie, B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 1993, 75, 1179–1186. [Google Scholar] [CrossRef]
- Holgersson, J.; Löfling, J. Glycosyltransferases involved in type 1 chain and Lewis antigen biosynthesis exhibit glycan and core chain specificity. Glycobiology 2006, 16, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Löfling, J.; Diswall, M.; Eriksson, S.; Borén, T.; Breimer, M.E.; Holgersson, J. Studies of Lewis antigens and H. pylori adhesion in CHO cell lines engineered to express Lewis b determinants. Glycobiology 2008, 18, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lofling, J.; Holgersson, J. Core saccharide dependence of sialyl Lewis X biosynthesis. Glycoconj. J. 2009, 26, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gustafsson, A.; Breimer, M.E.; Kussak, A.; Holgersson, J. Anti-pig antibody adsorption efficacy of α-Gal carrying recombinant P-selectin glycoprotein ligand-1/immunoglobulin chimeras increases with core 2 β1, 6-N-acetylglucosaminyltransferase expression. Glycobiology 2005, 15, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Cherian, R.M.; Gaunitz, S.; Nilsson, A.; Liu, J.; Karlsson, N.G.; Holgersson, J. Shiga-like toxin binds with high avidity to multivalent O-linked blood group P1 determinants on mucin-type fusion proteins. Glycobiology 2014, 24, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Hiraoka, N.; Petryniak, B.; Nakayama, J.; Ellies, L.G.; Rabuka, D.; Hindsgaul, O.; Marth, J.D.; Lowe, J.B.; Fukuda, M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell 2001, 105, 957–969. [Google Scholar]
- Xu, X.; Nagarajan, H.; Lewis, N.E.; Pan, S.; Cai, Z.; Liu, X.; Chen, W.; Xie, M.; Wang, W.; Hammond, S.; et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011, 29, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.C.; Rademacher, C. Glycan terminator. Nat. Struct. Mol. Biol. 2009, 16, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Grabenhorst, E.; Schlenke, P.; Pohl, S.; Nimtz, M.; Conradt, H.S. Genetic engineering of recombinant glycoproteins and the glycosylation pathway in mammalian host cells. Glycoconj. J. 1999, 16, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, N.; Yongky, A.; Johnson, K.C.; Fu, H.-Y.; Jacob, N.M.; Le, H.; Yusufi, F.N.K.; Lee, D.Y.; Hu, W.-S. Global insights into the Chinese hamster and CHO cell transcriptomes. Biotechnol. Bioeng. 2015, 112, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, H. Human glycogene cloning: focus on β3-glycosyltransferase and β4-glycosyltransferase families. Curr. Opin. Struct. Biol. 2006, 16, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Stanley, P. Regulatory mutations in CHO cells induce expression of the mouse embryonic antigen SSEA-1. Cell 1983, 35, 303–309. [Google Scholar] [CrossRef]
- Datti, A.; Dennis, J.W. Regulation of UDP-GlcNAc:Galβ1-3GalNAc-R β1-6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc) in Chinese hamster ovary cells. J. Biol. Chem. 1993, 268, 5409–5416. [Google Scholar] [PubMed]
- Amado, M.; Almeida, R.; Carneiro, F.; Levery, S.B.; Holmes, E.H.; Nomoto, M.; Hollingsworth, M.A.; Hassan, H.; Schwientek, T.; Nielsen, P.A.; et al. A family of human β3-galactosyltransferases: Characterization of four members of a UDP-galactose: β-N-acetyl-glucosamine/β-N-acetyl-galactosamine β-1,3-galactosyltransferase family. J. Biol. Chem. 1998, 273, 12770–12778. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Holgersson, J. Recombinant Galα1,3Gal-substituted mucin/immunoglobulin chimeras: A superior absorber of anti-pig antibodies. Transplant. Proc. 2000. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Thomsson, K.A.; Holmén-Larsson, J.M.; Ångström, J.; Johansson, M.E.V.; Xia, L.; Hansson, G.C. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology 2012, 22, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.; de Souza-e-Silva, U.; Paulson, J.C. Purification of a Gal β 1 to 4GlcNAc α 2 to 6 sialyltransferase and a Gal β 1 to 3(4)GlcNAc α 2 to 3 sialyltransferase to homogeneity from rat liver. J. Biol. Chem. 1982, 257, 13835–13844. [Google Scholar] [PubMed]
- Gustafsson, A.; Sjoblom, M.; Strindelius, L.; Johansson, T.; Fleckenstein, T.; Chatzissavidou, N.; Lindberg, L.; Angstrom, J.; Rova, U.; Holgersson, J. Pichia pastoris-produced mucin-type fusion proteins with multivalent O-glycan substitution as targeting molecules for mannose-specific receptors of the immune system. Glycobiology 2011, 21, 1071–1086. [Google Scholar] [CrossRef]
- Schulz, B.L.; Packer, N.H.; Karlsson, N.G. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 2002, 74, 6088–6097. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherian, R.M.; Jin, C.; Liu, J.; Karlsson, N.G.; Holgersson, J. A Panel of Recombinant Mucins Carrying a Repertoire of Sialylated O-Glycans Based on Different Core Chains for Studies of Glycan Binding Proteins. Biomolecules 2015, 5, 1810-1831. https://doi.org/10.3390/biom5031810
Cherian RM, Jin C, Liu J, Karlsson NG, Holgersson J. A Panel of Recombinant Mucins Carrying a Repertoire of Sialylated O-Glycans Based on Different Core Chains for Studies of Glycan Binding Proteins. Biomolecules. 2015; 5(3):1810-1831. https://doi.org/10.3390/biom5031810
Chicago/Turabian StyleCherian, Reeja Maria, Chunsheng Jin, Jining Liu, Niclas G. Karlsson, and Jan Holgersson. 2015. "A Panel of Recombinant Mucins Carrying a Repertoire of Sialylated O-Glycans Based on Different Core Chains for Studies of Glycan Binding Proteins" Biomolecules 5, no. 3: 1810-1831. https://doi.org/10.3390/biom5031810






